Abstract
Thioredoxin (TRx) is known to control redox homeostasis in cells. In recent years, a specific TRx binding protein called thioredoxin binding protein-2 (TBP-2) was found in other cell types and it appeared to negatively regulate TRx bioavailability and thereby control TRx biological function. In view of the sensitivity of lens transparency to redox status, proper regulation of TRx bioavailability is of the utmost importance. This study was conducted to examine the presence and function of TBP-2 in human lens epithelial cells (HLE B3). We cloned human lens TBP-2 from a human cDNA library (GenBank accession number AY 594328) and showed that it is fully homologous to the human brain TBP-2 gene. The recombinant TBP-2 protein was partially purified and mass spectrometric analysis confirmed its sequence homology to that of brain TBP-2. Immunoprecipitates obtained from HLE B3 cells using anti-TRx and anti-TBP-2 antibodies showed the presence of TRx and TBP-2 in immunoprecipitates indicating the formation of a TRx-TBP-2 complex in vivo. Furthermore, under H2O2-stress conditions, TRx gene expression was transiently up-regulated while TBP-2 gene expression was inversely down-regulated as seen in both HLE B3 cells and in the epithelial cell layers from cultured pig lenses. Cells with overexpressed TBP-2 showed lower TRx activity, grew slower and were more susceptible to oxidative stress-induced apoptosis. This is the first report of the presence of a TRx-specific binding protein in the lens. Our data suggest that TBP-2 is likely a negative regulator for the bioavailability, and therefore, the overall function of TRx in the lens.
Keywords: Thioredoxin, thioredoxin binding protein, oxidative stress, lens epithelial cells
INTRODUCTION
Thioredoxin (TRx) is a small 12-kDa ubiquitous protein with two vicinyl cysteine residues at the active site that provides a strong reducing potential (Holmgren, 1985). TRx dethiolates protein disulfide bonds and regulates the thiol/disulfide homeostasis in the cells. Oxidized TRx is recycled to reduced form by thioredoxin reductase (TR) and NADPH (Holmgren, 1977). In mammalian cells, TRx is known to have a wide range of physiological functions, including donation of electrons for ribonucleotide reductase (Laurent et al., 1964) and methionine sulfoxide reductase (Gonzalez et al., 1970), activation of glucocorticoid (Grippo et al., 1985) and androgen receptors (Wiegand et al., 1989), redox regulation of inflammation (Hayashi, et al. 1993) and apoptosis (Saitoh et al., 1998), and induction of cell proliferation by the reduction of AP-1 transcription factor (Akamatsu et al., 1997, Ueno et al., 1999). Additionally, TRx has antioxidant functions by providing electrons for peroxiredoxins that catalyze the reduction of H2O2 (Kang et al., 1998) and the regeneration of oxidatively damaged proteins (Fernando et al., 1992).
Because of the anatomical locale, the lens is vulnerable to oxidative stress either from the ultraviolet light or other environmental factors as well as the reactive oxygen species (ROS) generated in the mitochondria within the cells. Therefore, the lens depends on various antioxidants, and oxidation defense systems, to detoxify ROS and to keep the cellular environment in a reduced state, thereby maintaining lens transparency (Lou, 2003). Several well-defined oxidation damage repair systems have been identified in the lens in recent years (Lou, 2003). These systems include the GSH-dependent thioltransferase (glutaredoxin) and the NADPH-dependent thioredoxin/thioredoxin reductase systems. The former reduces protein-thiol mixed disulfides specifically while the later reduces protein disulfides and protein sulfenic acids. A third repair system has recently been found in the lens called methionine sulfoxide reductase. This repair system mainly reduces the methionine sulfoxide to methionine in oxidatively damaged proteins (Kantorow et al., 2004).
Unlike thioltransferase, thioredoxin in the lens is not well studied. Human lens TRx has been cloned and is known to participate in the oxidative defense of the lens (Yegorova et al., 2003). Bhuyan and associates have shown that the cytosolic isoenzyme TRx-1 was transiently upregulated in the lens of Emory mouse when exposed to UV radiation. Such treatment showed no effect on the mitochondrial isoform of TRx (TRx-2) (Bhuyan et al., 2002). Our laboratory has shown that exposure of human lens epithelial (HLE-B3) cells to H2O2 caused a transient up-regulation of TRx gene, corroborating the transient increase in enzyme activity and protein level (Yegorova et al., 2003). A similar result in transient TRx-1 up-regulation was found in the epithelium of cultured porcine lenses exposed to H2O2 (Moon et al., 2004). The mechanism of these oxidative stress-induced transient up-regulations of TRx is not understood and how TRx is regulated in cells under normal and stress conditions is an intriguing question.
In mammalian cells, several proteins are known to bind with TRx. For instance, TRx binds with the pre-apoptotic signaling protein, apoptosis activating kinase (ASK), and prevents apoptosis (Saitoh et al., 1998). TRx also binds to p40phox, a cytosolic component of the membrane-bound enzyme NADPH oxidase (NOX), and regulates NOX activity. Because of this binding and regulatory function p40phox is thus named thioredoxin-binding protein-1 (TBP-1) (Nishiyama et al., 1999a). In recent years another protein that specifically binds to TRx and regulates its activity has been identified. This protein is named thioredoxin binding protein-2 (TBP-2) (Nishiyama et al., 1999b), also called thioredoxin-interacting protein (Txnip), was identified in a yeast two-hybrid screen that had been designed to recognize proteins that bind to thioredoxin (Nishiyama et al., 1999b, Chen and DeLuca, 1994, Yamanaka et al., 2000, Junn et al., 2000). TBP-2 has a molecular mass of 46 kDa with 391 amino acid residues and is identical to vitamin D3 up-regulated protein 1 (VDUP-1), a protein inducible by 1, 25-dihydroxy vitamin D3 in human promyelocytic leukemia cells HL60 (Chen and DeLuca, 1994). TBP-2 is a cytosolic protein and is expressed in many mammalian tissues. High TBP-2 levels are often found in cells that also show low TRx levels (Nishiyama et al., 1999b).
The function of TBP-2 is not known but it may act as a negative regulator of TRx (Nishiyama et al., 1999b). Published data suggest that TBP-2 may be involved in cell differentiation and apoptosis (Junn et al., 2000) and in cell proliferation and aging (Yoshida et al. 2005). TBP-2 is also shown to have an anticancer role in mammary carcinomas (Young et al., 1996, Yang et al., 1998). Because of the important role of TRx in redox balance and lens clarity, it is essential to elucidate any regulation of the bioavailability of TRx that may be controlled by TBP-2. Therefore, we set forth to study the relationship of TBP-2 and TRx in the lens. We report in this paper that TBP-2 is present in lens cells and can negatively regulate TRx. We have cloned the human lens TBP-2 gene, isolated the recombinant protein, and demonstrated that TBP-2 is bound to TRx in the human lens epithelial B3 cells (HLE B3). TRx and TBP-2 expressions showed an inverse relationship both in HLE B3 cells and in the epithelia of cultured pig lenses under oxidative stress. TBP-2 overexpression in cells lowered TRx activity, retarded growth, and increased apoptosis in cells pre-exposed to oxidation. This is the first report that lens TRx is regulated in vivo.
MATERIALS AND METHODS
Cell culture and Materials
Human lens epithelial cells (HLE B3), propagated and immortalized as previously described (Andley et al. 1994) were generously provided by Dr. Usha Andley (Washington University, St. Louis, MO). These cells were grown in MEM medium supplemented with 20% fetal calf serum and 50 μg/ml gentamycin in 100 × 20 mm tissue culture plates in humid atmosphere with 5% CO2 at 37°C. Cells reached confluence with 6 × 106 cells / plate in 4 days. Fresh eyes from 6-8 months old pigs were obtained from Farmland Inc (Crete, NE). Human recombinant anti TBP-2 mouse monoclonal antibody was purchased from MBL international Corporation (Woburn, MA). All the other chemicals and reagents were standard commercial products of analytical grade.
Enzyme and other assays
Protein concentrations were determined by BCA following manufacture's protocol (Pierce Chemical Co., Rockford, IL) with bovine serum albumin as the standard. The activity of Trx was determined by a previously described method (Holmgren and Bjornstedt, 1995). Hydrogen peroxide concentration in lens organ culture medium was measured following Hildebrant et al. (Hildebrant et al., 1978).
Recombinant human lens TRx and anti- TRx antibody
Human cDNA for TRx was cloned into pET23a(+) vector, expressed in E. coli, and the TRx was purified using His-bind column as described earlier (Yegorova et al., 2003). Rabbit anti-TRx antibody was prepared by Proteintech Group, Inc. (Chicago, IL, USA) using purified recombinant human TRx. Antiserum was made by immunization of albino rabbit first with subcutaneous injection of 1 mg of recombinant human TRx (emulsified with Freund's complete adjuvant), followed by second injection ( 1mg TRx in Freund's incomplete adjuvant) eight weeks later. Antiserum was collected ten days after the booster and affinity purified by a TRx affinity column, prepared by linking TRx to agarose with cyanogen bromide.
Cloning of human lens TBP-2
To clone human lens TBP-2 cDNA, PCR was carried out with cDNA prepared from the total RNA extracted from HLE-B3 cells as a template. Primers were designed to clone TBP-2 into Escherichia coli expression system (forward primer 5'GAATTCGATGGT GATGTTCAAGAAGATC3'; reverse primer 5'CGCTCGAGTCACTGACAATTGTT GTTGA3'). Both primers were designed based on the known nucleotide sequence of human brain TBP-2 sequence (GenBank accession number NM_006472). The conditions of the PCR were: initial 94°C for 2 min., 30 cycles at 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. The obtained PCR fragments were separated on a 2% (wt/vol) agarose gel, and the band corresponding to 1176 bp were isolated and purified using a gel extraction kit (Qiagen, Valencia, CA). The purified PCR fragment were cloned downstream of the cytomegalovirus (CMV) promoter into PCR 3.1-Uni Vector (Invitrogen, San Diego, CA) and used to transform TOP 10F′ cells (Invitrogen). The transformants were selected on Luria-Bertani (LB)-coated plates with 50 μg/mL kanamycin. The recombinant plasmids designated as pCR-TBP-2 were analyzed for the presence and orientation of the insert by restriction enzymes Xho1 and EcoR1. Sequencing of the TBP-2 insert was performed and analyzed at the DNA Sequencing Facility the University of Nebraska-Lincoln (Lincoln, NE). The sequence was compared with published TBP-2 sequences using NCBI Blast, vector NTI, and GCG programs.
TBP-2 protein expression and purification
Human lens TBP-2 was expressed in E.coli by using pET-His expression system from Novagen (Madison, WI). To clone TBP-2 cDNA fragment into pET 28a(+) vector, primers were modified (forward 5'ACGCGTGCCATG GTG ATG TTC AAG AAG ATC3', reverse 5' CCATCGATTCACTGCACATTGTTGTTGAG 3') by introducing Mul1 and Cla1 restriction sites. The PCR product was then cloned into pET28a (+) between Mul1 and ClaI sites, and the positive expression plasmid pET-TBP-2(His) was used to transform into the BL21 (DE3) E.coli cells (Novagen). For the expression of recombinant TBP-2 protein, transformed BL21 (DE3) cells were grown at 37°C in LB medium with 100 μg/ml kanamycine until the absorbance at 600 nm reached to 0.4-0.6. The cells were induced for TBP-2 expression by 1mM isopropyl-1-thio-β-Dgalactopyranoside (IPTG) for 3-4 hrs and then harvested by centrifugation at 6,000 rpm for 30 min. The cell pellets were resuspended in 30 ml lysis buffer (BugBuster with Benzonaze Nuclease; Novagen), incubated for 20 min at room temperature, followed by centrifugation at 16,000 rpm for 45 min. The precipitate with the inclusion body fraction of the lyzed cells was used to purify TBP-2 using His Bind column (Novagen) according to the manufacturer's protocol. The size and purity of recombinant TBP-2 protein was confirmed by SDS-PAGE and the identity of the protein was confirmed by protein sequencing (Protein sequencing facility, University of Nebraska, Lincoln).
Immunoprecipitation of TBP-2-TRx complex by anti-TRx and anti-tbp-2 antibodies
Both anti-TBP-2 monoclonal antibody and anti-TRx antibodies were used for the immunoprecipitation of TRx-TBP-2 complex in vivo. HLE B3 cell lysate was incubated for 3 hrs at 4°C either with 10 μl (2 μg) of anti-TRx antibodies or with 50 μl (50 μg) of anti-TBP-2 antibodies, followed by adding 20 μl Protein-A Agarose beads (Santa Cruz, CA) for overnight incubation at 4°C. Immunoprecipitate was collected by centrifugation at 2,500 rpm for 5 min at 4°C, washed 4 times with ice-cold washing buffer (150 mM NaCl, 1% Tween 20, 1% deoxycholate, and 20 mM Tris HCL pH 7.5), and then resuspended in 40 μl of 1X electrophoresis sample buffer. Seize® X Protein A Immunoprecipitation kit (PIERCE, IL) was used to immunoprecipitate TRx-TBP-2 complex according to manufacture's protocol. These immunoprecipitates, which were freed from antibodies used for the immunoprecipitation were then used for Western blot analysis.
Effect of H2O2 on TBP-2 expression in HLE B3 cells
HLE B3 cells (4.2 x 106) were used for the study. Prior to H2O2 treatment, the cells were gradually serum-starved by incubating overnight in MEM with 2% FBS and then in serum-free MEM for another 30 min before subjecting to a bolus of 0.1 mM H2O2 for 0, 5, 10, 15, 20, and 30 min. At each time point medium was removed for analysis of H2O2 concentration. Cell lysates were made with 500 μL of lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40 (vol/vol), 1 mM Na3VO4, 0.25% Na-deoxycholate, 1 mM PMSF, 1 mM NaF, and 1 μg/mL each of aprotinin, pepstatin, and leupeptin) and used for immunoblot analysis for TBP-2 and TRx.
Effect of H2O2 on TBP-2 and TRx protein expression in cultured pig lenses
Fresh pig lenses were surgically removed from the eye balls and immediately pre-incubated in 4 ml TC-199 medium for 2 hrs at 37°C in a CO2 incubator. The lenses were exposed to medium with and without 0.2 mM H2O2 (0.1 mM H2O2 and 2.31 units of glucose oxidase for final and constant H2O2 level) for 0, 2, 4, 6, 9, 12, 18, and 24 hrs with 3 lenses per each time point. Fifty μl of the medium was removed at each time interval and used for H2O2 assay. At the same time points, three lenses were removed, rinsed with PBS, epithelial layers peeled off surgically and immediately frozen on dry ice. The pooled three epithelial layers were homogenized in 200 μl ice-cold buffer containing 10 mM Tris-HCL (pH 7.5), 100 mM NaCl, 1 mM EDTA, 10 mM β-mercaptoethanol, 1μg/ml aprotinin, and 100 μg/ml PMSF using Polytron glass hand homogenizer. The homogenate was centrifuged at 13,000 g for 25 min at 4° C, and the supernatant was used for immunoblot analysis.
Extraction of Total RNA and detection of mRNA for TRx and TBP-2 by RT-PCR
In a separate experiment, H2O2-treated and the control untreated pig lenses were used for RT-PCR analysis. The epithelial layers from each time point were peeled off as described above and immediately transferred to RNAlater (QIAGEN Inc., Valencia, CA 91355) RNA stabilization reagent and kept submerged at room temperature for 6 hrs. The total mRNA was then extracted using RNeasy RNA extraction mini kit (QIAGEN), following the manufacturer's protocol. Aliquots containing equal amounts (1μg) of mRNA were reverse transcribed using cloned AMV first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA 92008). Following primers were designed and synthesized to detect TRx, TBP-2 and β-actin cDNA, respectively. TRx: (Forward) 5'-GCTGCCAAGATGGTG AA GCAGATT-3', (Reverse) 5'-GCAACATCCTGACAGTCATCCACA-3'; TBP-2: (Forward) 5'-GAATTCGATGGTGATGTTCAAGAAGATC3' (Reverse) 5'-CGCTCGA GTCACTGACAATTGTTGTTGA3'; β-actin: (Forward) 5'-GTGGGGCGCCCAG GCACCA-3', (Reverse) 5'-CTCCTTAATGTCACGCAGGATTTC-3'. The sizes for the amplification products of TRx, TBP-2, and β-actin were 206 bp, 1176 bp, and 420 bp, respectively. Equal amounts of synthesized cDNA were amplified by PCR using Taq DNA polymerase (Invitrogen) to detect mRNA for TRx, TBP-2 and β-actin. The PCR conditions used were 94° C for 1 min, 50° C for 1 min and 72° C for 1 min and 35 cycles. Aliquots were taken from PCR mixtures and analyzed by 1% agarose gel electrophoresis.
Real-Time PCR
Total mRNA was extracted from HLE B3 cells. cDNA was synthesized from 50ng of total RNA with AMV First-Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CL) according to the manufacturer's instructions. Primers and FAM-labeled probes for human TRx, TBP-2 and β-actin were obtained from Applied Biosystems (Branchburg, NJ). Quantitative real-time PCR (QPCR) was then performed using a real-time detection system called Icycler IQ (Bio-Rad, Hercules, CL). Briefly; reactions were performed in 96-well optical reaction plates of 25 μl volume, containing cDNA equivalent to 50ng of DNase-digested RNA,12.5 μl of TaqMan Universal Master mixture and optimized concentrations of forward and reverse primers. The mRNA expression of TRx and TBP-2 were normalized to the level of GAPDH mRNA as described previously (Livak and Schmittgen 2001).
Western Blot analysis
Tissue or cell homogenates were separated by 10% SDS-PAGE, transferred to a membrane (TransBlot, Bio-Rad), and probed with either anti-TRx, or anti-TBP-2 antibodies diluted in TBST buffer (10 mM Tris-HCl [ph 7.5], 100 mM NaCl and 0.1 % Tween 20), followed by treatment with either goat anti-rabbit IgG-horseradish peroxidase (for membrane probed with rabbit anti human Trx) or goat anti-mouse IgG2b-horseradish Peroxidase (for membranes probed with mouse anti human TBP-2 antibody). Immunodetection was performed with chemiluminescent reagent (Santa Cruz Biotechnology). The immunoblot was analyzed with an imaging system (Fluor-S MAX MultImager, Bio-Rad, Richmond, CA). Densitometric analyses of immunoblots were carried out using an image processing and analysis program, Scion Image (Scion Corporation, Frederick, MD).
Over-expression of TBP-2 in HLE-B3 cells
Sense cDNA for TBP-2 was introduced into the multi-cloning site of Geneticin (G418 sulfate)-resistant mammalian expression vector pcDNA3.1 (+) to construct plasmids. The plasmids were then transfected into HLE-B3 cells using Lipofectamine plus reagent (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Cells transfected with the cDNA were incubated with transfection medium for one day and the cells were passaged into new plates with fresh culture medium. The cells were grown for two days before transferring to fresh medium containing 1 mg/ml geneticin for selection and the cells were fed every four days. After 4 weeks of selection, the cells were passaged into new plates containing fresh medium with 400 μg/ml geneticin and grown to 50-60% confluency before use.
Cell proliferation
To investigate the proliferation of control and TBP-2 over-expressed HLE B3 cells, 1×105 cells were plated in 6 well plates, and the number of cells was counted by using a haemacytometer. The assay was performed over a period of 4 days.
Effect of H2O2 on apoptosis of control and TBP-2 over-expressed HLE-B3 cells
Control and TBP-2 over-expressing HLE B3 cells were cultured in MEM with 20% FBS in 60 mm dishes. When cells were 50-60 % confluent, the serum-containing medium was removed and cells were washed two times with PBS before exposing to serum-free medium with and without 0.1 mM H2O2 for 1 hr. The cells were then placed in fresh MEM with 20% FBS, incubated for another 16 hrs followed by analysis for apoptosis. To quantify apoptosis Annexin V apoptosis detection kit was used (Biovision, Mountain View, CA). The H2O2 pre-treated cells were trypsinized and washed with 20% FBS containing MEM once and then once with PBS. Then the cells were stained with Annexin V-FITC according to manufacturer's protocol and analyzed by flow cytometry (FACScan flow cytometer; Becton Dickinson, Franklin Lakes, NJ). Excitation was set at 558 nm and the emission was measured at 530 nm in 10,000 gated cells using linear amplification. The arithmetic mean fluorescence channel was derived by CellQuest® software (Becton Dickinson, NJ).
RESULTS
Cloning of human lens TBP-2
Reverse transcriptase PCR carried out using total mRNA extracted from HLE B3 cells and primers specific for TBP-2 resulted in a single 1176 bp fragment (data not shown). This cDNA fragment was purified and sequenced. The complete composite sequence of the ORF consisted of 1176 bp, encoding a protein of 391 amino acids with an estimated molecular mass of 46 kDa. The multiple sequence alignment obtained by the clustalW program (data not shown) indicated that the coding region of the human lens TBP-2 gene sequence was identical to that of human brain TBP-2 (Chen and DeLuca, 1994). This nucleotide sequence for human lens TBP-2 gene has been deposited in the GenBank database with accession number AY 594328.
Expression and purification of recombinant human lens TBP-2
Recombinant TBP-2 was partially purified from E coli cells expressing recombinant TBP-2 using a His-bind column. SDS-PAGE analysis of the purified protein showed a single band of 46 kDa, which reacted positively with anti-human TBP-2 monoclonal antibody (data not shown). Mass spectrometric analysis of the band cut from SDS-PAGE indicated that the amino acid sequence of this protein matched to the known sequence of TBP-2 (data not shown) from other sources (Nishiyama et al., 1999b, Chen and DeLuca, 1994).
Immunoprecipitation of TRx with TBP-2 using anti-TRx antibodies
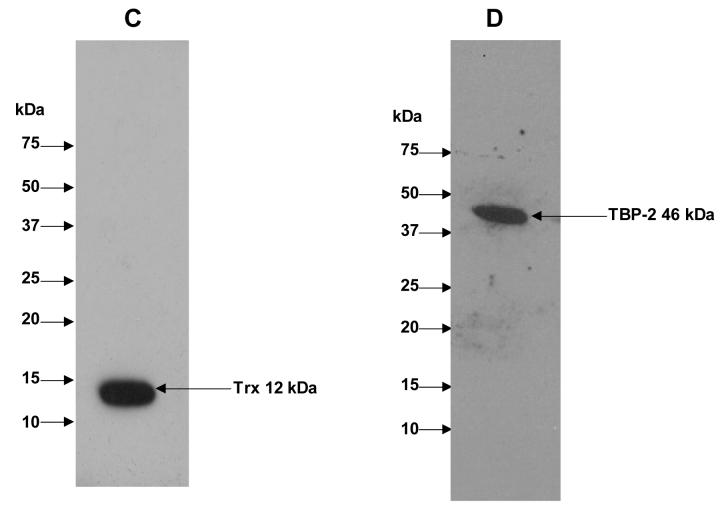
To examine the interaction between TBP-2 and TRx in HLE B3 cells under normal physiological conditions, HLE B3 lysates were immunoprecipitated using either anti-TRx or anti-TBP-2 antibodies. The immunoprecipitated proteins were eluted from protein A agarose using SDS-loading buffer and analyzed by SDS-PAGE, followed by staining with Coomassie brilliant blue R-250. As shown in Fig. 1A, SDS-PAGE separated immunoprecipitate obtained using anti-TRx antibody shows well-separated bands at 50 kDa and 25 kDa representing IgG heavy chain and IgG light chain, respectively. The SDS-PAGE also showed a third band at 12 kDa corresponded to TRx and a fourth band at 46 kDa corresponded to TBP-2 (Fig. 1A). Immunoprecipitate obtained using anti-TBP-2 antibody also showed the presence of TBP-2 and TRx (Fig. 1B). Western blot analysis of the immunoprecipitates with anti-TRx and anti-TBP-2 antibodies showed that the 12 kDa protein band reacted with anti-TRx antibody (Fig. 1C) while the 46 kDa protein reacted with anti-TBP-2 antibody (Fig. 1D), confirming the presence of both TBP-2 and TRx in the immunoprecipitated complex from the HLE B3 cells. Furthermore, the 46 kDa protein band observed in SDS-PAGE (Fig. 1A) was sequence analyzed by mass spectrometry, which further confirmed its identity as TBP-2 (data not shown). These data suggest that TBP-2 interacts with TRx by forming a TRx-TBP-2 complex in HLE B3 cells under normal physiological conditions.
Figure 1. Immunoprecipitation of TBP-2-TRx complex by anti-TRx and anti-TBP-2 antibodies.
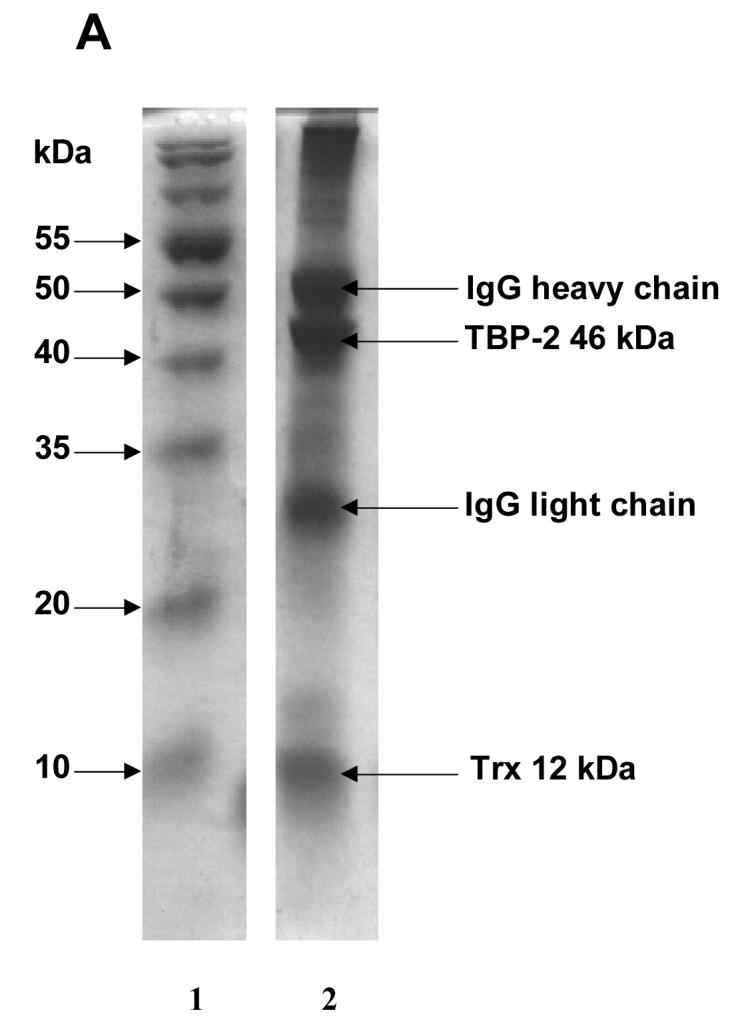
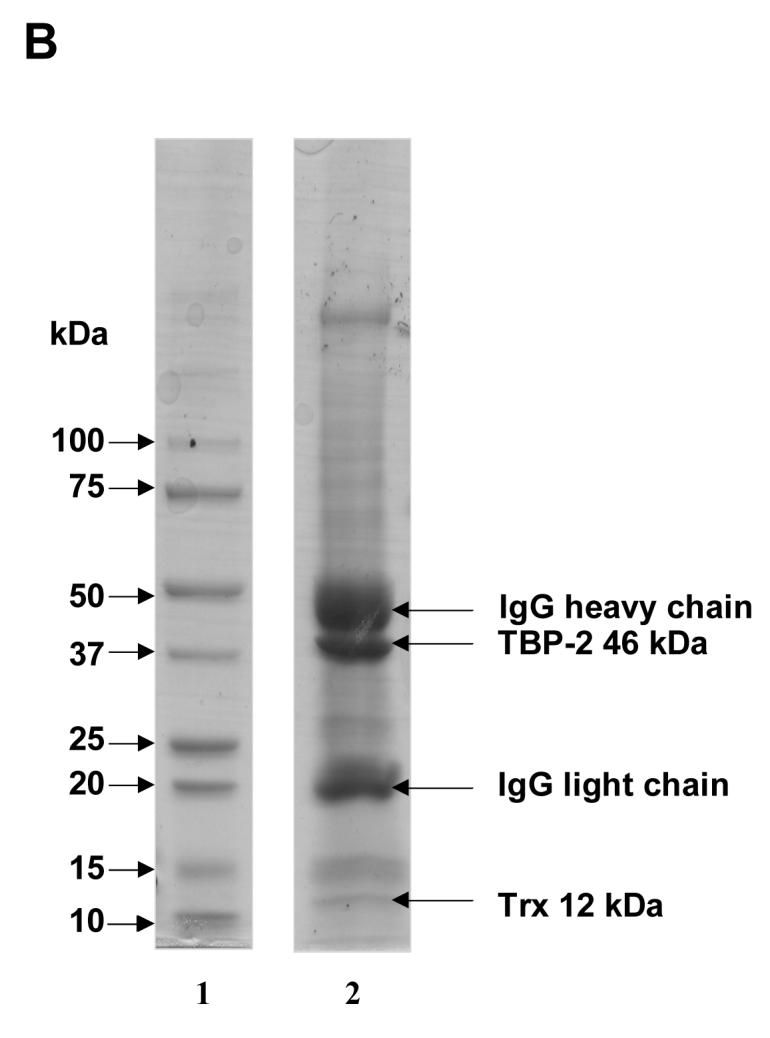
HLE B3 cell lysate was incubated with either anti-TRx or anti-TBP-2 antibody. Protein-A Agarose was added and incubated as described in the Methods. Immunoprecipitate was then collected by centrifugation. A, SDS-PAGE analysis of immunoprecipitate obtained using anti-TRx antibody. Lane 1, Molecular weight marker; lane 2 Immunoprecipitate. B, SDS-PAGE of immunoprecipitate with anti-TBP-2 antibody. Lane 1, Molecular weight marker; lane 2 Immunoprecipitate. C, Western blot of the immunoprecipitate obtained with anti-TRx antibody. D, Western blot analysis of the immunoprecipitate obtained with anti-TBP-2 antibody. For western blot analysis immunoprecipitation was done using Seize® X Protein A Immunoprecipitation kit (PIERCE, IL).
Effect of H2O2 on TBP-2 and TRx mRNA and protein expression in HLE B3 cells
Oxidative stress was able to transiently increase TRx expression both in HLE B3 cells and in the epithelium of organ cultured pig lenses (Yegorova et al., 2003, Moon et al., 2004). Therefore, if TBP-2 can negatively regulate the bioavailability of TRx, oxidative stress may suppress the expression of TBP-2 to allow more available free and functional TRx in the cells. To test this hypothesis, we studied the effect of H2O2 on the expression of TRx and TBP-2 in HLE B3 cells using immunoblot analysis. As shown in Fig. 2A, the cellular TRx protein level is transiently up-regulated during the 30 min treatment with a bolus of 0.1 mM H2O2. TRx was elevated within 5 min after H2O2 exposure and reached maximum by 10 min before returning to the basal level at 30 min. In contrast, TBP-2 protein level shows a transient down-regulation with a pattern that is inversely related to that of TRx. The constant intensity of β-actin shown in the Western blot confirmed that equal amounts of total protein were used for the analysis.
Figure 2. The effect of a 0.1 mM bolus of H2O2 on TRx and TBP-2 protein and mRNA expression in HLE B3 cells.
A, Western blot analysis of cell lysates. HLE B3 cells were treated with H2O2 for indicated time points. Then cells were lysed and subjected to SDS-PAGE (40 μg of protein). The separated proteins were transferred to a nitrocellulose membrane and probed with antibody specific to TBP-2, TRx and β-actin (internal control). Immune complexes were visualized with the use of suitable secondary antibodies. B, Real-time PCR quantification of TRx and TBP-2 mRNA expression. HLE B3 cells were treated with a 0.1 mM bolus of H2O2 for indicated times, cells collected at each time point, mRNA extracted and reverse transcribed as described in methods section. Quantification of mRNA for TRx (◆) and TBP-2 (■) was carried out by real-time PCR as a function of time as described. β-actin was used as an internal control. Data analysis was done by ΔΔCt method. The results are based on the average of 4 determinations. Error bars indicate standard errors of the mean.
To clarify if the changes in TRx and TBP-2 proteins are related to their respective mRNA expression, we conducted real time PCR analysis. Fig. 2B shows the relative mRNA expression for TRx and TBP-2 in HLE B3 cells treated with a bolus of 0.1 mM H2O2. Similar to TRx protein expression there is a transient up-regulation of TRx mRNA expression, which reached to maximum by 10 min and gradually returned to the basal level by 30 min. The pattern of TBP-2 mRNA expression however shows a direct contrast to that of TRx mRNA and is similar to the pattern of TBP-2 protein expression in Figure 2A.
The effect of H2O2 on TRx and TBP-2 expressions in cultured pig lenses
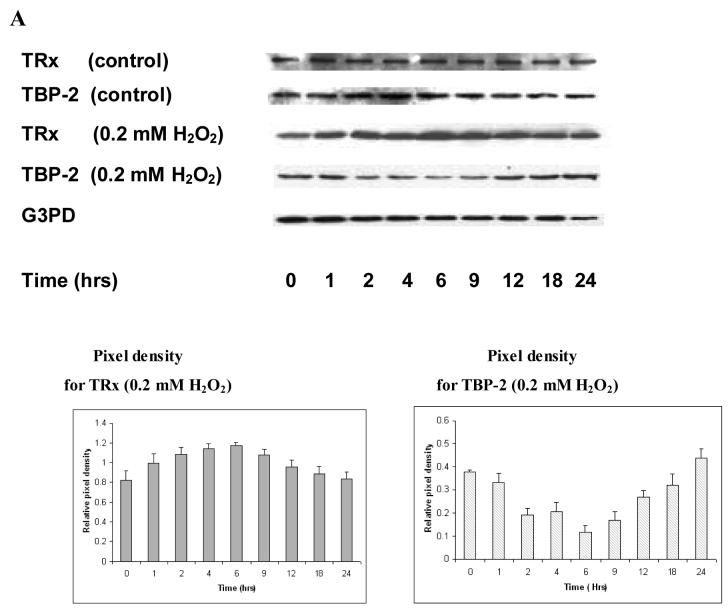
The relationship between TRx and TBP-2 expression under oxidative stress was also tested in intact pig lenses. As shown in Fig. 3A, the epithelial layers of control lenses (no oxidative stress) show constant levels of both TRx and TBP-2 during the entire 24 hr incubation. However, the epithelial layers of the lenses exposed to 0.2 mM H2O2 (constant level) display a transient up-regulation of TRx, in which the expression is elevated in the first hour, reached a maximum by 6 hrs and then gradually declined until it approached the basal level by 24 hrs. TBP-2 in the same samples showed a transient down-regulation with a reciprocal pattern in comparison to TRx. Since most of the TRx activity is located in lens epithelium, we only used lens epithelium for all the analyses. The constant level of G3PD in the control group indicates that equal amounts of proteins were used in the immunoblot analysis.
Figure 3. The effect of H2O2 on TRx and TBP-2 mRNA and protein expression in the epithelium of cultured pig lens.
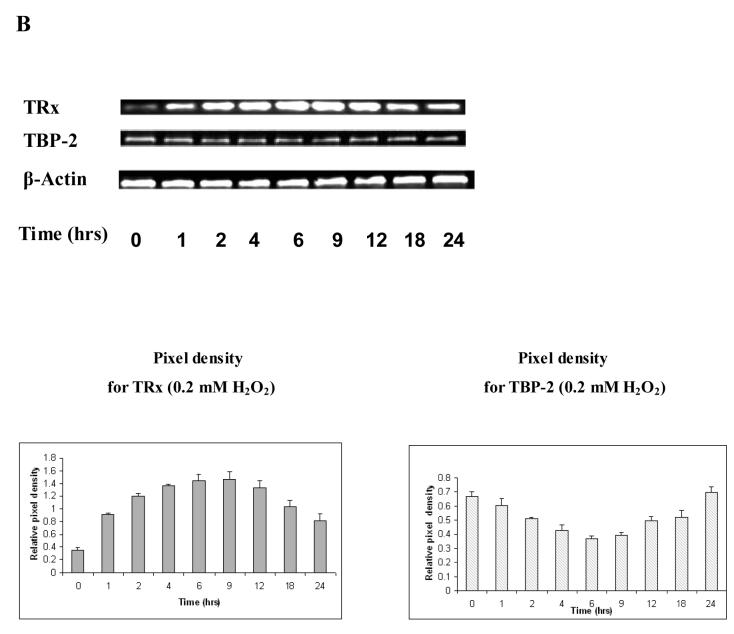
Fresh pig lenses were incubated in TC199 medium with or without 0.2 mM H2O2 for 24hours. The concentration of H2O2 was maintained using 2.31 unit of glucose oxidase in the medium. The lenses were taken at the indicated time points and the epithelial layers were removed. A, Effect of H2O2 on protein expression. The total soluble protein fraction was prepared and 40μg of protein from each time point was subjected to immunoblot analysis with antibodies specific to TRx and TBP-2. Glyceraldehyde-3-phosphate dehydrogenase (G3PD) was used as an internal control. B, Effect of H2O2 on mRNA expression. Lenses were removed at the indicated times and epithelial layers separated. Total RNA was extracted from the three pooled epithelial layers (in each group) and reverse transcribed. Primers were designed and synthesized to detect TBP-2, TRx and β-actin. Equal amounts of synthesized cDNA were amplified by PCR with synthesized primers. PCR products were analyzed by agarose gel electrophoresis. β-actin was amplified as an internal control.
We also studied the expression of mRNA for TRx and TBP-2 in the epithelial layers of pig lenses exposed to 0.2 mM H2O2. As shown in Figure 3B, TRx mRNA is clearly induced after one hr H2O2 exposure, reached to maximum at 6-12 hrs before declining at 18 - 24 hrs. TBP-2 mRNA shows a transient suppression that is reciprocal to the induction pattern of TRx during the 24 hr H2O2 exposure. β-Actin in these epithelial layers showed no change in mRNA expression, indicating that equal amounts of samples were used in each time point.
The effect of TBP-2 over-expression in HLE B3 cells
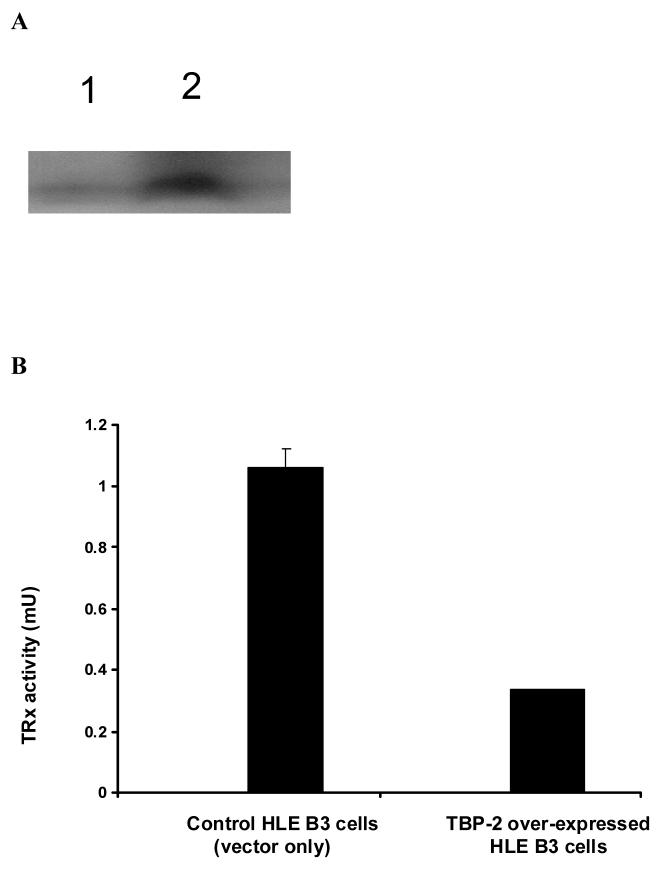
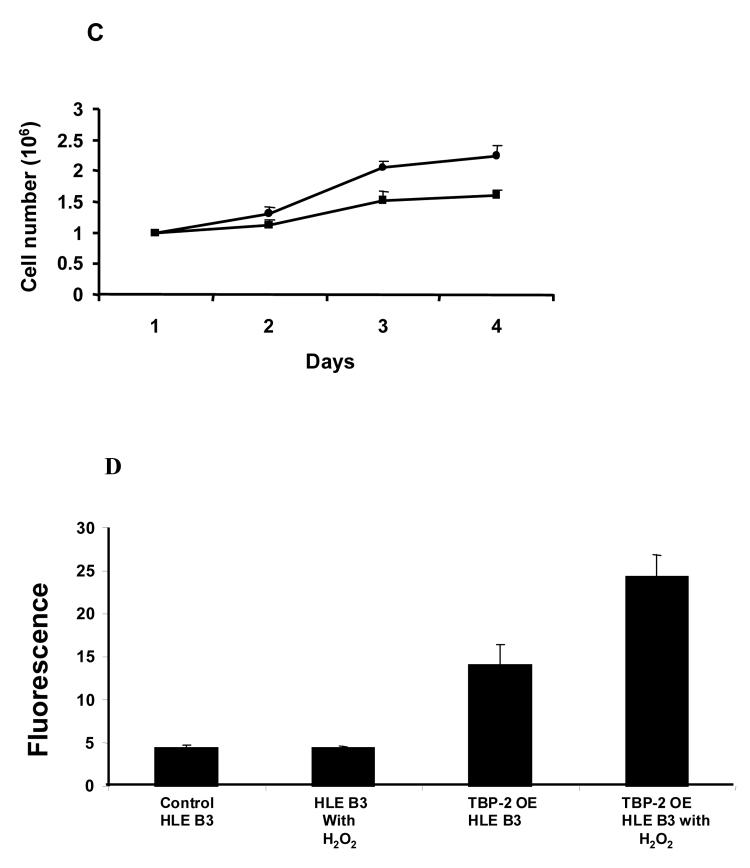
TBP-2 was successfully over-expressed in HLE B3 cells. As shown in the Western blot analysis (Fig. 4A), cells transfected with vector only displayed a low level of TBP-2 while cells transfected with cDNA for TBP-2 displayed at least 5-fold higher TBP-2 expression. Interestingly, the TRx activity in these TBP-2 over-expressed cells was suppressed to less than 1/3 of that of the vector control (Fig. 4B). Over-expression of TBP-2 in cells also affected cell proliferation. As shown in Figure 4C, during the course of 4-day incubation, the TBP-2 over-expressed cells grew slower compared to the control cells (vector only). At the end of the 4-day culturing, the cell population in TBP-2 over-expressed cells was only 70% of that of the control cells.
Figure 4. The effect of TBP-2 over-expression on HLE B3 cells.
A, Western blot analysis of TBP-2 expression in control HLE B3 lysate (lane 1) and TBP-2 over-expressed HLE B3 lysate (lane 2). B, Comparison of the TRx activity in control and TBP-2 over-expressed HLE B3 cells. Cells from both groups (2 × 106 cells from each group) were lysed and TRx activity determined as described. C, Comparison of growth rates of control (●) and TBP-2 over-expressed (■) cells during the 4-day growth period by cell counting. The results are based on the average of 3 determinations. Error bars indicate standard errors of the mean. D, Effect of TBP-2 over-expression on H2O2 mediated apoptosis. Control vector only cells and TBP-2 over-expressed cells (OE) were treated either with serum free MEM or with 100 μM H2O2 for 1 hr. Then the cells were washed with PBS and medium was replaced with 20% serum containing medium and incubated at 37°C for 16 hrs. Cells were trypsinized, washed with PBS, stained with Annexin V-FITC and analyzed by flow cytometry (for details see Materials and Methods). The results are based on the average of 3 determinations. Error bars indicate standard errors of the mean.
Over-expression of TBP-2 also affected cell apoptosis when cells were subjected to oxidative stress. As shown in Figure 4D, 0.1 mM of H2O2 treatment did not induce apoptosis in control cells (vector transfected cells) as determined by FACS analysis. However, TBP-2 over-expressed cells even without H2O2 stress already showed an increased level (3-fold) of apoptosis in comparison to the control (Fig. 4D). Exposing these cells to 0.1 mM H2O2 caused a further increase to 5-fold apoptosis over the control cells.
DISCUSSION
In this paper, we demonstrate for the first time that a regulatory system for thioredoxin is present in the lens epithelial cells. TBP-2 protein, which specifically binds TRx, is present in some mammalian cells and is likely a negative regulator for the intracellular bioavailability of TRx by forming a binding complex in the cytosol (Nishiyama et al., 1999b). TRx is a strong redox regulator and involves in many normal cellular functions. However, it is also known that TRx levels are high in many cancerous cells (Gallegos et al., 2000, Kahlos et al., 2001) while lower TRx levels are associated with some degenerative diseases such as Alzheimer (Lovell et al., 2000). Therefore, proper regulation of TRx bioavailability is of vital importance to the cells and tissues. It is not surprising that a delicate regulatory system is found in the lens, as the lens is extremely sensitive to oxidative stress and many oxidants are known to induce lens opacification (Lou, 2003). Our current study has shown, for the first time, the cloning of the human lens TBP-2 gene and the isolation of the recombinant TBP-2 protein. Even though it was difficult to purify the recombinant protein to homogeneity because the over-expressed TBP-2 was sequestered in the inclusion body of E. coli, we found that the sequence of the lens TBP-2 gene was identical to that of the brain (Chen and DeLuca, 1994) and the mass spectrometric analysis proved that the amino acid sequence of lens TBP-2 was identical to TBP-2 found in other cell types (Nishiyama et al., 1999b, Yoshida et al., 2005).
More importantly, we have found that TRx forms a complex with TBP-2 in HLE B3 cells, based on studies using anti-TRx and anti-TBP-2 antibodies. The immunoprecipitates from anti-TRx antibodies may contain other proteins besides TBP-2, as TRx is known to bind with proteins such as p40phox (Nishiyama et al., 1999a) and ASK-1 (Saitoh et al., 1998). However, when anti-TBP-2 antibody was used to immunoprecipitate TBP-2 from HLE B3 cell lysates, TRx was co-immunoprecipitated with TBP-2, thus confirming the in vivo binding of these two proteins. The amount of TRx in this anti-TBP-2 antibody-induced immuno-complex was not high (Figure 2B). It is expected that in normal conditions most TRx are present in the free-state, presumably used to maintain cellular redox homeostasis.
Interaction of TBP-2 and TRx has been demonstrated both in vivo and in vitro in other cell types (Nishiyama et al., 1999b). It has been reported that the reduced status of the TRx active site and the oxidized status of TBP-2 are critical for the interaction (Nishiyama et al., 1999b). Thus, the redox status of the cell may control TRx-TBP-2 binding. An earlier study has shown that the interaction of TBP-2 and TRx is similar to TRx binding with ASK-1 in which reduced cysteines at the active-site of TRx are required (Nishiyama et al., 1999b). Recently, Patwari et al. (2006) has further clarified that out of the 11 cysteine residues in the TBP-2 protein, only C247 was essential for binding with TRx active site cysteine via a disulfide bridge. Apparently TBP-2 is a unique cytosolic protein that can bind to TRx and negatively controls its activity. Other homologs of TBP-2, such as the intracellular scaffolding protein family of arrestin, could not bind to TRx even though they have the conserved C267 domain similar to human and mouse TBP-2 but lack the essential C247 in the structure (Patwari et al., 2006). Since human lens TRx and TBP-2 have high homology to TRx and TBP-2 found in other mammalian cells, we speculate that the nature of the binding between TRx and TBP-2 in the lens cells may be similar to other cell types.
Our hypothesis that TBP-2 acts as a TRx negative regulator in the lens is supported by the oxidative stress studies using both cultured cells and the epithelial layers from the cultured intact lenses. In each case, oxidative stress transiently up-regulated TRx as we have previously observed (Yegorova et al., 2003, Moon et al., 2004). It is reasonable to expect that cells under oxidative stress would require more TRx as an antioxidant to combat oxidative stress, or as an oxidation damage repair system to maintain redox homeostasis. Lowering cellular levels of TBP-2 could achieve this purpose, as less TBP-2 bound to the active site of TRx would facilitate higher levels of free TRx for antioxidation and repair functions. As shown in our studies, cells treated with a bolus of H2O2 increased TRx expression and created a higher availability of the unbound and active TRx by simultaneously suppressing TBP-2 expression. The intact pig lenses showed similar results as the HLE B3 cells in that TRx in the epithelium of the intact lens was up-regulated while TBP-2 was down-regulated within one hour of H2O2 exposure. This inverse relationship between TBP-2 and TRx lasted for 8 additional hours before the lens was overwhelmed by oxidative stress and lost transparency.
The same phenomenon of the up and down regulations of this protein pair has been seen in vascular smooth muscle cells treated with 200 μM H2O2 (Schulze et al., 2002). This negative regulatory role of TBP-2 is also supported by the findings that lowered activity and suppressed expression of TRx were found in TBP-2 transfected cells, and that recombinant TBP-2 directly inhibited the reducing activity of TRx (Nishiyama et al., 1999b). Consistent with the above observations, TBP-2 overexpressed cells have slower growth rate and less resistance against apoptotic agents (Junn et al., 2000). We were able to demonstrate the same pattern, in which TBP-2 over-expression in human lens epithelial cells suppressed TRx activity, cell growth and caused the cells to be more susceptible to oxidative stress-induced apoptosis. TRx is known to participate in the apoptotic signaling process by binding to the pro-apoptotic factor ASK-1 so that apoptosis would not proceed (Saitoh et al., 1998). TRx has also been shown as a key reducing agent for Ref-1, so that the reduced Ref-1 can activate AP-1 transcription factor for binding with its target DNA thereby regulating expression. AP-1 is known to stimulate various gene expressions related to cell proliferation in many cell types, including the lens epithelial cells (Krysan et al., 2002). Therefore, when there is more TBP-2 present in the cells, it is likely that more TBP-2 and TRx complexes are formed, resulting in less active TRx available to exercise many functions, including cell proliferation and anti-apoptotic activities.
Besides the down-regulation of TBP-2 during oxidative stress caused by H2O2, other stresses such as heat shock, UV, or TGF-β1 have been shown to up-regulate TBP-2 in murine T cell hybridoma (Junn et al., 2000). High glucose levels also stimulated TBP-2 expression in human aortic smooth muscle cells (Lovell et al., 2000). TBP-2 was found to be induced by SAHA, an anticancer agent (Butler et al., 2002), by 1, 25-dihydoxyvitamin D3, an agent known to induce differentiation and maturing of granulocytes (Chen and DeLuca, 1994) and by over-grown HL-60 cells (Junn et al., 2000). These observations support the hypothesis that TBP-2 regulates redox-dependent cell proliferation through interaction with TRx (Schulze et al., 2002).
In summary, we demonstrated the presence of TBP-2 in HLE B3 cells and evaluated the role of TBP-2 in relation to TRx under H2O2 stress conditions. We speculate that TBP-2 may play a major role in negatively regulating TRx in normal physiological as well as under various stress conditions. Further study is warranted in this area of research.
ACKNOWLEDGEMENTS
We appreciate Dr. Stefan Löfgren for his assistance in dissecting the pig lenses for the study, and Joel Lechner for reading of this manuscript. This research was supported by NIH RO1 10595 (MFL).
Footnotes
Presented in part at the annual meeting for the Association for Research in Vision and Ophthalmology at Fort Lauderdale, FL, May, 2005. This research was conducted as a partial fulfillment of the master's degree for NL.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akamatsu Y, Ohno T, Hirota K, Kagoshima H, Yodoi J, Shigesada K. Redox regulation of the DNA binding activity in transcription factor PEBP2. The roles of two conserved cysteine residues. J. Biol. Chem. 1997;272:14497–14500. doi: 10.1074/jbc.272.23.14497. [DOI] [PubMed] [Google Scholar]
- Andley UP, Rhim JS, Chylack LT, Jr., Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1994;35:3094–3102. [PubMed] [Google Scholar]
- Bhuyan KC, Reddy PG, Bhuyan DK. Thioredoxin genes in lens: regulation by oxidative stress. Methods Enzymol. 2002;347:421–435. doi: 10.1016/s0076-6879(02)47042-5. [DOI] [PubMed] [Google Scholar]
- Butler LM, Zhou X, Xu WS, Scher HI, Rifkind RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc. Natl. Acad. Sci. USA. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KS, DeLuca HF. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994;1219:26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Fernando MR, Nanri H, Yoshitake S, Nagata-Kuno K, Minakami S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur. J. Biochem. 1992;209:917–922. doi: 10.1111/j.1432-1033.1992.tb17363.x. [DOI] [PubMed] [Google Scholar]
- Gallegos A, Raffel J, Bhattacharyya AK, Powis G, Silberstein DS. Increased expression of thioredoxin in human primary and metastatic colon cancer. Amer. Assoc. Cancer Res. 2000;41:189. [Google Scholar]
- Gonzalez PP, Baldesten A, Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J. Biol. Chem. 1970;245:2371–2374. [PubMed] [Google Scholar]
- Grippo JF, Holmgren A, Pratt WB. Proof that the endogenous, heat-stable glucocorticoid receptor-activating factor is thioredoxin. J. Biol. Chem. 1985;260:93–97. [PubMed] [Google Scholar]
- Hayashi T, Ueno Y, Okamoto T. Oxidative regulation of nuclear factor kappa B. Involvement of a cellular reducing catalyst thioredoxin. J. Biol. Chem. 1993;268:11380–11388. [PubMed] [Google Scholar]
- Hildebrant AG, Roots I, Tjoe M, Heinemeyer G. Hydrogen peroxide in hepatic microsomes. Methods Enzymol. 1978;52:342–350. doi: 10.1016/s0076-6879(78)52037-5. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its functions in disulfide reduction. J. Biol. Chem. 1977;252:4600–4606. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu. Rev. Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J. Immunol. 2000;164:6287–95. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- Kahlos K, Soini Y, Saily M, Koistinen P, Kakko S, Paakko P, Holmgren A, Kinnula VL. Up-regulation of thioredoxin and thioredoxin reductase in Human malignant pleural mesthelioma. Int. J. Cancer. 2001;95:198–204. doi: 10.1002/1097-0215(20010520)95:3<198::aid-ijc1034>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J. Biol. Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- Kantorow M, Hawse JR, Cowell TL, Benhamed S, Pizarro GO, Reddy VN, Hejtmancik JF. Methionine sulfoxid reductase A is important for lens cell viability and resistance to oxidative stress. Pro. Nat. Acad. Sci. USA. 2004;101:9654–9659. doi: 10.1073/pnas.0403532101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysan K, Lou MF. Human thioltransferease (TTase) gene is controlled by AP-1 and mediated through redox signaling. Invest. Ophthalmol. Vis. Sci. 2002;43:1876–1883. [PubMed] [Google Scholar]
- Laurent TC, Moore EC, Reichard P. Enzymatic synthesis of deoxyribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J. Biol. Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lou MF. Redox regulation in the lens. Prog. Retin. Eye. Res. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Chengsong X, Gabbita SP, Markesbery WR. Decreased thioredoxin and increased thioredoxin reductase levels in alzheimer's disease brain. Free Red. Biol. Med. 2000;28:418–427. doi: 10.1016/s0891-5849(99)00258-0. [DOI] [PubMed] [Google Scholar]
- Moon S, Fernando MR, Lou MF. Induction of thioltransferase and thioredoxin/thioredoxin reductase systems in cultured porcine lenses under oxidative stress. Invest. Ophthalmol. Vis. Sci. 2005;46:3783–3789. doi: 10.1167/iovs.05-0237. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Ohno T, Iwata S, Matsui M, Hirota K, Masutani H, Nakamura H, Yodoi J. Demonstration of the interaction of thioredoxin with p40phox, a phagocyte oxidase component, using a yeast two-hybrid system. Immunol. Lett. 1999a;68:155–159. doi: 10.1016/s0165-2478(99)00045-0. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Matsui M, Iwata S, Hirota K, Masutani H, Nakamura H, Takagi Y, Sono H, Gon Y, Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999b;274:21645–50. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- Patwari P, Higgins LJ, Chutkow WA, Yoshioka J, Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze PC, De Keulenaer GW, Yoshioka J, Kassik KA, Lee RT. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ. Res. 2002;91:689–695. doi: 10.1161/01.res.0000037982.55074.f6. [DOI] [PubMed] [Google Scholar]
- Ueno M, Masutani H, Arai RJ, Yamauchi A, Hirota K, Sakai T, Inamoto T, Yamaoka Y, Yodoi J, Nikaido T. Thioredoxin-dependent redox Regulation of p53-mediated p21 activation. J. Biol. Chem. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- Wiegand M, Ofenloch HB, Eisele KJ. Reactivation of the androgen receptor from murine preputial gland by thioredoxin or GSH. Steroid Biochem. 1989;32:53–58. doi: 10.1016/0022-4731(89)90013-7. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Maehira F, Oshiro M, Asato T, Yanagawa Y, Takei H, Nakashima Y. A possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem. Biophys Res. Commun. 2000;271:796–800. doi: 10.1006/bbrc.2000.2699. [DOI] [PubMed] [Google Scholar]
- Yang X, Young LH, Voigt JM. Expression of a vitamin D-regulated gene (VDUP-1) in untreated- and MNU-treated rat mammary tissue. Breast cancer Res. Treat. 1998;48:33–44. doi: 10.1023/a:1005929714900. [DOI] [PubMed] [Google Scholar]
- Yegorova S, Liu A, Lou MF. Human lens thioredoxin: Molecular cloning, and functional characterization. Invest. Ophthalmol. Vis. Sci. 2003;44(8):3263–3271. doi: 10.1167/iovs.02-1322. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Nakamura H, Masutani H, Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann. N. Y. Acad. Sci. 2005;1055:1–12. doi: 10.1196/annals.1323.002. [DOI] [PubMed] [Google Scholar]
- Young LH, Yang X, Voigt JM. Alteration of gene expression in rat mammary tumors induced by N-methyl-N-nitrosourea. Mol. Carcinog. 1996;15:251–60. doi: 10.1002/(SICI)1098-2744(199604)15:4<251::AID-MC2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]