Abstract
Erk1/Erk2 MAP kinases are key regulators of cell behaviour and their activation is generally associated with tyrosine kinase signalling. However, TGF-β stimulation also activates Erk MAP kinases through an undefined mechanism, albeit to a much lower level than receptor tyrosine kinase stimulation. We report that upon TGF-β stimulation, the activated TGF-β type I receptor (TβRI) recruits and directly phosphorylates ShcA proteins on tyrosine and serine. This dual phosphorylation results from an intrinsic TβRI tyrosine kinase activity that complements its well-defined serine-threonine kinase function. TGF-β-induced ShcA phosphorylation induces ShcA association with Grb2 and Sos, thereby initiating the well-characterised pathway linking receptor tyrosine kinases with Erk MAP kinases. We also found that TβRI is tyrosine phosphorylated in response to TGF-β. Thus, TβRI, like the TGF-β type II receptor, is a dual-specificity kinase. Recruitment of tyrosine kinase signalling pathways may account for aspects of TGF-β biology that are independent of Smad signalling.
Keywords: Erk, MAP kinase, receptor, ShcA/TGF-β
Introduction
Cell behaviour is regulated by growth factors that activate transmembrane receptor kinases. The substrates of these kinases initiate enzymatic cascades that ultimately regulate gene transcription. Although growth factors associate with specific receptors, the pathways initiated by these kinases are highly interconnected. Entire classes of receptors phosphorylate identical substrates, whereas multiple pathways converge on key intracellular effectors. Prominent among these effectors are the Erk1/Erk2 MAP kinases. Erk1 and Erk2 phosphorylate an array of transcription factors, thereby regulating cell proliferation, apoptosis and differentiation (Qi and Elion, 2005). MAP kinases represent the final cytoplasmic components of signalling pathways initiated by receptor tyrosine kinases and G protein-coupled receptors.
Shc adaptor proteins are substrates of receptor tyrosine kinases, and their phosphorylation initiates signalling events that culminate in Erk activation (Ravichandran, 2001). Among the three related Shc proteins, ShcA is ubiquitously expressed, whereas ShcB and ShcC are restricted to cells of neural origin. ShcA, in turn, is expressed as three isoforms. The prototype p52ShcA consists of an N-terminal PTB domain followed by CH1 and SH2 domains. p66ShcA is identical to p52ShcA except for an added N-terminal CH2 domain, whereas p46ShcA results from N-terminal truncation of the p52ShcA PTB domain. The PTB and SH2 domains both bind tyrosine-phosphorylated peptides, and either may associate with activated receptor kinases (Kavanaugh and Williams, 1994). Tyrosine phosphorylation enables p52ShcA to bind the Grb2 adaptor and the Sos GTP-exchange factor (van der Geer et al, 1996). The ShcA/Grb2/Sos complex converts Ras into its active GTP-bound form, leading to the sequential activation of c-Raf, MEK and Erk1/Erk2. In contrast, p66ShcA antagonises Erk activation, possibly by sequestering Grb2 (Migliaccio et al, 1997), and mediates an oxidative stress signalling function (Migliaccio et al, 1999). p46ShcA is targeted to mitochondria (Ventura et al, 2004), where its role is unclear.
TGF-β stimulation also activates Erk MAP kinases, albeit to a much lower level than receptor tyrosine kinases (Mulder, 2000). TGF-β proteins are key regulators of development and tissue differentiation (Attisano and Wrana, 2002), and signal through complexes of type II (TβRII) and type I (TβRI) receptors that are characterised as serine-threonine kinases (Chen et al, 1995). However, TβRI and TβRII kinase domains share homologies with tyrosine kinases (Manning et al, 2002), and TβRII autophosphorylates on tyrosine as well as on serine and threonine (Lawler et al, 1997). Engagement of the receptor complex activates TβRI, which phosphorylates and activates Smad2 and Smad3 (Derynck and Zhang, 2003). These proteins then complex with Smad4, translocate to the nucleus, and associate with DNA-binding complexes to regulate gene transcription.
The Smad pathway does not explain Erk activation by TGF-β. The kinetics of this process vary with cell type and culture conditions (Massagué, 2000). In some cell lines, delayed activation suggests an indirect response requiring protein translation (Simeone et al, 2001), whereas in others, activation is rapid and comparable to signalling by mitogenic factors such as EGF (Olsson et al, 2001). Although TGF-β induces modest Ras activation consistent with low level Erk induction (Mulder, 2000), the mechanisms underlying this induction are unclear.
Despite its low level, Erk activation is important to TGF-β signalling. First, Erk activation and Smad signalling are both necessary for TGF-β-induced epithelial-mesenchymal transformation (Davies et al, 2005), a key event in neoplastic invasion and metastasis. Second, Erk MAP kinases phosphorylate receptor-activated Smads to regulate their nuclear translocation (Kretzschmar et al, 1999). Finally, Erk substrates interact with Smads to regulate gene expression (Mucsi et al, 1996). Thus, the mechanism by which TGF-β activates Erk MAP kinases is an unresolved issue of considerable interest (Massagué, 2000). We now report that upon TGF-β stimulation, TβRI phosphorylates ShcA on serine and, to a lesser degree, on tyrosine to activate Erk MAP kinases.
Results
TGF-β rapidly induces serine and tyrosine phosphorylation of ShcA
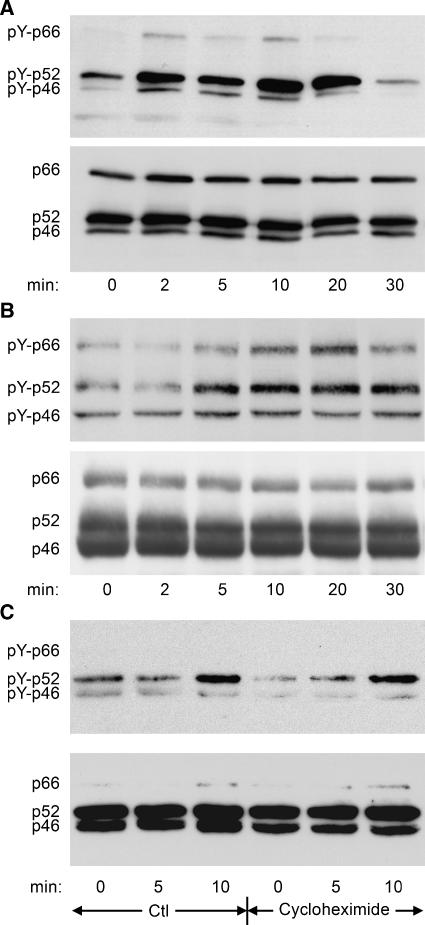
TGF-β-induced ShcA tyrosine phosphorylation was assessed in Mv1Lu mink epithelial cells and 3T3-Swiss mouse fibroblasts using anti-phosphotyrosine antibodies. TGF-β stimulated rapid tyrosine phosphorylation of all three isoforms that peaked after 5–20 min (Figure 1A and B; quantified in Supplementary Figure 1). The tyrosine phosphorylation of ShcA induced by TGF-β was generally higher in 3T3-Swiss than in Mv1Lu cells, but much lower than that induced by EGF (data not shown; Figure 2A). The rapid ShcA phosphorylation suggested that new gene expression was not required. This was confirmed by persistent TGF-β-induced ShcA tyrosine phosphorylation in the presence of the protein synthesis inhibitor cycloheximide (Figure 1C).
Figure 1.
TGF-β induces ShcA tyrosine phosphorylation. (A, B) Anti-phosphotyrosine Western blot of ShcA immunoprecipitated from lysates of Mv1Lu cells (A) or 3T3-Swiss cells (B) treated with 4 ng/ml TGF-β for the indicated times (upper panels), together with ShcA immunoblots of the same membranes (lower panels). (C) Anti-phosphotyrosine Western blot of ShcA immunoprecipitated from lysed 3T3-Swiss cells treated with or without cycloheximide before and concomitant with stimulation with 4 ng/ml TGF-β for the indicated times. The same membrane was reprobed for ShcA (lower panel).
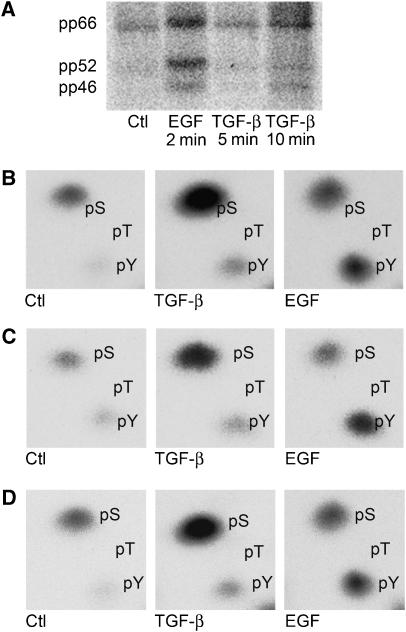
Figure 2.
TGF-β induces ShcA phosphorylation on serine and tyrosine. (A) Autoradiogram of 3T3-Swiss cells cultured in the presence of 32P-[PO4] and treated with 4 ng/ml TGF-β, 20 ng/ml EGF, or neither (Ctl) for the indicated times. (B–D) Phosphoamino acid analysis of in vivo 32P-phosphorylated p66ShcA (B), p52ShcA (C), or p46ShcA (D) isolated from the membrane shown in (A). The 32P-labelled amino acids migrated in the same positions as unlabelled phosphoserine and phosphotyrosine added to the reaction mixture. No 32P-labelled phosphothreonine was detected.
TGF-β-induced ShcA phosphorylation in vivo was characterised by phosphoamino acid analysis. 32P-labelled ShcA was immunoprecipitated, isolated by SDS–PAGE (Figure 2A), and acid hydrolysed. The labelled amino acids were then resolved by two-dimensional electrophoresis. For all three ShcA isoforms, serine and, to a much lesser extent, tyrosine phosphorylation increased rapidly after TGF-β stimulation (Figure 2B–D). In contrast, only tyrosine was phosphorylated by EGF stimulation.
Roles of type II and type I receptors in TGF-β-induced ShcA phosphorylation
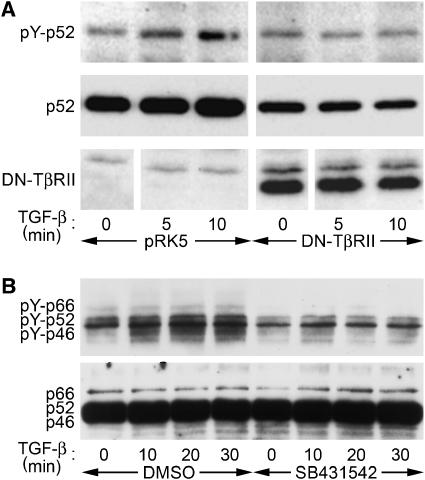
TGF-β binding induces TβRII to phosphorylate TβRI, which in turn phosphorylates Smad proteins. To address the role of TβRII in TGF-β-induced ShcA phosphorylation, we expressed tagged ShcA in Mv1Lu cells together with a cytoplasmic truncation of TβRII, a kinase-deficient point mutant of TβRII, or a control vector. TGF-β-induced ShcA tyrosine phosphorylation was abrogated by truncated (Figure 3A) or kinase-deficient TβRII (data not shown), indicating that TβRII signalling is essential for this response.
Figure 3.
TβRII and TβRI receptors are required for TGF-β-induced ShcA tyrosine phosphorylation. (A) Anti-phosphotyrosine Western blot of HA-tagged p52ShcA immunoprecipitated from Mv1Lu cells that do or do not coexpress a cytoplasmically truncated, dominant-negative version of TβRII. (B) Anti-phosphotyrosine blot of ShcA immunoprecipitated from 3T3-Swiss cells treated with 4 ng/ml TGF-β for the indicated times after 30 min pretreatment with 10 μM TβRI inhibitr SB431542 or DMSO solvent.
We also treated 3T3-Swiss cells with SB431542, a specific inhibitor of TβRI (Inman et al, 2002). SB431542 treatment prevented TGF-β from inducing ShcA tyrosine phosphorylation (Figure 3B; quantified in Supplementary Figure 2). Together, these data indicate that upon activation by TβRII, TβRI induces ShcA phosphorylation.
ShcA proteins interact with TGF-β receptors
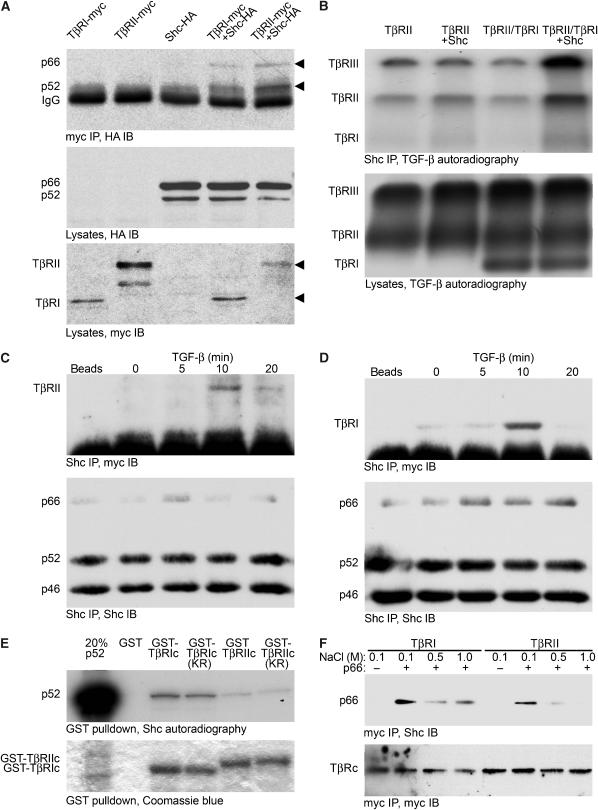
Rapid ShcA phosphorylation following TGF-β stimulation led us to hypothesise that ShcA proteins physically interact with TGF-β receptors. To evaluate this postulate, COS cells were transfected to coexpress p66ShcA and p52ShcA with TβRII or TβRI. p46ShcA was not evaluated as its role in signalling is unclear. As shown in Figure 4A, p66ShcA and p52ShcA co-precipitated with TβRII and TβRI, with p66ShcA co-precipitating less efficiently than p52ShcA. As p66ShcA differs from p52ShcA only in the addition of an N-terminal CH2 domain, we conclude that this domain is not required for receptor interaction and may decrease interaction efficiency.
Figure 4.
ShcA interacts with TGF-β receptors. (A) p66ShcA and p52ShcA co-precipitate with TGF-β receptors in COS cells. Cells expressing myc-tagged TβRI or TβRII, and/or HA-tagged p66ShcA and p52ShcA, as indicated, were subjected to myc immunoprecipitation followed by HA immunoblotting (top). Expression of ShcA and TGF-β receptors was monitored by HA (middle) or myc (bottom) Western analysis of cell lysates. (B) ShcA interacts with cell surface TGF-β receptors. 3T3-Swiss cells were transfected to express TβRII and/or TβRI with or without p66ShcA, and cell surface receptors were radiolabelled with 125I-TGF-β1 followed by chemical crosslinking. Immunoprecipitation of both endogenous and transfected ShcA followed by autoradiography (top panel) demonstrated co-precipitation of 125I-labelled TGF-β receptors with ShcA. 125I-labelled TGF-β receptors did not precipitate when the ShcA antibody was replaced with non-immune IgG (data not shown). Autoradiography of total cell lysates (below) showed equivalent levels of 125I-labelled TGF-β receptors. (C, D) Ligand-dependent interaction of endogenous ShcA and TGF-β receptors in mammalian cells. 3T3-Swiss cells were treated with 4 ng/ml TGF-β for the indicated times, lysed, and subjected to ShcA immunoprecipitation followed by TβRII (C) or TβRI (D) immunoblotting. Control precipitations without primary antibody are shown (‘beads'). Subsequent ShcA immunoblots (lower panels) confirmed equivalent precipitation efficiencies. (E) In vitro association of p52ShcA with TβRI and TβRII cytoplasmic domains. In vitro translated 35S-labelled p52ShcA was incubated with GST, GST-fused TβRI or TβRII cytoplasmic domains, or their kinase-deficient point mutants (TβRIc, TβRIIc, TβRIc KR, and TβRIIc KR, respectively). 35S-labelled ShcA adsorbed to the GST fusion proteins was visualised by SDS–PAGE and autoradiography (upper panel). To confirm identity, 35S-labelled p52ShcA was loaded in lane 1 at 20% of the volume used in the binding assay. Equivalent GST fusion protein loading was confirmed by Coomassie blue staining. (F) ShcA association with TβRI and TβRII cytoplasmic domains in vitro depends on NaCl concentration. Myc-tagged proteins incorporating either TβRIc or TβRIIc were expressed in COS cells, isolated by myc immunoprecipitation, and bound to recombinant p66ShcA. The complexes were washed in otherwise identical buffers containing the indicated NaCl concentrations and subjected to ShcA immunoblotting (above). Myc Western analysis of the blot confirmed equivalent recovery of TβRIc and TβRIIc (below).
We also assessed whether ShcA interacts with TGF-β receptors at the cell surface. 3T3-Swiss cells expressing TβRII with or without coexpressed TβRI were incubated with 125I-TGF-β1. The radiolabelled ligand was then chemically crosslinked to its receptors, and endogenous ShcA was immunoprecipitated. 125I-TGF-β1 binds TβRII or TβRII in complex with TβRI (TβRII/RI), but not TβRI alone (Yamashita et al, 1994). Under these conditions, 125I-TGF-β1 also crosslinks to TβRIII. As shown in Figure 4B, 125I-TGF-β-labelled TβRII interacted with endogenous ShcA. Increased expression of p66ShcA (or p52ShcA; data not shown) enhanced the co-precipitation of TβRII alone or TβRI co-expressed with TβRII, whereas the total level of 125I-TGF-β-labelled receptors remained unaffected (Figure 4B, lanes 2 and 4). Because 125I-TGF-β1 co-precipitated with ShcA more efficiently when TβRI was coexpressed (Figure 4B, lane 4 versus lane 3), we suggest that ShcA interacts more efficiently with the TβRII/RI complex than with TβRII alone.
In nontransfected 3T3-Swiss cells, the interaction between endogenous ShcA and TβRII or TβRI was greatly enhanced by TGF-β stimulation (Figure 4C and D). This association was maximal at 10 min, coincident with peak TGF-β-induced ShcA phosphorylation. The low level interaction of ShcA with TβRI in the absence of exogenous TGF-β likely reflects autocrine TGF-β signalling, which is common in cultured cells (Derynck et al, 2001).
The preferential interaction of ShcA with TβRI was also assessed in vitro. In these assays, in vitro translated, 35S-labelled p52ShcA interacted more efficiently with wild-type or kinase-deficient glutathione-S-transferase (GST)-fused TβRI cytoplasmic domains than with TβRII cytoplasmic domains (Figure 4E). The interactions of p52ShcA with the cytoplasmic domains of TβRI or TβRII in vitro were attenuated by increasingly stringent NaCl concentrations. Again, the interaction of ShcA with TβRI was more stable than with TβRII (Figure 4F). These results suggest that ShcA associates specifically and directly with TGF-β receptor cytoplasmic domains, and more strongly with TβRI than with TβRII.
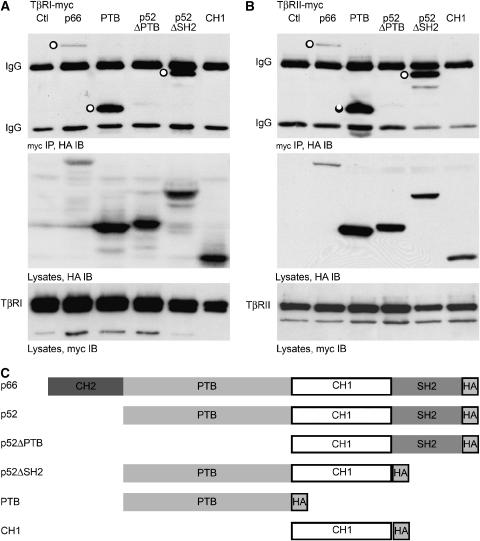
p66ShcA and p52ShcA share a PTB domain, a CH1 domain, and an SH2 domain (Migliaccio et al, 1997). To determine which domains interact with TGF-β receptors in vivo, we evaluated several ShcA truncations. Deletion of the PTB domain abolished the interaction, whereas deletion of the SH2 domain did not detectably alter the interaction of ShcA with TβRI or TβRII (Figure 5). The CH1 domain alone did not interact with either receptor, whereas the PTB domain alone interacted efficiently with both. This association of the PTB domain with TβRI was confirmed in vitro (Supplementary Figure 3). We conclude that the PTB domain, present in all ShcA isoforms, is required for efficient association with TGF-β receptors.
Figure 5.
ShcA associates with TGF-β receptors through its PTB domain. p66ShcA differs from p52ShcA only by an N-terminal addition of a CH2 domain; otherwise, both p66ShcA and p52ShcA are comprised of PTB and SH2 phosphotyrosine-binding domains flanking a central CH1 domain. HA-tagged p66ShcA, ShcA fragments, or p52ShcA deficient in the SH2 domain (ΔSH2) or the PTB domain (ΔPTB) were expressed in 293 cells together with myc-tagged cytoplasmic domains of TβRI or TβRII. (A, B) HA Western blot of myc immunoprecipitates (top), HA immunoblot (centre), and myc immunoblot (bottom) of cells co-transfected with TβRI-myc (A) or TβRII-myc (B) and ShcA constructs. ‘○' indicates ShcA construct co-precipitated with TGF-β receptor. (C) Schematic representation of ShcA expression constructs used.
TGF-β receptors directly phosphorylate ShcA
The physical association of ShcA with TGF-β receptors (Figures 3 and 4) and the rapidity with which TGF-β induced ShcA phosphorylation despite translational inhibition (Figure 1) suggested that activated TGF-β receptors directly phosphorylate ShcA. This function may be assigned to TβRI, as TGF-β-induced ShcA phosphorylation was inhibited by SB431542 (Figure 3B). Phosphorylation of ShcA on serine and tyrosine by TβRI in vitro would indicate that TβRI, like TβRII (Lawler et al, 1997), is a dual-specificity kinase.
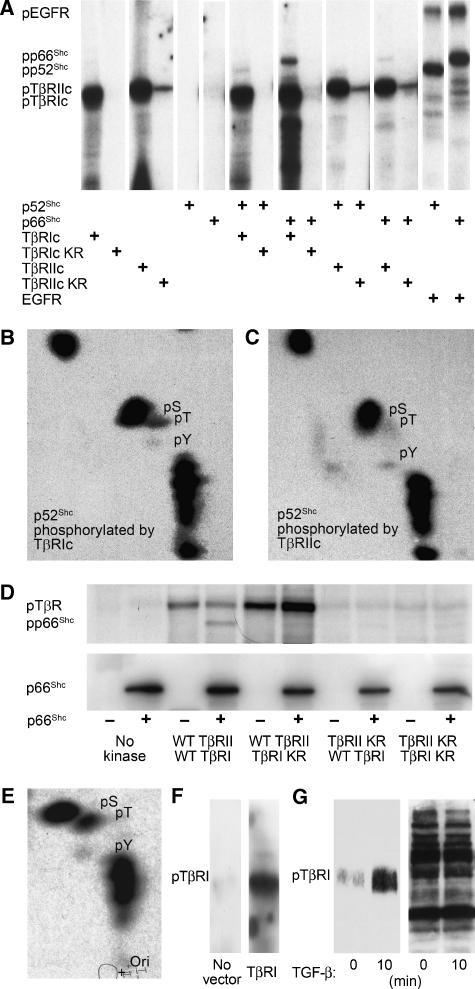
To assess this possibility, we expressed the TβRI and TβRII cytoplasmic domains in Escherichia coli as GST fusion proteins and immobilised the purified proteins to glutathione-Sepharose. Incubation of purified p52ShcA or p66ShcA (also made in E. coli) with TβRI or TβRII kinase in the presence of γ-32P-ATP resulted in ShcA phosphorylation in vitro (Figure 6A; p66ShcA data not shown). Phosphoamino acid analysis revealed that both kinases phosphorylated p52ShcA (Figure 6B and C) and p66ShcA (data not shown) predominantly on serine, with low levels of tyrosine and threonine phosphorylation. The level of tyrosine relative to serine phosphorylation was much lower in vitro than in vivo, and the ShcA threonine phosphorylation observed in vitro was absent in TGF-β-stimulated cells (compare Figure 6B and C with Figure 2B and C). Lysates from untransformed E. coli did not phosphorylate ShcA (data not shown). As each reaction contained only purified kinase and substrate proteins, these results indicate that TβRI and TβRII function as dual specificity kinases of ShcA. The reduced tyrosine kinase activities of the TGF-β receptors in these experiments suggest, not surprisingly, that the conditions in vitro did not fully replicate conditions in vivo.
Figure 6.
TGF-β receptors directly phosphorylate ShcA. (A) ShcA phosphorylation by purified GST-fused TβRI or TβRII cytoplasmic domains in vitro. Recombinant p52ShcA or p66ShcA was incubated with GST-fused wild type and/or kinase-inactive mutants (TβRIc, TβRIc KR, TβRIIc, or TβRIIc KR) or human EGF receptor in the presence of γ-32P-ATP. The radiolabelled, autophosphorylated cytoplasmic domains, and ShcA proteins are indicated. (B, C) Phosphoamino acid analyses of p52ShcA phosphorylated by TβRI (B) or TβRII (C) cytoplasmic domains, as shown in panel (A). The locations of radiolabelled phosphoserine (pS), phosphotyrosine (pY), and phosphothreonine (pT) correlated with those of unlabelled phosphoamino acids added to the reaction mixture. (D) In vitro phosphorylation of E. coli-derived p66ShcA by TβRII-TβRI cytoplasmic chimeras generated in insect cells. Wild-type (WT) or kinase-inactivated (KR) receptor cytoplasmic domains were incubated with or without p66ShcA. The radiolabelled p66ShcA and autophosphorylated RII-RI chimeras are visualised in the upper panel. ShcA immunoblotting confirmed equivalent p66ShcA levels in the reaction mixtures (lower panel). (E) Phosphoamino acid analyses of p66ShcA phosphorylated by wtRII-wtRI, as shown in panel (D). (F) Phosphotyrosine immunoblot of in vitro kinase reaction products containing myc immunoprecipitates from COS cells transfected with control pRK5 plasmid or pRK5 encoding myc-tagged TβRI. (G) TGF-β induces tyrosine phosphorylation of endogenous TβRI, as assessed by TβRI immunoblot of anti-pTyr immunoprecipitates from Mv1Lu cells treated with TGF-β for 10 min (left). Equal precipitation efficiency was demonstrated by phosphotyrosine immunoblotting of the precipitates (right).
We also examined the phosphorylation of purified ShcA by a constitutively active chimera of the TβRII and TβRI cytoplasmic domains (RII-RI) that mimics the activated TβRII/TβRI complex in vivo (Feng and Derynck, 1996). Myc-tagged RII-RI chimeras comprised of wild-type kinases or chimeras in which one or both kinases were inactivated by point mutations were expressed from baculoviruses in insect cells and purified by affinity chromatography. As shown in Figure 6D, the chimera composed of two wild-type kinases (wtRII-wtRI) phosphorylated both itself and purified p66ShcA produced in E. coli. In contrast, the chimera of wild-type TβRII kinase fused to an inactive TβRI kinase (wtRII-RIKR) failed to phosphorylate p66ShcA, but phosphorylated itself in vitro similar to wtRII-wtRI. The chimera of inactivated TβRII kinase fused to wild-type TβRI kinase and the chimera of two inactive kinases showed minimal autophosphorylation and did not phosphorylate ShcA (Figure 6D). These data indicate that the TβRI kinase phosphorylates ShcA, and that TβRII mediates the RII-RI autophosphorylation. Phosphoamino acid analysis of p66ShcA phosphorylated by wtRII-wtRI chimera in vitro again revealed predominant serine phosphorylation and much lower levels of tyrosine and threonine phosphorylation (Figure 6E). These data suggest that TβRI, like TβRII (Lawler et al, 1997), possesses intrinsic tyrosine kinase activity and that ShcA phosphorylation by TβRI in vitro requires TβRII kinase activity.
As ShcA associated with the TGF-β receptor complex following TGF-β stimulation (Figure 4D) through its phosphotyrosine-binding PTB domain (Figure 5; Supplementary Figure 3), we postulated that TβRI is tyrosine phosphorylated upon TGF-β binding. Anti-phosphotyrosine Western analysis showed that TβRI immunoprecipitated from transfected COS cells was tyrosine phosphorylated following in vitro kinase reactions (Figure 6F). To confirm this finding, Mv1Lu cells were stimulated with TGF-β for 10 min, and TβRI phosphorylation was evaluated by phosphotyrosine immunoprecipitation and TβRI immunoblotting. TβRI was rapidly tyrosine phosphorylated after TGF-β stimulation (Figure 6G). As TβRI is phosphorylated by TβRII, it is unclear whether this tyrosine phosphorylation results from TβRI autophosphorylation or from TβRII dual-specificity kinase activity (Lawler et al, 1997).
TGF-β induces signalling downstream of ShcA activation
In canonical ShcA signalling, receptor tyrosine kinases phosphorylate p52ShcA on tyrosine to induce its association with Grb2 and Sos. This heterotrimer sequentially activates Ras, Raf, MEK1/2 and the Erk1/2 MAP kinases (Pelicci et al, 1992). Although TGF-β induces ShcA phosphorylation on serine as well as on tyrosine, our observations suggest that TGF-β activates the ShcA pathway. Low levels of ShcA tyrosine phosphorylation following TGF-β stimulation (relative to EGF) led us to predict that TGF-β activates the ShcA pathway much less efficiently than EGF.
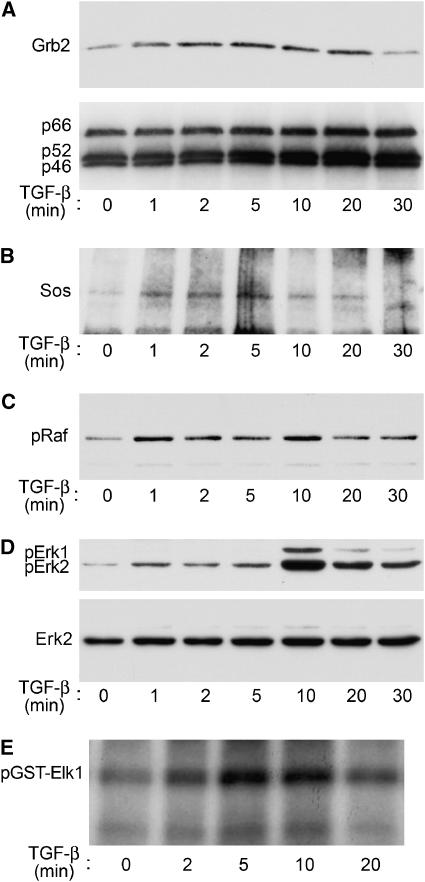
We evaluated whether TGF-β stimulation enabled ShcA to bind Grb2 and Sos by stimulating 3T3-Swiss cells with TGF-β and immunoprecipitating the endogenous ShcA at defined times. The ShcA immunoprecipitates were then assessed for Grb2 and Sos association by Western analysis. As apparent in Figure 7A and B (and quantified in Supplementary Figure 4), TGF-β induced ShcA to associate with Grb2 and Sos with kinetics similar to TGF-β-induced ShcA phosphorylation (Figure 1). The amounts of Grb2 or Sos associated with ShcA were considerably less than in response to EGF (data not shown).
Figure 7.
TGF-β activates the ShcA pathway in 3T3-Swiss cells. (A) TGF-β induces ShcA to associate with Grb2. Cells were stimulated with TGF-β for the indicated times, lysed, and subjected to ShcA immunoprecipitation followed by Grb2 Western analysis (upper panel). Reprobing the same membrane for ShcA confirmed equivalent precipitation efficiency (lower panel). (B) TGF-β induces ShcA to associate with Sos. The upper portion of the membrane shown in (A) was immunoblotted using an anti-Sos antibody. (C) TGF-β induces c-Raf phosphorylation, as assessed by phosphoRaf immunoblotting of stimulated cell lysates. (D) TGF-β induces Erk1/2 phosphorylation. The membrane in (C) was immunoblotted using a phosphoErk1/2-specific antibody (above). Reprobing the blot for Erk2 showed equivalent Erk expression and loading (below). (E) TGF-β activates Erk1/2. Stimulated cell lysates were incubated in vitro with Elk1-GST and γ32P-ATP; 32P-labelled Elk1-GST was visualised by autoradiography.
ShcA activation by receptor tyrosine kinases results in rapid phosphorylation of Raf. We therefore examined the effect of TGF-β on Raf phosphorylation in 3T3-Swiss cells using antibodies specific for phosphorylated Raf. As shown in Figure 7C, TGF-β induced rapid phosphorylation of Raf.
Finally, we evaluated the phosphorylation and activation of Erk1/2 MAP kinases. 3T3-Swiss cells were stimulated with TGF-β, and Erk1/2 phosphorylation was assessed using phospho-Erk-specific antibodies. TGF-β induced rapid Erk phosphorylation that peaked 5–10 min after addition of TGF-β (Figure 7D). This was similar to the kinetics of ShcA phosphorylation following TGF-β stimulation (Figure 1). Erk MAP kinase activation was also assessed by an Erk-specific in vitro kinase assay using immobilised Elk1 fused to GST as substrate (Figure 7E). In this assay, Erk kinase activity peaked 5–10 min after exposure to TGF-β, consistent with TGF-β-induced Erk phosphorylation. Together, these data suggest that TGF-β induces events immediately downstream of ShcA activation.
Direct induction of MAP kinase by TGF-β is mediated by ShcA
As TGF-β activates the MAP kinase pathway downstream of ShcA, we hypothesised that abrogating ShcA function would attenuate TGF-β-induced Erk activation. We overexpressed truncation mutants of ShcA that are expected to confer dominant inhibition of ShcB and ShcC as well as ShcA (Ravichandran, 2001). This approach was prompted by our observation that ShcA inactivation resulted in compensatory upregulation of ShcB and ShcC (data not shown), which may explain why mitogen-induced signalling is only modestly decreased in ShcA-deficient fibroblasts (Lai and Pawson, 2000). As ShcA binds TβRI through its PTB domain, we evaluated a PTB-deficient p52ShcA mutant (p52ShcAΔPTB). This construct, which retains its Grb2 docking sites (van der Geer et al, 1996), does not associate with TβRI (Figure 5), co-precipitates with wild-type ShcA, and prevents TβRI from binding wild-type ShcA (Supplementary Figure 5). We also examined an SH2-deficient p52ShcA mutant (p52ShcAΔSH2). Although the interaction of ShcA with the EGF receptor, like TβRI, depends on its PTB domain, the SH2 domain is required for EGF receptor-mediated ShcA phosphorylation (Gotoh et al, 1995). The mechanism for this requirement is uncertain.
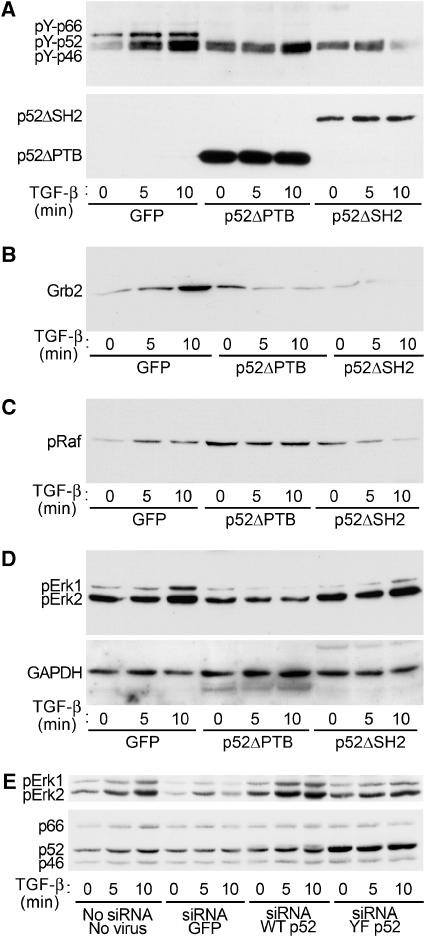
As shown in Figure 8A (and quantified in Supplementary Figure 6), expression of p52ShcAΔPTB or p52ShcAΔSH2 in 3T3-Swiss cells attenuated TGF-β-induced ShcA tyrosine phosphorylation, suggesting dominant-negative interference. Consistent with these observations, p52ShcAΔPTB or p52ShcAΔSH2 expression reduced TGF-β-induced Grb2 recruitment to ShcA (Figure 8B) and Raf phosphorylation (Figure 8C). In the latter experiments, basal Raf phosphorylation was increased for unknown reasons. Finally, TGF-β-induced Erk1/2 phosphorylation was decreased in the presence of either p52ShcA mutant, with p52ShcAΔPTB being more effective than p52ShcAΔSH2 (Figure 8D). Attenuation of TGF-β-induced Erk1/2 phosphorylation by p52ShcAΔPTB was observed in both 3T3-Swiss (Figure 8D) and Mv1Lu (data not shown) cells. We conclude that dominant-negative mutants of p52ShcA interfere with TGF-β responses downstream of ShcA activation.
Figure 8.
Erk activation by TGF-β is inhibited by inactive ShcA mutants and ShcA downregulation. 3T3-Swiss cells were infected with adenoviruses expressing GFP (control), HA-tagged p52ShcA lacking the PTB domain (p52ShcAΔPTB), or HA-tagged p52ShcA lacking the SH2 domain (p52ShcAΔSH2). Cells were then stimulated with TGF-β for the indicated times and lysed. (A) TGF-β-induced tyrosine phosphorylation of endogenous ShcA is attenuated by expression of p52ShcA truncations. Endogenous ShcA was immunoprecipitated and subjected to phosphotyrosine Western analysis (above). HA immunoblotting of this membrane confirmed expression of p52ShcA truncations and equivalent precipitation efficiency (below). (B) TGF-β-induced Grb2 association with endogenous ShcA is attenuated by p52ShcA truncations. The membrane in (A) was subjected to Grb2 Western analysis. (C) TGF-β-induced Raf phosphorylation is attenuated by p52ShcA truncations. Cell lysates from the experiment shown in (A) were subjected to phosphoRaf Western blot. (D) TGF-β-induced Erk phosphorylation is attenuated by p52ShcA truncations. The membrane shown in (C) was subjected to phosphoErk1/2 Western blot (above). The membrane was reprobed for GAPDH to confirm equivalent loading (below). (E) TGF-β-induced Erk activation is attenuated by ShcA silencing and restored by ectopic expression of wild-type, but not mutant p52ShcA. 3T3-Swiss cells were exposed to an siRNA targeted to the common region of ShcA together with adenoviruses encoding either green fluorescent protein (GFP), wild-type p52ShcA, or p52ShcA in which the Grb2 binding sites are mutated. Both p52ShcA constructs incorporate a silent mutation in the siRNA target site. PhosphoErk immunoblot of cells stimulated with TGF-β for the indicated times (above). ShcA immunoblot confirming partial downregulation and virus-mediated expression (below).
To confirm that ShcA is required for TGF-β-induced Erk activation, 3T3-Swiss cells were transfected with an siRNA that silences all three ShcA isoforms (Kisielow et al, 2002). Cells were concomitantly infected with adenoviruses encoding wild-type p52ShcA or green fluorescent protein. In addition, we generated a vector encoding a mutant p52ShcA with tyrosines 239, 240, and 317 replaced by phenylalanines. These amino acids are phosphorylated by tyrosine kinases to become Grb2 binding sites, and their ablation prevents Grb2 binding (van der Geer et al, 1996). The p52ShcA expression vectors also incorporated a silent four-base mutation within the siRNA target sequence that conferred resistance to the siRNA. Transfection of ShcA siRNA resulted in partial ShcA downregulation that was nonetheless sufficient to abrogate TGF-β-induced Erk activation (Figure 8E). This abrogation was reversed by expression of wild-type, but not Grb2 binding-deficient p52ShcA (Figure 8E). These data confirm that ShcA is necessary for TGF-β-induced Erk1/2 activation, and implicate the same ShcA tyrosines that mediate receptor tyrosine kinase signalling.
Discussion
These data suggest that upon activation, TβRI directly phosphorylates ShcA on serine and tyrosine to induce its association with Grb2/Sos, thus initiating the pathway known to link receptor tyrosine kinases to Erk MAP kinases. Although phosphorylated by both TβRI and TβRII in vitro, ShcA interacted with and was phosphorylated by TβRI more efficiently than TβRII. In vivo, TGF-β-induced ShcA phosphorylation was abrogated by the TβRI inhibitor SB431542. Finally, a chimera comprised of an inactive TβRI kinase and a functional TβRII kinase abolished ShcA phosphorylation in vitro without affecting chimera autophosphorylation. The requirement of a functional TβRII for ShcA phosphorylation in vivo is consistent with the TGF-β-dependent activation of TβRI by TβRII. The mechanism of ShcA phosphorylation by TGF-β receptors thus recapitulates that of Smad activation. However, Smad proteins are phosphorylated only on serine (Macias-Silva et al, 1996).
Unlike TβRII (Lawler et al, 1997), neither tyrosine kinase activity nor tyrosine phosphorylation have been reported for TβRI. However, the TβRII and TβRI kinases share structural similarities with tyrosine kinases (Manning et al, 2002). Moreover, two critical autophosphorylated tyrosines in TβRII (Tyr336 in subdomain V and Tyr424 in subdomain VIII) are conserved in TβRI. TβRII, like most dual specificity kinases (Hanks and Hunter, 1995), has low levels of tyrosine relative to serine-threonine kinase activity (Lawler et al, 1997), and this is also true for TβRI. The tyrosine kinase activities of dual specificity kinases also tend to manifest as autophosphorylation (Hanks and Hunter, 1995), as observed for TβRII (Lawler et al, 1997). This may also be true for TβRI, although it might also be phosphorylated by TβRII. In either case, TβRI tyrosine phosphorylation may enable its association with the ShcA PTB domain (Kavanaugh and Williams, 1994). Whereas the canonical PTB domain-binding NPXpY motif is not present in TβRI, a structurally similar motif is recognised and conserved in both TβRI and TβRII. Motifs that associate with the ShcA PTB domain typically contain a large hydrophobic residue, a phosphotyrosine located five amino acids downstream, and intervening arginine and proline residues that impose a β-turn (Trub et al, 1995). In TβRII, this structure is provided by the autophosphorylated Tyr424 (Lawler et al, 1997) located five bases downstream of a Val. Tyr424 and Val419 are highly conserved across TβRI and TβRII and correspond to Tyr378 and Val373 in human TβRI (Huse et al, 1999). Among the dual specificity kinases, only MEK kinases are known to phosphorylate substrates on tyrosine and serine (Lindberg et al, 1992), as we now report for TβRI.
Receptor tyrosine kinases initiate ShcA signalling by phosphorylating p52ShcA at tyrosines 239, 240, and 317 (van der Geer et al, 1996), thereby creating binding sites for Grb2. The resulting ShcA/Grb2/Sos complex then translocates to the plasma membrane and activates Ras (Lotti et al, 1996). Similarly, TGF-β induces low levels of ShcA/Grb2 association that correlate with low levels of Erk activation, suggesting that the same tyrosines mediate TGF-β signalling. This inference is supported by our finding that siRNA-mediated ShcA downregulation attenuated TGF-β-induced Erk activation, and that this attenuation was reversed by ectopic expression of wild-type p52ShcA, but not Tyr239,240,317Phe p52ShcA. Thus, Erk activation by TGF-β stimulation is at least partially mediated by ShcA phosphorylation at these tyrosines, with consequent ShcA/Grb2/Sos complex formation. Furthermore, TGF-β-induced Erk activation was inhibited by ShcA truncations that may sequester wild-type ShcA. However, mutation of p52ShcA at all three tyrosines does not fully abrogate EGF-induced Erk activation (Thomas and Bradshaw, 1997); in fact, enhanced MAP kinase activity suggests alternative Erk activation and compensatory regulation. Such mechanisms may explain the increased Raf phosphorylation in cells expressing p52ShcAΔPTB and the increased basal Erk2 phosphorylation in cells expressing p52ShcAΔSH2 (Figure 8C and D). Conversely, ShcA signalling that is dependent on Tyr239,240 phosphorylation, but independent of Ras activation has been reported (Gotoh et al, 1996). These alternative mechanisms may contribute to Erk activation by TβRI.
It is unclear how serine phosphorylation affects ShcA function. Because TGF-β can attenuate Erk activation by receptor tyrosine kinases (Berrou et al, 1996), we speculate that ShcA serine phosphorylation may inhibit its participation in tyrosine kinase signalling. TGF-β-induced serine phosphorylation may also activate ShcA functions that do not lead to MAP kinase activation. Upon serine phosphorylation of its CH2 domain, p66ShcA mediates an oxidative stress response. Cells deficient in p66ShcA are resistant to H2O2-induced cell death, and susceptibility is restored by expressing wild-type p66ShcA, but not a serine phosphorylation-deficient mutant (Migliaccio et al, 1999). As TGF-β enhances H2O2-induced apoptosis (Yasuda et al, 2003) and is expressed in response to H2O2 (Frippiat et al, 2001), we speculate that TGF-β-induced ShcA phosphorylation may contribute to the oxidative stress response.
TGF-β signalling may also depend on the relative expression of ShcA isoforms. p52ShcA mediates Ras activation by receptor tyrosine kinases (Pelicci et al, 1992), whereas p66ShcA antagonises Ras and Erk activation (Migliaccio et al, 1997). Moreover, p66ShcA expression varies widely during fetal development (Lee et al, 1998). As TGF-β receptors phosphorylate all three ShcA isoforms, their activation of Erk MAP kinases may be regulated by ShcA isoform expression.
The repertoire of TGF-β signalling should now be expanded to include responses primarily associated with receptor tyrosine kinases. The identification of ShcA activation as a direct link between TGF-β stimulation and Erk MAP kinase signalling further confirms the highly interconnected nature of intracellular signalling.
Materials and methods
Cell culture, transfections, and adenoviral infections
3T3-Swiss, HEK-293, COS, and Mv1Lu cells (ATCC, Manassas, VA) were cultured in MEM-α or DMEM with 10% fetal calf serum. Cells were grown to 75% confluence in six-well plates before transfection or infection. Transfections were performed using LipofectAMINE 2000 (Invitrogen, Carlsbad, CA). For adenoviral infections, 5 × 107 p.f.u. were added to each well (MOI=20). Cells were rested for 48 h, starved overnight, and stimulated with 4 ng/ml TGF-β1 (Leinco, St Louis, MO) or 20 ng/ml EGF (R&D Systems, Minneapolis, MN). In selected experiments, cells were treated with 10 μM SB431542 (Sigma, St Louis, MO). To downregulate ShcA expression, cells cultured to 60% confluence in six-well plates were treated for 4 h with 3 μl/well of 50 μM siRNA suspended in 4 μl Oligofectamine (Invitrogen). Cells were incubated overnight with adenoviruses encoding siRNA-resistant p52ShcA or GFP, then rested 1 day before starvation and TGF-β stimulation.
Mammalian expression plasmids and siRNA-mediated ShcA downregulation
p66ShcA and p52ShcA cDNAs were isolated from a fetal mouse λgt-11 library (Clontech, Palo Alto, CA) using ShcA antibody. High-fidelity PCR (Advantage HF, Clontech or pFU, Stratagene, La Jolla, CA) was used to truncate proteins, add restriction sites, append transcription initiation or termination sites, and incorporate epitope tags. ShcA deletion mutants were generated by truncating the coding region along domain boundaries (Luzi et al, 2000).The p52ShcAΔSH2 mutant encodes amino acids 1–373 with a C-terminal HA-tag and stop codon. The p52ShcAΔPTB truncation encodes amino acids 208–469 with a start codon, Kozak sequence and C-terminal HA-tag. Vectors expressing the PTB (amino acids 1-233) or CH1 (amino acids 234–373) domains incorporate start codons, Kozak sequences, C-terminal HA tags, and stop codons. For mammalian expression, constructs were subcloned into pRK5 (BD Pharmingen, San Diego, CA) at EcoRI and HindIII.
Constructs expressing TβRI and TβRII, their cytoplasmic domains (TβRIc and TβRIIc), and the cytoplasmically truncated TβRII (DN-TβRII) have been described (Feng et al, 1995). The TβRIc KR kinase was inactivated by mutating Lys230 to Arg (Chen et al, 1995). Similarly, TβRIIc KR was inactivated by mutating Lys277 to Arg (Wrana et al, 1992). C-terminal Myc- or FLAG-tags were added before subcloning into pRK5 at EcoRI and HindIII. Plasmids were purified by endotoxin-free maxiprep (Qiagen, Chatsworth, CA).
Dominant inhibitory ShcA adenoviruses were constructed by subcloning HA-tagged p52ShcAΔSH2 and p52ShcAΔPTB into pShuttle-CMV-GFP (Stratagene) at KpnI and HindIII. Constructs incorporating myc epitopes and either TβRIc or TβRIIc were subcloned into pShuttle-CMV-GFP at HindIII and NotI. A control virus expressing only GFP was also produced. The plasmids were recombined with pAdEasy-1 (Stratagene) in E. coli BJ5183 and expanded in HEK 293 packaging cells.
ShcA expression was silenced with siRNA against nucleotides 677–697 of p52ShcA, a region common to all three isoforms (Kisielow et al, 2002). To restore p52ShcA function, site-directed mutagenesis (QuikChange, Stratagene) was used to generate a wild-type p52ShcA transcript with the siRNA target mutated from CTGTCA to CTCAGT, thereby leaving the amino-acid sequence unchanged. A second p52ShcA construct was developed in which the Grb2 docking sites at tyrosines 239, 240, and 317 were mutated to phenylalanines. Constructs were subcloned into pShuttle as above.
Protein expression in E. coli and insect cells
For bacterial expression of p66ShcA, p52ShcA, and the cytoplasmic domains such as TβRIc, TβRIc KR, TβRIIc, and TβRIIc KR, the coding sequences were flanked with EcoRI and NotI sites using high-fidelity PCR and cloned into the pGEX-6P-1 GST fusion vector (Pharmacia Amersham, Piscataway, NJ). The plasmids were transformed into E. coli BL21 De3 Lys S (Stratagene), the expressed fusion proteins adsorbed to glutathione Sepharose 4B, and the GST segment removed with Factor Xa protease (Pharmacia Amersham).
A constitutively active chimera of the TβRI and TβRII cytoplasmic domains (Feng and Derynck, 1996) was produced in Sf9 cells using the Bac-To-Bac baculovirus system (Invitrogen). The sequence encoding the RII-RI chimera was cloned from pRK5-(RII-I)C into pFastBac I (Invitrogen) together with a C-terminal myc epitope. Chimeras incorporating inactivating point mutations in one or both kinase domains were made using PCR-based mutagenesis. In the wtRII-RIKR chimera, Lys230 in the TβRI kinase domain was replaced by Arg (Chen et al, 1995). In RIIKR-wtRI, Lys277 in the TβRII kinase domain was replaced by Arg (Wrana et al, 1992). The RIIKR-RIKR chimera incorporated both mutations. Chimeras were purified using myc (9E10) affinity matrix (Covance Babco, Berkeley, CA).
Immunoprecipitations and immunoblotting
Anti-phosphotyrosine, anti-ShcA, anti-Sos, anti-Grb1, and horseradish peroxidase-conjugated anti-rabbit and anti-mouse IgG antibodies were from BD Transduction Laboratories (Lexington, KY). Anti-phosphoErk1/2 antibody was from Biosource (Camarillo, CA). Anti-myc, anti-HA, and anti-FLAG antibodies were provided by Covance Babco. Anti-TβRI and anti-phosphoRaf antibodies were from Cell Signaling Technologies (Beverly, MA). Anti-TβRII antibody was from Upstate (Charlottesville, VA). Immobilised anti-mouse IgG and anti-rabbit IgG antibodies were from EY Laboratories (San Mateo, CA).
Cells were lysed in RIPA buffer (150 mM NaCl, 1% NP-40, 0.25% deoxycholate, 0.1% SDS, 50 mM Tris (pH 8.0), 2 mM EDTA, 1 mM NaVO4, 10 mM NaF and Complete protease inhibitor (Roche, Indianapolis, IN)), and the lysate protein concentrations quantified and equalised using Micro BCA (Pierce, Rockford, IL). For phosphotyrosine and co-precipitation analyses, proteins were precipitated overnight at 4°C with 1 μg primary antibody and 10 μl immobilised anti-mouse or anti-rabbit IgG (EY Laboratories) per 100 μg of total protein. Immunoblots were visualised by chemiluminescence (Pierce).
ShcA kinase assays
ShcA phosphorylation by TGF-β receptors in vitro was evaluated using myc-tagged TβRI or TβRII immunoprecipitated from transfected 293 cells, GST-fused TβRIc or TβRIIc generated in E. coli, or chimeric TβRII-TβRI cytoplasmic domains. Receptor proteins were incubated with p52ShcA or p66ShcA purified from E. coli (see above) in 25 mM HEPES (pH 7.4), 2 mM MnCl2, 10 mM MgCl2, 0.01% Triton X-100, 100 μM Na3VO4, 50 mM NaF, 20 μM DTT, 10 μM ATP, and 5 μCi/reaction γ-32P ATP at 37°C for 30 min. Labelled reaction products were visualised by autoradiography.
Phosphoamino acid analyses
Radiolabelled proteins were resolved by SDS–PAGE, transferred to polyvinylidene difluoride membrane and isolated by autoradiography and band excision. Proteins were hydrolysed in 6 N HCl at 110°C for 1 h, resolved by two-dimensional thin-layer electrophoresis (Cooper et al, 1983), and visualised by autoradiography.
Interaction of ShcA with cell surface TGF-β receptors
ShcA association with cell surface TGF-β receptors was assessed as described previously (Lyons et al, 1991). Briefly, 3T3-Swiss cells were incubated with 125I-TGF-β1 for 10 min on ice and treated with 100 μM disuccimidyl suberate (Pierce) for 15 min. Cells were solubilised in 1% Triton X-100, 10 mM Tris–HCl (pH 7.4), 1 mM EDTA before ShcA immunoprecipitation. Labelled receptors were visualised by autoradiography.
Signalling assays
Association of ShcA with Grb2 or Sos was assessed by ShcA immunoprecipitation followed by Grb2 or Sos immunoblotting. Raf phosphorylation was evaluated by phosphoRaf immunoblotting. Erk1/2 phosphorylation was assayed by phosphoErk1/2 immunoblot. Erk1/2 activity was assessed by in vitro kinase assay (Cano et al, 1995) using Elk1307–428 fused to GST as substrate (provided by RA Hipskind). Cells were lysed in 10 mM Tris (pH 7.05), 30 mM Na4P2O7, 1% Triton X-100, 50 mM NaCl, 5 mM ZnCl2, 100 μM Na3VO4, 50 mM NaF, 1 mM DTT, 5 μM okadaic acid and Complete protease inhibitor. Lysates were equalised for protein content and Erk MAP kinases precipitated with Elk1-GST coupled to glutathione-agarose at 4°C for 4 h. Reactions were performed in 20 mM HEPES (pH 7.6), 2 mM MgCl2, 20 mM β-glycerophosphate, 10–20 mM p-nitrophenylphosphate, 100 μM Na3VO4, 50 nM okadaic acid, 50 mM NaF, 2 mM DTT, Complete protease inhibitor, 20 mM ATP, and 5 μCi/reaction γ-32P ATP at 25°C for 30 min. Labelled complexes were visualised by autoradiography.
Supplementary Material
Supplementary Figures
Acknowledgments
MKL and SMS thank Mahvash Navazesh and Charles Shuler for their support. This research was supported by NIH grants P01-HL60231 (D Warburton, PI), project III, and RO1-CA63101 to RD, RO1-HL62317 to MKL, and postdoctoral fellowships by the Leukemia and Lymphoma Society, the Tobacco-Related Disease Research Program and American Heart Association to CP, PSL, and JQ, respectively.
References
- Attisano L, Wrana JL (2002) Signal transduction by the TGF-β superfamily. Science 296: 1646–1647 [DOI] [PubMed] [Google Scholar]
- Berrou E, Fontenay-Roupie M, Quarck R, McKenzie FR, Lévy-Toldano S, Tobelem G, Bryckaert M (1996) Transforming growth factor β1 inhibits mitogen-activated protein kinase induced by basic fibroblast growth factor in smooth muscle cells. Biochem J 316: 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E, Hazzalin CA, Kardalinou E, Buckle RS, Mahadevan LC (1995) Neither ERK nor JNK/SAPK MAP kinase subtypes are essential for histone H3/HMG-14 phosphorylation or c-fos and c-jun induction. J Cell Sci 108: 3599–3609 [DOI] [PubMed] [Google Scholar]
- Chen RH, Moses HL, Maruoka EM, Derynck R, Kawabata M (1995) Phosphorylation-dependent interaction of the cytoplasmic domains of the type I and type II transforming growth factor-β receptors. J Biol Chem 270: 12235–12241 [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sefton BM, Hunter T (1983) Detection and quantification of phosphotyrosine in proteins. Methods Enzymol 99: 387–402 [DOI] [PubMed] [Google Scholar]
- Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I (2005) Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-β1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem 95: 918–931 [DOI] [PubMed] [Google Scholar]
- Derynck R, Akhurst RJ, Balmain A (2001) TGF-β signaling in tumor suppression and cancer progression. Nat Genet 29: 117–129 [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425: 577–584 [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R (1996) Ligand-independent activation of transforming growth factor (TGF) β signaling pathways by heteromeric cytoplasmic domains of TGF-β receptors. J Biol Chem 271: 13123–13129 [DOI] [PubMed] [Google Scholar]
- Feng XH, Filvaroff EH, Derynck R (1995) Transforming growth factor-β (TGF-β)-induced down-regulation of cyclin A expression requires a functional TGF-β receptor complex. Characterization of chimeric and truncated type I and type II receptors. J Biol Chem 270: 24237–24245 [DOI] [PubMed] [Google Scholar]
- Frippiat C, Chen QM, Zdanov S, Magalhäes JP, Remacle J, Toussaint O (2001) Subcytotoxic H2O2 stress triggers a release of transforming growth factor-β1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem 276: 2531–2537 [DOI] [PubMed] [Google Scholar]
- Gotoh N, Muroya K, Hattori S, Nakamura S, Chida K, Shibuya M (1995) The SH2 domain of Shc suppresses EGF-induced mitogenesis in a dominant negative manner. Oncogene 11: 2525–2533 [PubMed] [Google Scholar]
- Gotoh N, Tojo A, Shibuya M (1996) A novel pathway from phosphorylation of tyrosine residues 239/240 of Shc, contributing to suppress apoptosis by IL-3. EMBO J 15: 6197–6204 [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9: 576–596 [PubMed] [Google Scholar]
- Huse M, Chen YG, Massagué J, Kuriyan J (1999) Crystal structure of the cytoplasmic domain of the type I TGFβ receptor in complex with FKBP12. Cell 96: 425–436 [DOI] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol 62: 65–74 [DOI] [PubMed] [Google Scholar]
- Kavanaugh WM, Williams LT (1994) An alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science 266: 1862–1865 [DOI] [PubMed] [Google Scholar]
- Kisielow M, Kleiner S, Nagasawa M, Faisal A, Nagamine Y (2002) Isoform-specific knockdown and expression of adaptor protein ShcA using small interfering RNA. Biochem J 363: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Timokhina I, Massagué J (1999) A mechanism of repression of TGFβ/Smad signaling by oncogenic Ras. Genes Dev 13: 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KM, Pawson T (2000) The ShcA phosphotyrosine docking protein sensitizes cardiovascular signaling in the mouse embryo. Genes Dev 14: 1132–1145 [PMC free article] [PubMed] [Google Scholar]
- Lawler S, Feng XH, Chen RH, Maruoka EM, Turck CW, Griswold-Prenner I, Derynck R (1997) The type II transforming growth factor-β receptor autophosphorylates not only on serine and threonine but also on tyrosine residues. J Biol Chem 272: 14850–14859 [DOI] [PubMed] [Google Scholar]
- Lee MK, Zhao J, Smith S, Tefft JD, Bringas P Jr, Hwang C, Warburton D (1998) The Shc 66 and 46 kDa isoforms are differentially downregulated at parturition in the fetal mouse lung. Pediatr Res 44: 850–859 [DOI] [PubMed] [Google Scholar]
- Lindberg RA, Quinn AM, Hunter T (1992) Dual-specificity protein kinases: will any hydroxyl do? Trends Biochem Sci 17: 114–119 [DOI] [PubMed] [Google Scholar]
- Lotti LV, Lanfrancone L, Migliaccio E, Zompetta C, Pelicci G, Salcini AE, Falini B, Pelicci PG, Torrisi MR (1996) Shc proteins are localized on endoplasmic reticulum membranes and are redistributed after tyrosine kinase receptor activation. Mol Cell Biol 16: 1946–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi L, Confalonieri S, di Fiore PP, Pelicci PG (2000) Evolution of Shc functions from nematode to human. Curr Opin Genet Dev 10: 668–674 [DOI] [PubMed] [Google Scholar]
- Lyons RM, Miller DA, Graycar JL, Moses HL, Derynck R (1991) Differential binding of transforming growth factor-β1, -β2, and -β3 by fibroblasts and epithelial cells measured by affinity cross-linking of cell surface receptors. Mol Endocrinol 5: 1887–1896 [DOI] [PubMed] [Google Scholar]
- Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL (1996) MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell 87: 1215–1224 [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298: 1912–1934 [DOI] [PubMed] [Google Scholar]
- Massagué J (2000) How cells read TGF-β signals. Nat Rev Mol Cell Biol 1: 169–178 [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG (1999) The p66Shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402: 309–313 [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti Furga G, Pawson T, di Fiore PP, Lanfrancone L, Pelicci PG (1997) Opposite effects of the p52Shc/p46Shc and p66Shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J 16: 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucsi I, Skorecki KL, Goldberg HJ (1996) Extracellular signal-regulated kinase and the small GTP-binding protein, Rac, contribute to the effects of transforming growth factor-β1 on gene expression. J Biol Chem 271: 16567–16572 [DOI] [PubMed] [Google Scholar]
- Mulder KM (2000) Role of Ras and Mapks in TGFβ signaling. Cytokine Growth Factor Rev 11: 23–35 [DOI] [PubMed] [Google Scholar]
- Olsson N, Piek E, Sundstrom M, ten Dijke P, Nilsson G (2001) Transforming growth factor-β-mediated mast cell migration depends on mitogen-activated protein kinase activity. Cell Signal 13: 483–490 [DOI] [PubMed] [Google Scholar]
- Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, Nicoletti I, Pawson T, Pelicci PG (1992) A novel transforming protein (Shc) with an SH2 domain is implicated in mitogenic signal transduction. Cell 70: 93–104 [DOI] [PubMed] [Google Scholar]
- Qi M, Elion EA (2005) MAP kinase pathways. J Cell Sci 118: 3569–3572 [DOI] [PubMed] [Google Scholar]
- Ravichandran KS (2001) Signaling via Shc family adapter proteins. Oncogene 20: 6322–6330 [DOI] [PubMed] [Google Scholar]
- Simeone DM, Zhang L, Graziano K, Nicke B, Pham T, Schaefer C, Logsdon CD (2001) Smad4 mediates activation of mitogen-activated protein kinases by TGF-β in pancreatic acinar cells. Am J Physiol Cell Physiol 281: C311–C319 [DOI] [PubMed] [Google Scholar]
- Thomas D, Bradshaw RA (1997) Differential utilization of ShcA tyrosine residues and functional domains in the transduction of epidermal growth factor-induced mitogen-activated protein kinase activation in 293T cells and nerve growth factor-induced neurite outgrowth in PC12 cells. Identification of a new Grb2-2-Sos1 binding site. J Biol Chem 272: 22293–22299 [DOI] [PubMed] [Google Scholar]
- Trub T, Choi WE, Wolf G, Ottinger E, Chen Y, Weiss M, Shoelson SE (1995) Specificity of the PTB domain of Shc for β turn-forming pentapeptide motifs amino-terminal to phosphotyrosine. J Biol Chem 270: 18205–18208 [DOI] [PubMed] [Google Scholar]
- van der Geer P, Wiley S, Gish GD, Pawson T (1996) The Shc adaptor protein is highly phosphorylated at conserved, twin tyrosine residues (Y239/240) that mediate protein-protein interactions. Curr Biol 6: 1435–1444 [DOI] [PubMed] [Google Scholar]
- Ventura A, Maccarana M, Raker VA, Pelicci PG (2004) A cryptic targeting signal induces isoform-specific localization of p46Shc to mitochondria. J Biol Chem 279: 2299–2306 [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J (1992) TGFβ signals through a heteromeric protein kinase receptor complex. Cell 71: 1003–1014 [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Franzen P, Miyazono K, Heldin CH (1994) Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-β. J Biol Chem 269: 20172–20178 [PubMed] [Google Scholar]
- Yasuda K, Aoshiba K, Nagai A (2003) Transforming growth factor-β promotes fibroblast apoptosis induced by H2O2. Exp Lung Res 29: 123–134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures