Abstract
The NF-κB transcription factor is normally transiently activated by proinflammatory cytokines and bacterial lipopolysaccharide (LPS); however, persistent NF-κB activation is commonly observed in inflammatory disease and malignancy. The ubiquitin editing enzyme A20 serves an essential role in the termination of TNF-α- and LPS-mediated NF-κB signaling by inactivating key signaling molecules. However, little is known about how A20 is regulated and if other molecules play a role in the termination of NF-κB signaling. Here we demonstrate that Tax1-binding protein 1 (TAX1BP1) is essential for the termination of NF-κB and JNK activation in response to TNF-α, IL-1 and LPS stimulation. In TAX1BP1-deficient mouse fibroblasts, TNF-α-, IL-1- and LPS-mediated IKK and JNK activation is elevated and persistent owing to enhanced ubiquitination of RIP1 and TRAF6. Furthermore, in the absence of TAX1BP1, A20 is impaired in RIP1 binding, deubiquitination of TRAF6 and inhibition of NF-κB activation. Thus, TAX1BP1 is pivotal for the termination of NF-κB and JNK signaling by functioning as an essential regulator of A20.
Keywords: A20, Tax, NF-κB, RIP1, TAX1BP1, TRAF6
Introduction
NF-κB/Rel is a family of enhancer binding proteins that play a central role in cell growth, survival, development and innate and adaptive immunity (Hayden and Ghosh, 2004). The mammalian NF-κB is composed of five subunits, RelA (p65), p50, c-Rel, p52 and RelB, which form various homo- and hetero-dimeric protein complexes. The NF-κB proteins all contain a Rel homology domain that has been shown to confer DNA binding, nuclear localization and dimerization properties. NF-κB is held inactive in the cytosol by a family of inhibitory proteins known as IκBs that mask the nuclear localization signals of NF-κB (Rothwarf and Karin, 1999). Proinflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), viral-encoded proteins, such as the human T-cell leukemia virus type I (HTLV-I) Tax, and bacterial lipopolysaccharide (LPS) all activate the IκB kinase (IKK), which is composed of two catalytic subunits, IKKα and IKKβ, and a regulatory component, IKKγ (Hacker and Karin, 2006). The activated IKK phosphorylates IκBs, triggering their proteolysis through the ubiquitin/proteasome pathway, facilitating nuclear import of NF-κB and activation of target genes (Karin and Ben-Neriah, 2000).
Signaling events upstream of IKK have been characterized in great detail for the TNF-α and IL-1 signaling pathways. TNF-α binding to the TNF receptor 1 (TNFR1) results in the recruitment of the adaptor protein TRADD, which subsequently recruits a signaling complex consisting of TNF receptor-associated factor 2 (TRAF2), TRAF5 and RIP1 (Tada et al, 2001). TRAF2 or RIP1 then plays a role in the recruitment of the IKK complex to TNFR1, leading to oligomerization and activation (Devin et al, 2001). Binding of IL-1 to the IL-1R triggers the recruitment of the adaptor protein MyD88 to the receptor (Medzhitov et al, 1998). MyD88 then recruits the kinases IRAK1 and IRAK4, which play an essential role in the recruitment of TRAF6, triggering its oligomerization and autoubiquitination via lysine 63 (K63)-linked ubiquitin chains (Deng et al, 2000). Downstream of TRAF6, TGF-β- activated kinase 1 (TAK1) and the adaptor proteins TAB2, TAB3 mediate the activation of the IKK complex (Sato et al, 2005; Shim et al, 2005). TAK1 has also been reported to be important for TNF-α-mediated NF-κB activation (Takaesu et al, 2003). Also, TRAF6 is an essential component for NF-κB activation downstream of CD40 and Toll-like receptors (Lomaga et al, 1999). In addition to NF-κB activation, both TNF-α and IL-1 are potent activators of c-jun N-terminal kinase (JNK), which regulates the activation of AP-1 transcription factors, including c-jun and ATF-2 (Song et al, 1997).
NF-κB is tightly regulated by numerous mechanisms to maintain transient activation in order to prevent inflammation-induced tissue damage or malignancy associated with persistent NF-κB activation (Karin and Greten, 2005). The NF-κB inhibitor IκBα is an NF-κB target gene, thus providing a mechanism for the replenishment of the pool of degraded IκBα and the removal of NF-κB from the nucleus (Sun et al, 1993). Ubiquitination plays important regulatory roles in several steps of NF-κB signaling events, and thus is an important target for several negative regulators of NF-κB. The cylindromatosis tumor suppressor (CYLD) has been shown to inhibit IKK downstream of LPS, CD40 and IL-1 by cleaving K63-linked ubiquitin chains on TRAF2, TRAF6 and IKKγ (Trompouki et al, 2003). CYLD also deubiquitinates and modulates the subcellular localization of Bcl-3 (Massoumi et al, 2006). CYLD-deficient mice exhibit defective T-cell development owing to impaired regulation of the ubiquitination of the Lck kinase (Reiley et al, 2006). Another deubiquitinating enzyme (DUB) that is an important regulator of NF-κB is A20, which is a transcriptional target of NF-κB (Krikos et al, 1992). A20-deficient mice develop severe inflammation and cachexia and die prematurely (Lee et al, 2000). A20−/− murine embryonic fibroblasts (MEFs) exhibit persistent NF-κB and IKK activation in response to TNF-α stimulation (Lee et al, 2000). At least three targets of A20 have been identified, TRAF6, RIP1 and IKKγ (Boone et al, 2004; Wertz et al, 2004; Mauro et al, 2006). A20 contains an amino-terminal ovarian tumor (OTU) domain and seven carboxy-terminal zinc–finger domains (Evans et al, 2004). A20 inhibits RIP1 by removing K63-linked ubiquitin chains via the OTU domain, and also catalyzes K48-linked ubiquitin chains via the zinc-fingers, leading to RIP1 degradation (Wertz et al, 2004). Thus, A20 functions as a negative regulator by acting as a dual-function DUB and E3 ligase.
Tax1-binding protein 1 (TAX1BP1), also known as T6BP or TXBP151, is an 86 kDa protein that contains three central coiled-coil domains and two C-terminal zinc-finger domains (Ling and Goeddel, 2000). In addition, TAX1BP1 contains an N-terminal novel membrane binding domain termed SKIP carboxyl homology (SKICH) (Gurung et al, 2003). TAX1BP1 was cloned as an HTLV-I Tax-interacting protein by a yeast two-hybrid screen (Jin et al, 1997; Gachon et al, 1998). TAX1BP1 also interacts with TRAF6 in an IL-1-inducible manner, which is dependent on IRAK1 (Ling and Goeddel, 2000). TAX1BP1 has also been shown to interact with A20 by a yeast two-hybrid screen (De Valck et al, 1999). TAX1BP1 inhibits TNF- and Fas-mediated apoptosis, and antisense TAX1BP1 inhibits the anti-apoptotic effect of A20, suggesting that TAX1BP1 may mediate the function of A20 (De Valck et al, 1999). Furthermore, TNF-α- or Fas-mediated apoptosis led to cleavage of TAX1BP1 by caspases, which was inhibited by the broad-spectrum caspase inhibitor zVAD-fmk or by expression of the viral-encoded caspase inhibitor CrmA (De Valck et al, 1999). In a recent study, it was demonstrated that TAX1BP1 acts as a nuclear receptor coactivator and is functionally repressed by HTLV-I Tax (Chin et al, 2007). To determine the physiological function of TAX1BP1, we generated TAX1BP1 knockout mice by a gene trapping strategy. Although homozygous null TAX1BP1 mice are not viable, TAX1BP1-deficient MEFs exhibit elevated and persistent NF-κB and JNK activation in response to TNF-α, IL-1 and LPS stimulation. RIP1 and TRAF6 ubiquitination was highly upregulated in TAX1BP1-deficient MEFs owing to impaired A20 function. Therefore, our results suggest that TAX1BP1 functions as an essential regulator of A20.
Results
TAX1BP1 is a negative regulator of NF-κB
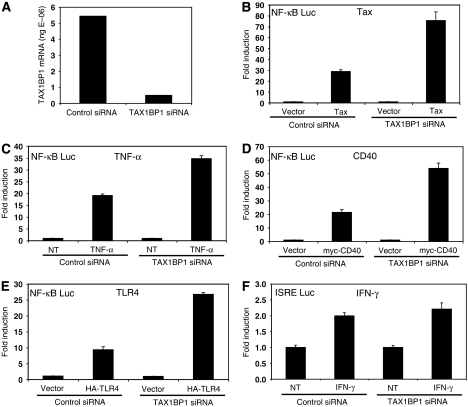
TAX1BP1 has been demonstrated to interact with TRAF6 and A20; however, the functional significance of these interactions is currently unknown (De Valck et al, 1999; Ling and Goeddel, 2000). Therefore, we initially examined the role of TAX1BP1 in NF-κB activation by siRNA-mediated knockdown in 293T cells. Transfection of TAX1BP1 siRNA effectively knocked down TAX1BP1 expression compared to a control scrambled siRNA, as determined by qRT–PCR (Figure 1A). We then determined the functional effect of TAX1BP1 knockdown on NF-κB activation by a variety of inducers, including TNF-α stimulation, and overexpression of the HTLV-I Tax oncoprotein, CD40 or TLR4. Knockdown of TAX1BP1 enhanced NF-κB activation by Tax and TNF-α treatment (Figure 1B and C). TAX1BP1 knockdown also led to enhanced NF-κB activation by IL-1 treatment (data not shown). Similarly, diminished expression of TAX1BP1 led to increased activation of NF-κB via CD40 (Figure 1D) and TLR4 (Figure 1E). The functional effect of TAX1BP1 knockdown appears to be specific for NF-κB, since IFN-γ activation of an ISRE reporter was unaffected by TAX1BP1 siRNA (Figure 1F). Collectively, these results indicate that TAX1BP1 functions as a negative regulator of NF-κB.
Figure 1.
TAX1BP1 is a negative regulator of NF-κB. (A) 293T cells were transfected with 60 pmol scrambled (control) or TAX1BP1 siRNA (Ambion) together with 600 ng pCMV4. After 72 h, total RNA was isolated and converted to cDNA for qRT–PCR using a TAX1BP1 TaqMan probe. Absolute values of TAX1BP1 mRNA are indicated. (B–E) 293T cells were transfected with control or TAX1BP1 siRNA, κB-TATA Luc (0.1 μg) and pRL-tk (0.01 μg). In addition, pCMV4-Tax (2 μg), myc-CD40 (0.5 μg) and HA-TLR4 (1 μg) was transfected in panels B, D and E respectively. Cells were treated with TNF-α (20 ng/ml) for 7 h in panel C. Cells were harvested and dual luciferase assays were performed. (F) Transfection of 293T cells was performed as described above except that the reporter construct used was ISRE-Luc (0.25 μg) and treatment was IFN-γ (100 ng/ml).
Generation of TAX1BP1 knockout mice and MEFs
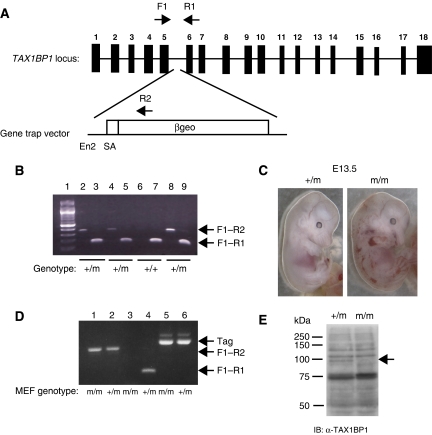
To determine the function of TAX1BP1 in vivo, we generated TAX1BP1 knockout mice using a gene trapping strategy (Stryke et al, 2003). Mouse embryonic stem (ES) cells with the TAX1BP1 genomic locus disrupted by viral-mediated insertion were obtained from BayGenomics (cell line RRJ464). The β-galactosidase/neomycin (βgeo) cassette was inserted in an intron between exons 5 and 6, generating a truncated transcript consisting of exons 1–5 fused to βgeo (Figure 2A). The mouse TAX1BP1 locus consists of 18 exons, with the first 5 exons coding for amino acids 1–204, consisting of the SKICH domain but lacking all coiled-coil and zinc-finger domains. TAX1BP1 mutant ES cells were microinjected into C57BL/6 blastocysts to create chimeric mice. Male chimeras were bred with female C57BL/6 mice and pups were screened for germline transmission of the mutant allelle. Heterozygous mice (designated TAX1BP1+/m) were identified by PCR (Figure 2B), indicating successful germline transmission. We also noticed a faint band when genotyping TAX1BP1+/m mice approximately 200 bp higher than the predicted product obtained with primers specific for exons 5 and 6 (Figure 2B, lane 9). Sequencing of this band revealed an additional transcript consisting of a 206 bp insertion of the ampicillin (AMP) resistance gene in the reverse orientation derived from the targeting vector. This insertion is also between exons 5 and 6 and encodes stop codons in all three reading frames. This type of alternative splicing was also described with gene trap-mutated Ndfip1 in a recent publication by Oliver et al (2006). Thus, the mutant TAX1BP1 locus encodes an exon 1–5/βgeo transcript and an alternatively spliced transcript consisting of full-length TAX1BP1 with a 206 bp AMP insertion between exons 5 and 6.
Figure 2.
Generation of TAX1BP1 knockout mice by gene trapping. (A) Genomic organization of the TAX1BP1 locus. PCR primers for genotyping are indicated by arrows. (B) PCR genotyping strategy for mice. The expected sizes of the PCR fragments are 275 bp with primers F1 and R2, and 154 bp with primers F1 and R1. RT–PCR using two sets of primers was performed with mRNA isolated from tails of four pups born from litters of a TAX1BP1 male chimera bred with a female C57BL/6 mouse. (C) TAX1BP1m/m embryos exhibit hemorrhaging at E13.5. Photographs of E13.5 TAX1BP1m/m and TAX1BP1+/m embryos. (D) The expression of TAX1BP1 and SV40 large T antigen (Tag) in TAX1BP1+/m and TAX1BP1m/m MEFs was determined by RT–PCR. The same primers (F1, R1 and R2) used in panel B were used to genotype MEFs. Primers specific to Tag were used to amplify a 500 bp fragment from Tag. (E) Whole-cell lysates from TAX1BP1+/m and TAX1BP1m/m MEFs were subjected to immunoblotting with polyclonal αTAX1BP1.
Heterozygous mice were viable, fertile and exhibited no gross developmental abnormalities. Extensive matings were performed between heterozygote mice, and of a total of 123 mice genotyped, there were 42 TAX1BP1+/+, 81 TAX1BP1+/m and 0 TAX1BP1m/m mice, indicating that TAX1BP1 deficiency leads to embryonic lethality. By E13.5, TAX1BP1m/m embryos developed systemic hemorrhaging (Figure 2C) and/or multiple cardiac defects (data not shown) resulting in lethality beginning at E13.5. A more detailed characterization of TAX1BP1+/m mice and TAX1BP1m/m embryos will be described elsewhere. To examine the function of TAX1BP1 in cellular signaling pathways, we generated MEFs from E12.5 TAX1BP1+/m and TAX1BP1m/m embryos. TAX1BP1m/m primary MEFs grew poorly in culture; therefore, we immortalized TAX1BP1m/m and control TAX1BP1+/m MEFs with SV40 large T antigen. RT–PCR and Western blotting with a polyclonal α-TAX1BP1 antibody (C-terminal specific) confirmed the absence of full-length TAX1BP1 (Figure 2D and E) and the presence of Tag in both TAX1BP1m/m and TAX1BP1+/m MEFs (Figure 2D). We were also unable to detect either full-length TAX1BP1 or the predicted TAX1BP1 1–204/βgeo fusion in the MEFs with an N-terminal-specific TAX1BP1 antibody (data not shown). Thus, the expression of the fusion protein is likely too low to detect by Western blotting in the MEFs.
Elevated and persistent activation of NF-κB in TAX1BP1m/m MEFs
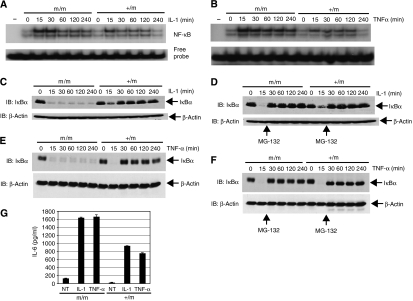
Since our in vitro data (Figure 1B–E) suggested that TAX1BP1 may be a negative regulator of NF-κB, we examined NF-κB activation in response to IL-1 and TNF-α stimulation in MEFs by electrophoretic mobility shift assay (EMSA) using a 32P-labeled NF-κB probe. As seen in Figure 3A and B, TAX1BP1m/m MEFs exhibited elevated NF-κB DNA binding at all of the time points; however, there was no difference in untreated cells. Interestingly, NF-κB DNA binding was persistent and was unable to be terminated throughout the time course of the stimulations, an observation that is remarkably similar to A20−/− MEFs treated with TNF-α (Lee et al, 2000). There was also no difference between TAX1BP1+/m and TAX1BP1+/+ MEFs (data not shown); therefore, all subsequent experiments were performed with TAX1BP1+/m and TAX1BP1m/m MEFs. We next examined stimulus-induced degradation of the NF-κB inhibitor IκBα. In control TAX1BP1+/m MEFs, both TNF-α and IL-1 triggered rapid degradation of the IκBα pool, which was replenished by resynthesized IκBα, since IκBα is an NF-κB target gene (Sun et al, 1993) (Figure 3C and E). Strikingly, in TAX1BP1m/m MEFs, there was a rapid loss of IκBα but it was not replenished at later times. To examine if the lack of IκBα expression at later time points was due to constitutive degradation, we added the proteasome inhibitor MG-132 at the 15 min time point of treatment. Indeed, MG-132 treatment restored expression of IκBα at later time points for both IL-1 and TNF-α stimulation (Figure 3D and F). Again, this observation is very similar to what was observed with A20−/− MEFs, at least for TNF-α stimulation (Lee et al, 2000).
Figure 3.
TAX1BP1 is required for the termination of IL-1- and TNF-α-mediated NF-κB activation. (A, B) TAX1BP1m/m and TAX1BP1+/m MEFs were stimulated with either IL-1 (20 ng/ml) in panel A or TNF-α (20 ng/ml) in panel B for the indicated times. Nuclear extracts were prepared and subjected to NF-κB EMSA. (C, E) TAX1BP1m/m and TAX1BP1+/m MEFs were stimulated with either IL-1 (panel C) or TNF-α (panel E) for the indicated times. Whole-cell extracts were subjected to immunoblotting with anti-IκBα and β-actin antibodies. (D, F) TAX1BP1m/m and TAX1BP1+/m MEFs were treated with IL-1 (panel D) and TNF-α (panel F) for the indicated times together with MG-132 (25 μM) at the 15 min time point. Whole-cell extracts were subjected to immunoblotting with anti-IκBα and β-actin antibodies. (G) TAX1BP1m/m and TAX1BP1+/m MEFs were treated with either IL-1 or TNF-α for 20 h. Cell supernatants were collected and subjected to an IL-6 ELISA. The error bars represent the standard error of the mean (s.e.m.) of triplicate samples.
Because TAX1BP1m/m MEFs exhibited enhanced and persistent NF-κB activation, we expected that expression of NF-κB-regulated genes would be upregulated in these cells. As a representative NF-κB target gene, we chose to examine IL-6 protein levels. We measured IL-6 protein levels in the supernatants of IL-1- and TNF-α-treated MEFs, and TAX1BP1m/m MEFs clearly produced more IL-6 compared to TAX1BP1+/m MEFs (Figure 3G). Thus, persistent NF-κB activation is associated with increased expression of IL-6 in TAX1BP1m/m MEFs.
IKK and JNK are persistently activated in response to TNF-α and IL-1 in TAX1BP1m/m MEFs
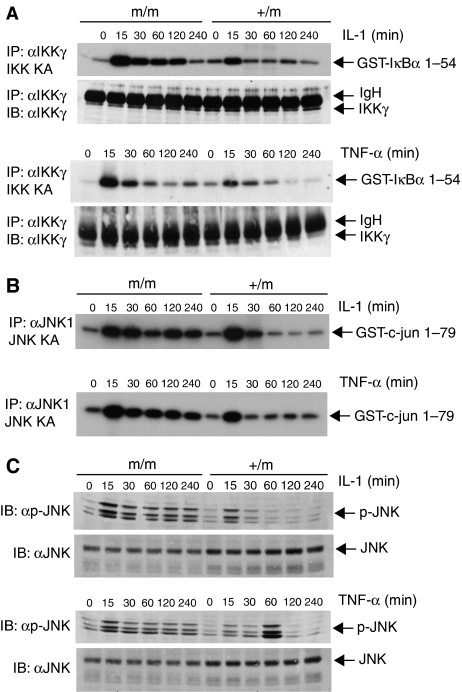
To determine if the enhanced NF-κB DNA binding and IκBα degradation in TAX1BP1m/m MEFS was due to elevated IKK kinase activity, we next performed in vitro kinase assays. TAX1BP1+/m and TAX1BP1m/m MEFs were stimulated with IL-1 or TNF-α for various time points, whereupon cells were lysed and the IKK complex was immunoprecipitated with anti-IKKγ antibody. An in vitro kinase assay was performed in the presence of γ-32P using GST-IκBα 1–54 as a substrate. As seen in Figure 4A, TAX1BP1m/m MEFs exhibited elevated and persistent IKK kinase activity after both IL-1 and TNF-α stimulation.
Figure 4.
TAX1BP1 is essential for the downregulation of IKK and JNK activation upon IL-1 and TNF-α stimulation. (A) TAX1BP1m/m and TAX1BP1+/m MEFs were stimulated with IL-1 (20 ng/ml) or TNF-α (20 ng/ml) for the indicated times. Lysates were immunoprecipitated with anti-IKKγ for an in vitro kinase assay using GST-IκBα 1–54 as substrate. The amount of immunoprecipitated IKKγ was determined by immunoblotting for IKKγ. IgH represents the immunoglobulin heavy chain. (B, C) TAX1BP1m/m and TAX1BP1+/m MEFs were stimulated with IL-1 or TNF-α for the indicated times. In panel B, lysates were immunoprecipitated with anti-JNK1 (Santa Cruz) for an in vitro kinase assay using GST-c-jun 1–79 as substrate. In panel C, lysates were immunoblotted with either anti-phospho-JNK or anti-JNK.
We next examined JNK activation by in vitro kinase assays. TAX1BP1+/m and TAX1BP1m/m MEFs were stimulated with IL-1 and TNF-α for various times, and lysates were immunoprecipitated with anti-JNK1 followed by an in vitro kinase assay with a c-jun 1–79 substrate. Interestingly, TAX1BP1m/m MEFs also displayed enhanced and persistent JNK kinase activity in response to IL-1 and TNF-α treatment (Figure 4B). Results obtained with the JNK in vitro kinase assays were also confirmed by immunoblotting with a phospho-JNK antibody. Both TNF-α- and IL-1-induced phosphorylation of JNK were unable to be terminated in the TAX1BP1m/m MEFs (Figure 4C). Thus, absence of full-length TAX1BP1 in the TAX1BP1m/m MEFs leads to enhanced and persistent activation of both IKK and JNK in response to IL-1 and TNF-α treatment.
Retroviral-mediated transfer of TAX1BP1 in TAX1BP1m/m MEFs restores transient NF-κB and JNK activation
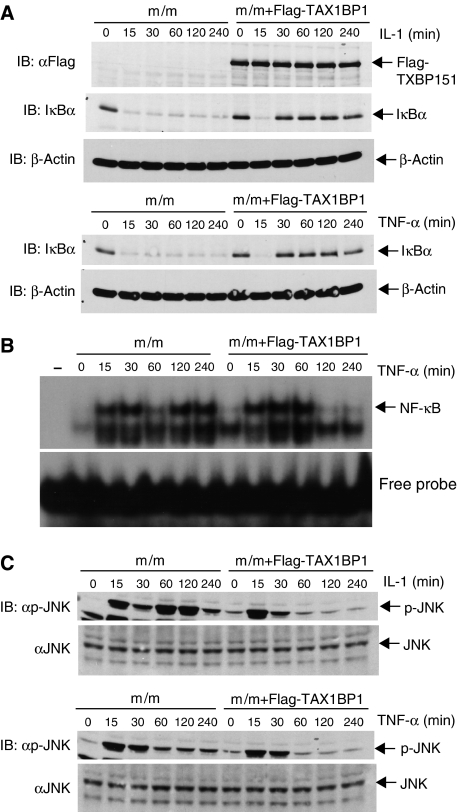
To ensure that the differences in NF-κB activation that we observed with the MEFs were due to loss of full-length TAX1BP1, we introduced wild-type TAX1BP1 back into TAX1BP1m/m MEFs by retroviral-mediated gene transfer. TAX1BP1m/m MEFs were infected with recombinant retroviruses expressing Flag-TAX1BP1 or empty vector and selected for 2 weeks in the presence of G418. TAX1BP1m/m MEFs infected with empty vector exhibited a sustained loss of IκBα in response to IL-1 or TNF-α (Figure 5A) similar to TAX1BP1m/m MEFs (Figure 3C and E). Importantly, expression of Flag-TAX1BP1 resulted in the reappearance of IκBα at 30 min and later time points after stimulation with IL-1 or TNF-α (Figure 5A), suggesting that NF-κB activation was properly terminated. This notion was confirmed by EMSA showing that Flag-TAX1BP1 expression correlated with transient NF-κB DNA binding in response to TNF-α stimulation (Figure 5B). We next examined JNK activation in the reconstituted MEFs. TAX1BP1m/m MEFs expressing Flag-TAX1BP1 exhibited transient JNK activation in response to IL-1 or TNF-α unlike the vector control MEFs (Figure 5C). Thus, TAX1BP1 clearly plays a role in the downregulation of cytokine-mediated NF-κB and JNK activation.
Figure 5.
Retroviral-mediated gene transfer of wild-type TAX1BP1 restores transient NF-κB and JNK activation in TAX1BP1m/m MEFs. (A) TAX1BP1m/m MEFs were infected with recombinant retroviruses expressing either Flag-TAX1BP1 or pCLXSN empty vector. Cells were selected with G418 and stimulated with either IL-1 (20 ng/ml) or TNF-α (20 ng/ml) for the indicated times. Whole-cell lysates were subjected to immunoblotting with anti-Flag to detect exogenous TAX1BP1, anti-IκBα and β-actin. (B) NF-κB EMSA was performed with reconstituted TAX1BP1m/m MEFs. Cells were stimulated with TNF-α for the indicated times and nuclear extracts were used for EMSA. (C) Reconstituted TAX1BP1m/m MEFs were treated with IL-1 or TNF-α for the indicated times. Immunoblotting was performed with anti-JNK and anti-phospho-JNK antibodies.
Enhanced LPS- and Tax-mediated NF-κB activation in TAX1BP1m/m MEFs
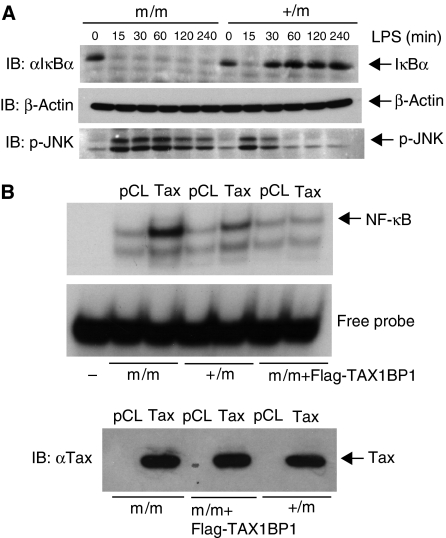
We next examined LPS- and Tax-induced NF-κB activation in TAX1BP1m/m and TAX1BP1+/m MEFs. LPS treatment of TAX1BP1+/m MEFs led to a transient NF-κB activation characterized by early IκBα degradation followed by resynthesis at later time points (Figure 6A). However, in TAX1BP1m/m MEFs, LPS treatment promoted the sustained loss of IκBα (Figure 6A) exactly as was observed with TNF-α and IL-1 treatment (Figure 3C and E). LPS stimulation also triggered persistent activation of JNK in TAX1BP1m/m MEFs (Figure 6A). Clearly, TAX1BP1 is an important negative regulator of TLR4 signaling to NF-κB and JNK.
Figure 6.
TAX1BP1 inhibits LPS- and Tax-mediated NF-κB activation. (A) TAX1BP1m/m and TAX1BP1+/m MEFs were stimulated with LPS (1 μg/ml) for the indicated times. Whole-cell extracts were subjected to immunoblotting with anti-IκBα, p-JNK and β-actin antibodies. (B) TAX1BP1m/m, TAX1BP1+/m and TAX1BP1m/m MEFs reconstituted with Flag-TAX1BP1 were infected with either pCLXSN or pCLXSN-Tax expressing retroviruses. After 4 days, the expression of Tax was confirmed by immunoblotting and nuclear extracts were subjected to an NF-κB EMSA.
Next, we examined Tax-mediated activation of NF-κB in the absence of TAX1BP1. Expression of Tax by retroviral-mediated gene transfer led to NF-κB activation in TAX1BP1+/m MEFs that was enhanced in TAX1BP1m/m MEFs (Figure 6B). Interestingly, Tax was unable to activate NF-κB in TAX1BP1m/m MEFs reconstituted with Flag-TAX1BP1 (Figure 6B), suggesting that the relative amounts of Tax and TAX1BP1 may determine the levels of NF-κB activation induced by Tax. Thus, loss of full-length TAX1BP1 in MEFs is also associated with enhanced NF-κB activation by Tax.
TAX1BP1 inhibits TRAF6 ubiquitination in an A20-dependent manner
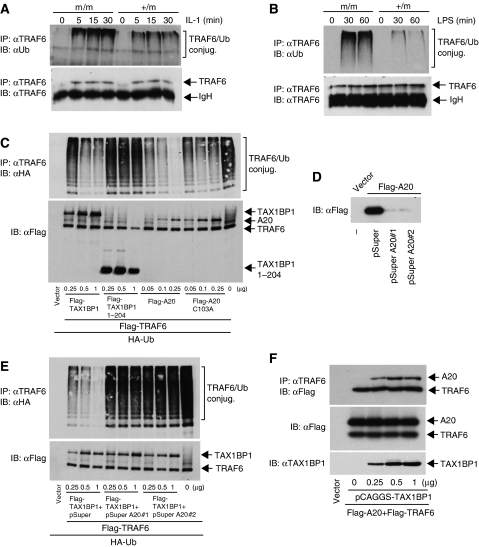
TAX1BP1 interacts with TRAF6 in an IL-1-dependent manner (Ling and Goeddel, 2000), although the functional significance of the interaction is not understood. Therefore, we monitored the activation of TRAF6 in response to IL-1 stimulation in TAX1BP1+/m and TAX1BP1m/m MEFs. TRAF6 activation results in its autoubiquitination via K63-linked polyubiquitin chains (Lamothe et al, 2007). Thus, TAX1BP1+/m and TAX1BP1m/m MEFs were stimulated with IL-1, and TRAF6 ubiquitination assays were performed by immunoprecipitating TRAF6 and immunoblotting for ubiquitin. As expected, TRAF6 was inducibly ubiquitinated by IL-1 stimulation in TAX1BP1+/m MEFs. Interestingly, TRAF6 ubiquitination was elevated in TAX1BP1m/m MEFs treated with IL-1 despite similar amounts of TRAF6 immunoprecipitated (Figure 7A). We next examined the role of TAX1BP1 in LPS-mediated TRAF6 ubiquitination. In TAX1BP1m/m MEFs, LPS triggered much higher levels of TRAF6 ubiquitination compared to control TAX1BP1 MEFs (Figure 7B). These results suggest that TAX1BP1 functions as a negative regulator of TRAF6 ubiquitination in response to IL-1 and LPS stimulation.
Figure 7.
TAX1BP1 promotes the deubiquitination of TRAF6 in an A20-dependent manner. (A) TAX1BP1m/m and TAX1BP1+/m MEFs were treated with IL-1 (20 ng/ml) for the indicated times. Cells were lysed and TRAF6 was immunoprecipitated with anti-TRAF6 followed by immunoblotting with anti-ubiquitin or anti-TRAF6. (B) TAX1BP1m/m and TAX1BP1+/m MEFs were treated with LPS (1 μg/ml) and TRAF6 ubiquitination was examined as described in panel A. (C) 293T cells were transfected with Flag-TRAF6 (1 μg) and HA-Ub (0.5 μg) together with Flag-TAX1BP1 (0.25, 0.5 and 1 μg), Flag-TAX1BP1 1–204 (0.25, 0.5 and 1 μg), Flag-A20 (0.1, 0.25 and 0.5 μg) or Flag-A20 C103A (0.1, 0.25 and 0.5 μg). After 36 h, cells were lysed and immunoprecipitated with anti-TRAF6 followed by immunoblotting with anti-HA. Lysates were examined for TRAF6, TAX1BP1 and A20 expression by immunoblotting with anti-Flag. (D) 293T cells were transfected with vector or Flag-A20 (1 μg) together with pSuper (2 μg) or pSuper A20 nos. 1 or 2 siRNAs (2 μg). After 36 h, cells were lysed and subjected to immunoblotting with anti-Flag. (E) 293T cells were transfected with Flag-TRAF6, HA-Ub and Flag-TAX1BP1 as above together with 2 μg of pSuper or pSuper A20 siRNAs. The IPs and immunoblotting were performed as in panel C. Lysates were examined for TRAF6 and TAX1BP1 expression by immunoblotting with anti-Flag. (F) TAX1BP1 promotes an interaction between A20 and TRAF6. 293T cells were transfected with Flag-A20 (1 μg) and Flag-TRAF6 (1 μg) together with pCAGGS-TAX1BP1 (0, 0.25, 0.5 and 1 μg). After 36 h, cells were lysed and TRAF6 was immunoprecipitated followed by immunoblotting with anti-Flag. Lysates were examined for A20, TRAF6 and TAX1BP1 expression.
To further delineate the role of TAX1BP1 in the regulation of TRAF6 ubiquitination, we transfected Flag-TRAF6 together with TAX1BP1 in 293T cells and performed TRAF6 ubiquitination assays. In support of the data obtained in the MEFs, overexpression of TAX1BP1 inhibited TRAF6 ubiquitination in a dose-dependent manner (Figure 7C). This effect was not observed with a TAX1BP1 deletion mutant, 1–204, the predicted protein product in TAX1BP1m/m MEFs that lacks all coiled-coil and zinc-finger domains. Overexpression of A20, but not a catalytically inactive mutant A20 C103A, also potently inhibited TRAF6 ubiquitination. Since TAX1BP1 lacks any known domain associated with deubiquitinating activity, we reasoned that TAX1BP1 may function via A20. To address this possibility, we used a pSuper plasmid expressing two different A20 siRNAs (pSuper A20 nos. 1 and 2) previously shown to be effective at reducing A20 expression (Storz et al, 2005; Lin et al, 2006). Transfection of pSuper A20 nos. 1 and 2, but not pSuper vector, was effective at knocking down the expression of transfected Flag-A20 (Figure 7D). Knockdown of A20 by both siRNAs completely abrogated TAX1BP1-mediated inhibition of TRAF6 ubiquitination (Figure 7E); thus, TAX1BP1 clearly requires A20 to promote the deubiquitination of TRAF6. We then examined if TAX1BP1 has any effect on the interaction of TRAF6 and A20. TRAF6 and A20 interacted weakly in 293T cells; however, increasing amounts of TAX1BP1 strongly promoted complex formation between TRAF6 and A20 (Figure 7F). TAX1BP1 may therefore function as an adaptor protein for A20 that ensures proper targeting to its substrates such as TRAF6.
TAX1BP1 interacts with RIP1 and negatively regulates TNF-α-mediated RIP1 ubiquitination
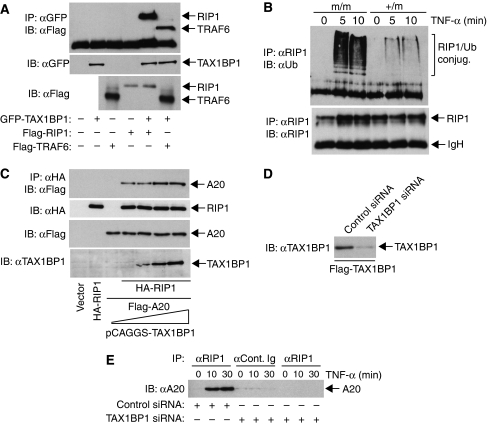
Although TRAF6 is a key target of TAX1BP1 in the IL-1R and TLR4 pathways, it is not clear what protein TAX1BP1 targets in the TNFR pathway. It is not likely TRAF2, since TAX1BP1 interacts specifically with TRAF6 but not TRAF2 (Ling and Goeddel, 2000). The target of A20 in the TNFR pathway is RIP1 (Wertz et al, 2004); therefore, we speculated that RIP1 may also serve as a target of TAX1BP1. First, we performed co-immunoprecipitation (Co-IP) assays to determine if TAX1BP1 interacts with RIP1. TAX1BP1 interacted with TRAF6 and RIP1 (Figure 8A). We next monitored the ubiquitination of RIP1 in TNF-α-stimulated MEFs. RIP1 was inducibly ubiquitinated in response to TNF-α in TAX1BP1+/m MEFs; however, the amount of ubiquitinated RIP1 was much higher in TAX1BP1m/m MEFs (Figure 8B). We next examined the effect of TAX1BP1 on the binding of A20 and RIP1. A20 interacted with RIP1, and increasing amounts of TAX1BP1 enhanced the interaction (Figure 8C). Since TAX1BP1 may be functioning as an adaptor molecule for A20, we next examined if TAX1BP1 was required for the interaction between RIP1 and A20 in TNF-α-stimulated cells. TAX1BP1 siRNA efficiently knocked down transfected Flag-TAX1BP1 (Figure 8D). TNF-α stimulation led to an inducible interaction between RIP1 and A20 that was completely dependent on TAX1BP1 expression (Figure 8E). Thus, TAX1BP1 interacts with RIP1, promotes an interaction of A20 with RIP1 and loss of TAX1BP1 leads to hyper-ubiquitination of RIP1 and deficient A20 binding after TNF-α stimulation. Collectively, these results strongly suggest that TAX1BP1 is an essential regulator of A20 in the TNFR pathway.
Figure 8.
TAX1BP1 interacts with RIP1 and negatively regulates RIP1 ubiquitination. (A) 293T cells were transfected with Flag-RIP1 (1 μg), Flag-TRAF6 (1 μg) and GFP-TAX1BP1 (1 μg). After 36 h, cells were lysed and immunoprecipitated with anti-GFP followed by immunoblotting with anti-Flag. Lysates were examined for TAX1BP1 expression by immunoblotting with anti-GFP. RIP1 and TRAF6 were detected by immunoblotting with anti-Flag. (B) TAX1BP1m/m and TAX1BP1+/m MEFs were treated with TNF-α (20 ng/ml) as indicated. Cells were lysed and RIP1 was immunoprecipitated with anti-RIP1 followed by immunoblotting with anti-ubiquitin or anti-RIP1. (C) 293T cells were transfected with Flag-A20 (1 μg) and HA-RIP1 (1 μg) together with pCAGGS-TAX1BP1 (0, 0.25, 0.5 and 1 μg). After 36 h, cells were lysed and RIP1 was immunoprecipitated with anti-HA followed by immunoblotting with anti-Flag. Lysates were examined for A20, RIP1 and TAX1BP1 expression by immunoblotting with anti-Flag, anti-HA and anti-TAX1BP1, respectively. (D) 293T cells were transfected with Flag-TAX1BP1 together with either control or TAX1BP1 siRNA. (E) 293T cells were transfected with control or TAX1BP1 siRNA. Cells were either untreated or treated with TNF-α (20 ng/ml) for the indicated times. Immunoprecipitations were performed with either anti-control Ig or anti-RIP1 followed by immunoblotting with anti-A20.
A20 is dependent on TAX1BP1 for the deubiquitination of TRAF6 and inhibition of NF-κB activation
According to our model, A20 requires TAX1BP1 to engage its targets RIP1 and TRAF6 for inactivation. Another recently described target of A20 may be NEMO/IKKγ (Mauro et al, 2006). Therefore, we examined the ubiquitination of IKKγ in TAX1BP1m/m MEFs in response to TNF-α and IL-1 stimulation. As observed with RIP1 and TRAF6, IKKγ ubiquitination was elevated in TAX1BP1m/m MEFs after TNF-α and IL-1 stimulation (Supplementary Figure S1). Thus, IKKγ is likely a target of TAX1BP1 in addition to RIP1 and TRAF6.
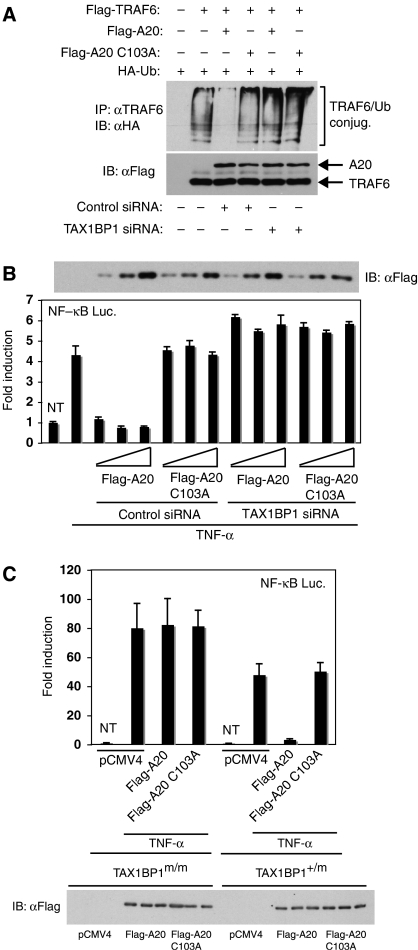
To provide further evidence that TAX1BP1 was an essential regulator of A20 function, we next examined A20-mediated deubiquitination of TRAF6 in the absence of TAX1BP1 expression. TRAF6 ubiquitination assays were performed in 293T cells together with ectopic A20 or A20 C103A. As expected, overexpression of A20, but not A20 C103A, abrogated TRAF6 ubiquitination (Figure 9A). Remarkably, the inhibitory effect of A20 was lost when TAX1BP1 expression was knocked down by siRNA (Figure 9A). Similarly, A20, but not A20 C103A, potently inhibited TNF-induced NF-κB activation in a manner completely dependent on TAX1BP1 expression (Figure 9B). We also examined the functional effects of A20 in TAX1BP1-deficient MEFs by NF-κB luciferase assays. In TAX1BP1+/m MEFs, A20, but not A20 C103A, efficiently inhibited TNF-α-mediated NF-κB activation (Figure 9C). However, in TAX1BP1m/m MEFs, A20 was impaired in the functional inhibition of NF-κB. Therefore, TAX1BP1 is an essential regulator of A20 and is required for A20-mediated deubiquitination of TRAF6 and inhibition of NF-κB.
Figure 9.
TAX1BP1 is essential for A20-mediated deubiquitination of TRAF6 and inhibition of NF-κB activation. (A) 293T cells were transfected with control siRNA or TAX1BP1 siRNA. The following day, the same cells were transfected with Flag-TRAF6 (1 μg) and HA-Ub (0.5 μg) together with Flag-A20 (0.25 μg) or Flag-A20 C103A (0.25 μg). After 36 h, cells were lysed and immunoprecipitated with anti-TRAF6 followed by immunoblotting with anti-HA. Lysates were examined for TRAF6 and A20 expression. (B) 293T cells were transfected with control or TAX1BP1 siRNA, κB-TATA Luc (0.1 μg), pRL-tk (0.01 μg), Flag-A20 or Flag-A20 C103A (10, 25 and 50 ng). Cells were harvested after 7 h treatment with TNF-α (20 ng/ml), and dual luciferase assays were performed. Lysates were immunoblotted with anti-Flag to detect ectopic A20. (C) TAX1BP1+/m and TAX1BP1m/m MEFs were transfected with 2 μg of pCMV4, Flag-A20 or Flag-A20 C103A together with 1 μg κB-TATA Luc and 0.1 μg pRL-tk. After 36 h, cells were stimulated with TNF-α (20 ng/ml), and dual luciferase assays were performed. Lysates were immunoblotted with anti-Flag to detect ectopic A20.
Discussion
In this report, we have demonstrated that TAX1BP1 plays a pivotal role in the termination of NF-κB and JNK signaling. TAX1BP1-deficient MEFs exhibited enhanced and persistent NF-κB activation in response to TNF-α, IL-1 and LPS treatment. Persistent signaling in the mutant MEFs was attributed to defective termination of IKK and JNK kinase activity due to impaired downregulation of RIP1 and TRAF6 ubiquitination. Importantly, reintroduction of full-length TAX1BP1 in mutant MEFs was sufficient to restore transient NF-κB and JNK signaling. Our study has also demonstrated that A20 requires TAX1BP1 to interact with RIP1 and to inhibit NF-κB; thus, TAX1BP1 is an essential regulator of A20.
TAX1BP1m/m mice are not viable owing to hemorrhaging and cardiac defects (Figure 2 and data not shown), suggesting that TAX1BP1 plays an essential role in vascular development. A number of gene-targeted mice, including Crk (Park et al, 2006), SLP-76 (Abtahian et al, 2003) and Tab1 (Komatsu et al, 2002) also succumb to hemorrhaging and cardiac defects during embryonic development. It will be interesting to determine if TAX1BP1 regulates any of these proteins, in particular Tab1, which is known to play a role in IL-1 signaling (Bertelsen and Sanfridson, 2007). Future studies will examine the integrity of blood vessels and the vascular network in TAX1BP1m/m embryos and if TAX1BP1 expression is required in endothelial cells or other cell types such as platelets to prevent hemorrhaging. One may envision that TAX1BP1 regulates signaling pathways either in endothelial or hematopoietic cells that control vascular development. Although TAX1BP1 and A20−/− MEFs are similarly impaired in the termination of TNF-mediated NF-κB and JNK signaling, the phenotypes of TAX1BP1 and A20 knockout mice are distinct (Lee et al, 2000). A20−/− mice develop severe inflammation and cachexia and die within weeks of birth (Lee et al, 2000). The distinct phenotypes between A20 and TAX1BP1 knockout mice suggest that TAX1BP1 likely exerts additional A20-independent functions. Future studies will examine the function of TAX1BP1 in individual immune cell subtypes and other tissues by conditional gene targeting.
TAX1BP1 was originally identified in a yeast two-hybrid screen as an HTLV-I Tax interacting protein (Jin et al, 1997; Gachon et al, 1998). Our data indicate that TAX1BP1 clearly exerts an inhibitory effect on Tax activation of NF-κB. However, it is unclear exactly why Tax interacts with TAX1BP1. Since Tax is ubiquitinated (Peloponese et al, 2004; Lamsoul et al, 2005) and ubiquitination of Tax regulates NF-κB activation, it is plausible that TAX1BP1 may modulate Tax ubiquitination. Another possibility is that Tax may impair the function of TAX1BP1. A recent study by Chin et al (2007) supports this hypothesis, since Tax impaired the nuclear receptor coactivator function of TAX1BP1. One may envision that Tax may also impair the NF-κB inhibitory function of TAX1BP1, thus explaining why NF-κB is persistently activated by Tax despite the presence of multiple negative regulatory mechanisms (Harhaj and Harhaj, 2005).
TAX1BP1 contains an N-terminal SKICH domain, three central coiled-coil domains and two C-terminal zinc-finger domains (Ling and Goeddel, 2000; Gurung et al, 2003). The predicted protein product in the TAX1BP1m/m mice (1–204/βgeo) lacks all of the coiled-coil domains and the zinc-finger domains. Thus, it is likely that one or more of these domains are critical for the inhibition of persistent NF-κB and JNK signaling. In support of this hypothesis, overexpression of TAX1BP1 1–204 was unable to mediate the deubiquitination of TRAF6 (Figure 7C). Coiled-coil domains are known to mediate protein/protein interactions, and HTLV-I Tax preferentially interacts with proteins that contain coiled-coil domains (Chun et al, 2000). Not surprisingly, Tax interacts with TAX1BP1 via the coiled-coil domains (Chin et al, 2007). TAX1BP1 has been shown to contain two independent domains that mediate TRAF6 binding, 1–320 containing the first coiled-coil domain and 321–747 containing the second and third coiled-coil domains (Ling and Goeddel, 2000). In addition, the second coiled-coil domain is responsible for TAX1BP1 self-association (Ling and Goeddel, 2000). Future studies will more precisely identify the regions of TAX1BP1 critical for NF-κB inhibition by retroviral-mediated reconstitution of TAX1BP1m/m MEFs.
The persistent NF-κB activation we have observed with TAX1BP1 mutant MEFs is strikingly similar to that observed with A20−/− MEFs (Lee et al, 2000). TAX1BP1 was identified in a yeast two-hybrid screen as a binding partner of both A20 and TRAF6. However, persistent NF-κB activation in A20−/− MEFs is observed with TNF-α and LPS, but not IL-1, stimulation (Lee et al, 2000; Boone et al, 2004). Thus, functional cooperation between TAX1BP1 and A20 may be specific for the downregulation of TNFR and TLR4 signaling pathways. This also raises the interesting possibility that TAX1BP1 may require a distinct DUB to exert negative regulatory effects on IL-1-mediated NF-κB and JNK activation. One candidate is CYLD, which has been shown to be a negative regulator of both JNK and IKK in IL-1 signaling (Reiley et al, 2004). In support of this hypothesis, we have recently observed an interaction between TAX1BP1 and CYLD under overexpression conditions (data not shown). The role of TAX1BP1 in CYLD regulation as well as the identification of putative DUBs regulating IL-1 signaling will be of great interest for future studies.
TAX1BP1 functions as a negative regulator of ubiquitination of key signaling molecules that are also targeted by A20. Given the lack of any known DUB domain in TAX1BP1, we propose that TAX1BP1 promotes the deubiquitination of TRAF6 and RIP1 via A20. This notion is supported by our finding that overexpressed TAX1BP1 inhibits TRAF6 ubiquitination, and this effect is abolished by siRNA-mediated knockdown of A20. Similarly, A20-mediated deubiquitination of TRAF6 is dependent on TAX1BP1 expression. TAX1BP1 has been reported to interact with A20 and TRAF6, and we demonstrate here a novel interaction with RIP1. We have also shown that TAX1BP1 promotes an interaction between A20 and its substrates TRAF6 and RIP1. Furthermore, TAX1BP1 expression is required for A20 binding to RIP1 and A20-mediated inhibition of NF-κB activation. Taken together, these data support a model whereby TAX1BP1 functions as an adaptor molecule for A20 by recruitment to specific targets such as TRAF6, RIP1 and possibly IKKγ. In the absence of TAX1BP1, A20 would be unable to engage TRAF6 or RIP1 for proper signal termination downstream of TLR4 and TNFR, respectively. TAX1BP1 would also speculatively serve an analogous role for another DUB in IL-1 signaling. Future studies will more precisely define the adaptor function of TAX1BP1, and identify additional targets of TAX1BP1 in inflammatory and innate immune signaling pathways.
Materials and methods
Generation of TAX1BP1 mutant mice
Gene trap mutated TAX1BP1 embryonic stem cells (RRJ464) were purchased from BayGenomics. ES cells were cultured and microinjected into C57BL/6 blastocysts. Chimeric male mice were bred with female C57BL/6 mice to transmit the mutated TAX1BP1 allele to the germline. The mice were maintained on a mixed 129 × C57BL/6 background. Genotyping was performed by RT–PCR using primers specific for exons 5 and 6 and βgeo. Animals were housed under specific pathogen-free conditions and experiments were in accordance with institutional guidelines and approved by the animal care and use committee at the University of Miami.
Isolation of MEFs
MEFs were obtained using a standard procedure (Rudolph et al, 2000). E12.5 embryos were dissected free of surrounding tissues, washed in PBS and the heads and livers were removed. The tissue was placed in trypsin/EDTA and disrupted by forcing through a 6 ml syringe followed by vigorous pipetting. TAX1BP1+/m and TAX1BP1m/m MEFs were immortalized with SV40 large T antigen.
Biological reagents and cell culture
MEFs and 293T cells were cultured in DMEM medium with standard formulations. Recombinant human IL-1β (201-LB) and TNF-α (210-TA) were purchased from R&D Systems (Minneapolis, MN). Interferon-γ, LPS and anti-Flag M2 monoclonal antibody were purchased from Sigma (Saint Louis, MO). The polyclonal anti-TAX1BP1 and monoclonal β-actin antibodies were purchased from Abcam (Cambridge, MA). Anti-IκBα, anti-JNK1, anti-TRAF6, anti-GFP and anti-IKKγ were from Santa Cruz. Anti-ubiquitin was from Stressgen/Assay Designs (San Diego, CA). Anti-RIP1 and anti-A20 antibodies were purchased from BD Biosciences/Pharmingen (San Diego, CA). Anti-JNK and anti-phospho-JNK were purchased from Cell Signaling (Beverly, MA). Control scrambled and TAX1BP1 synthetic siRNAs (138154) were purchased from Ambion (Austin, TX). GST-c-jun 1–79 and MG-132 were purchased from Calbiochem/EMD Biosciences (San Diego, CA).
Plasmid constructs
The pCMV4-Tax, pCLXSN-Tax, GST-IκBα 1–54 and NF-κB-TATA luciferase constructs have been described previously (Xiao et al, 2001; Harhaj et al, 2005). ISRE-Luc was purchased from Clontech (Mountain View, CA). TAX1BP1 was PCR amplified from 293T cell cDNA, digested with EcoR1 and Kpn1, and cloned into p3X-Flag-CMV-7.1 (Sigma). Flag-TAX1BP1 was PCR amplified, digested with BamH1 and Xho1 and cloned into pCLXSN to generate pCLXSN-Flag-TAX1BP1. GFP-TAX1BP1 was generated by subcloning a TAX1BP1 fragment digested with EcoR1 and Kpn1 into pEGFP-C1 (Clontech). pCAGGS TAX1BP1-L was obtained from BCCM/LMBP. CD40 was amplified by PCR, digested with HindIII and Xba1 and cloned into a pCDNA-Myc vector. Flag-TRAF6 was provided by Dr Khaled Tolba (University of Miami). Flag-A20 was a gift from Dr Cladius Vincenz (University of Michigan). HA-RIP1 and Flag-RIP1 were gifts from Dr Glen Barber (University of Miami). HA-TLR4 was provided by Dr Daniel Hwang (University of California, Davis). pSuper and pSuper A20 siRNA plasmids were provided by Dr Peter Storz (Mayo Clinic, Jacksonville). SV40 large T antigen plasmid was obtained from Dr David Ron (New York University).
Transfections and luciferase assays
Transient transfections in 293T cells and MEFs were performed with FuGENE 6 and FuGENE HD transfection reagents (Roche, Indianapolis, IN) respectively. For siRNA transfections, on day 1 293T cells were transfected with 60 pmol of siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). On day 2, other DNAs were transfected into the same cells with FuGENE 6 (Roche). Cells were harvested 36–48 h post-transfection and cell lysates were prepared in 1 × Passive Lysis Buffer (Promega, Madison, WI). Luciferase activity was assayed using the Dual Luciferase Assay system according to the manufacturer's instructions (Promega, Madison, WI). Error bars indicate the standard error of the mean (s.e.m.) of triplicate samples from a representative experiment.
EMSA
EMSA was performed as described previously (Harhaj et al, 2005). Briefly, 4 μg of nuclear extract was incubated with reaction buffer containing DTT (1 mM), poly (dI-dC) (1 μg), dialysis buffer (25 mM HEPES, pH 7.9, 10% glycerol, 100 mM KCl and 0.1 mM EDTA) and 32P-labeled NF-κB consensus probe for 15 min at room temperature. The DNA–protein complexes were resolved on a 5% polyacrylamide gel at 200 V in 0.25 × TBE buffer. The gels were dried under vacuum and subjected to autoradiography.
In vitro kinase assays
IKK and JNK kinase assays were performed as described previously (Xiao et al, 2001). Lysates were immunoprecipitated with either anti-IKKγ (for IKK KA) or anti-JNK (for JNK KA). GST-IκBα 1–54 was used as the substrate for the IKK KA whereas GST c-jun 1–79 was used as the substrate for the JNK KA.
ELISA
The IL-6 ELISA was performed according to the manufacturer's instructions (R&D, Minneapolis, MN). The supernatant from TNF-α- and IL-1-stimulated MEFs was used for the ELISA. The amount of IL-6 was calculated from a standard curve derived from recombinant IL-6.
Retroviral infections
Retroviral infections were performed as described (Harhaj et al, 2007). Either pCLXSN, pCLXSN-Tax or pCLXSN-Flag-TAX1BP1 together with pCL-Ampho and VSV-G was used to transfect 293T cells. The supernatant was filtered and used to infect MEFs. Cells were selected with G418 (400 ng/ml).
Quantitative real-time PCR
Total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA) and converted to cDNA. The PCR reactions were performed on a LightCycler Instrument (Roche Diagnostics) using the LightCycler® TaqMan Master kit (Roche) and gene-specific Hydrolysis (TaqMan) Probes. The TaqMan probe for human TAX1BP1 was purchased from Applied Biosystems (Foster City, CA). Absolute values were determined by comparison with a standard curve derived from a TAX1BP1 plasmid.
Co-IP and ubiquitination assays
Co-IP and ubiquitination assays were performed as described previously (Harhaj and Sun, 1999; Xiao et al, 2001). Briefly, transfected 293T cells or MEFs were lysed in RIPA buffer and immunoprecipitated with specific antibodies. Immunoprecipitates were washed three times with RIPA, and subjected to an extra wash with RIPA containing 1 M urea for ubiquitination assays. Immunoblotting was performed with the indicated antibodies for either Co-IPs or ubiquitination assays.
Western blotting
Western blotting was performed essentially as described previously (Harhaj et al, 2005). Whole-cell lysates were resolved by SDS/PAGE, transferred to nitrocellulose membranes, blocked in 5% milk, incubated with the indicated primary and secondary antibodies and then detected with Western Lightning Enhanced Chemiluminescence reagent (Perkin Elmer, Boston, MA).
Supplementary Material
Supplementary Figure S1
Acknowledgments
We greatfully acknowledge Drs Shao-Cong Sun, Glen Barber, David Ron, Cladius Vincenz, Daniel Hwang, Khaled Tolba and Peter Storz for plasmids, Dr Wayne Balkan (University of Miami Transgene/Gene Knockout Core Facility) for culturing ES cells and generating the chimeric mice, Dr Branislava Janic for breeding of chimeric mice, Dr Kuan-Teh Jeang for discussions and for sharing unpublished data and Kislay Parvatiyar for critical reading of the manuscript. The gene trap mutated ES cell line RRJ464 was obtained from BayGenomics, funded in part by the National Heart, Lung and Blood Institute (NHLBI).
References
- Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML (2003) Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299: 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen M, Sanfridson A (2007) TAB1 modulates IL-1α mediated cytokine secretion but is dispensable for TAK1 activation. Cell Signal 19: 646–657 [DOI] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052–1060 [DOI] [PubMed] [Google Scholar]
- Chin KT, Chun AC, Ching YP, Jeang KT, Jin DY (2007) Human T-cell leukemia virus oncoprotein tax represses nuclear receptor-dependent transcription by targeting coactivator TAX1BP1. Cancer Res 67: 1072–1081 [DOI] [PubMed] [Google Scholar]
- Chun AC, Zhou Y, Wong CM, Kung HF, Jeang KT, Jin DY (2000) Coiled-coil motif as a structural basis for the interaction of HTLV type 1 tax with cellular cofactors. AIDS Res Hum Retroviruses 16: 1689–1694 [DOI] [PubMed] [Google Scholar]
- De Valck D, Jin DY, Heyninck K, Van de Craen M, Contreras R, Fiers W, Jeang KT, Beyaert R (1999) The zinc finger protein A20 interacts with a novel anti-apoptotic protein which is cleaved by specific caspases. Oncogene 18: 4182–4190 [DOI] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ (2000) Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103: 351–361 [DOI] [PubMed] [Google Scholar]
- Devin A, Lin Y, Yamaoka S, Li Z, Karin M, Liu Z (2001) The alpha and beta subunits of IκB kinase mediate TRAF2-dependent IKK recruitment to TNFR1 in response to TNF. Mol Cell Biol 21: 3986–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PC, Ovaa H, Hamon M, Kilshaw PJ, Hamm S, Bauer S, Ploegh HL, Smith TS (2004) Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem J 378: 727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Peleraux A, Thebault S, Dick J, Lemasson I, Devaux C, Mesnard JM (1998) CREB-2, a cellular CRE-dependent transcription repressor, functions in association with Tax as an activator of the human T-cell leukemia virus type 1 promoter. J Virol 72: 8332–8337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung R, Tan A, Ooms LM, McGrath MJ, Huysmans RD, Munday AD, Prescott M, Whisstock JC, Mitchell CA (2003) Identification of a novel domain in two mammalian inositol-polyphosphate 5-phosphatases that mediates membrane ruffle localization. The inositol 5-phosphatase skip localizes to the endoplasmic reticulum and translocates to membrane ruffles following epidermal growth factor stimulation. J Biol Chem 278: 11376–11385 [DOI] [PubMed] [Google Scholar]
- Hacker H, Karin M (2006) Regulation and function of IKK and IKK-related kinases. Sci STKE 2006: re13. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Harhaj NS (2005) Mechanisms of persistent NF-κB activation by HTLV-I Tax. IUBMB Life 57: 83–91 [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Sun SC (1999) IKKγ serves as a docking subunit of the IκB kinase and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem 274: 22911–22914 [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Harhaj NS, Grant C, Mostoller K, Alefantis T, Sun SC, Wigdahl B (2005) Human T cell leukemia virus type I Tax activates CD40 gene expression via the NF-kappa B pathway. Virology 333: 145–158 [DOI] [PubMed] [Google Scholar]
- Harhaj NS, Sun SC, Harhaj EW (2007) Activation of NF-κB by the human T cell leukemia virus type I Tax oncoprotein is associated with ubiquitin-dependent relocalization of IκB kinase. J Biol Chem 282: 4185–4192 [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S (2004) Signaling to NF-κB. Genes Dev 18: 2195–2224 [DOI] [PubMed] [Google Scholar]
- Jin DY, Teramoto H, Giam CZ, Chun RF, Gutkind JS, Jeang KT (1997) A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor alpha. J Biol Chem 272: 25816–25823 [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol 18: 621–663 [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR (2005) NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5: 749–759 [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Shibuya H, Takeda N, Ninomiya-Tsuji J, Yasui T, Miyado K, Sekimoto T, Ueno N, Matsumoto K, Yamada G (2002) Targeted disruption of the Tab1 gene causes embryonic lethality and defects in cardiovascular and lung morphogenesis. Mech Dev 119: 239–249 [DOI] [PubMed] [Google Scholar]
- Krikos A, Laherty CD, Dixit VM (1992) Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by κB elements. J Biol Chem 267: 17971–17976 [PubMed] [Google Scholar]
- Lamothe B, Besse A, Campos AD, Webster WK, Wu H, Darnay BG (2007) Site-specific Lys-63-linked TRAF6 auto-ubiquitination is a critical determinant of IκB kinase activation. J Biol Chem 282: 4102–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsoul I, Lodewick J, Lebrun S, Brasseur R, Burny A, Gaynor RB, Bex F (2005) Exclusive ubiquitination and sumoylation on overlapping lysine residues mediate NF-κB activation by the human T-cell leukemia virus Tax oncoprotein. Mol Cell Biol 25: 10391–10406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A (2000) Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289: 2350–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Yang L, Nakhaei P, Sun Q, Sharif-Askari E, Julkunen I, Hiscott J (2006) Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J Biol Chem 281: 2095–2103 [DOI] [PubMed] [Google Scholar]
- Ling L, Goeddel DV (2000) T6BP, a TRAF6-interacting protein involved in IL-1 signaling. Proc Natl Acad Sci USA 97: 9567–9572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR et al. (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev 13: 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R (2006) Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-κB signaling. Cell 125: 665–677 [DOI] [PubMed] [Google Scholar]
- Mauro C, Pacifico F, Lavorgna A, Mellone S, Iannetti A, Acquaviva R, Formisano S, Vito P, Leonardi A (2006) ABIN-1 binds to NEMO/IKKγ and co-operates with A20 in inhibiting NF-κB. J Biol Chem 281: 18482–18488 [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2: 253–258 [DOI] [PubMed] [Google Scholar]
- Oliver PM, Cao X, Worthen GS, Shi P, Briones N, Macleod M, White J, Kirby P, Kappler J, Marrack P, Yang B (2006) Ndfip1 protein promotes the function of Itch ubiquitin ligase to prevent T cell activation and T helper 2 cell-mediated inflammation. Immunity 25: 929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Boyd K, Curran T (2006) Cardiovascular and craniofacial defects in Crk-null mice. Mol Cell Biol 26: 6272–6282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloponese JM Jr, Iha H, Yedavalli VR, Miyazato A, Li Y, Haller K, Benkirane M, Jeang KT (2004) Ubiquitination of human T-cell leukemia virus type 1 Tax modulates its activity. J Virol 78: 11686–11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley W, Zhang M, Sun SC (2004) Negative regulation of JNK signaling by the tumor suppressor CYLD. J Biol Chem 279: 55161–55167 [DOI] [PubMed] [Google Scholar]
- Reiley WW, Zhang M, Jin W, Losiewicz M, Donohue KB, Norbury CC, Sun SC (2006) Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol 7: 411–417 [DOI] [PubMed] [Google Scholar]
- Rothwarf DM, Karin M (1999) The NF-κB activation pathway: a paradigm in information transfer from membrane to nucleus. Sci STKE 1999: RE1. [DOI] [PubMed] [Google Scholar]
- Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW (2000) Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev 14: 854–862 [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S (2005) Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 6: 1087–1095 [DOI] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S (2005) TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 19: 2668–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HY, Regnier CH, Kirschning CJ, Goeddel DV, Rothe M (1997) Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of NF-κB and JNK/SAPK pathways at TRAF2. Proc Natl Acad Sci USA 94: 9792–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz P, Doppler H, Ferran C, Grey ST, Toker A (2005) Functional dichotomy of A20 in apoptotic and necrotic cell death. Biochem J 387: 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryke D, Kawamoto M, Huang CC, Johns SJ, King LA, Harper CA, Meng EC, Lee RE, Yee A, L'Italien L, Chuang PT, Young SG, Skarnes WC, Babbitt PC, Ferrin TE (2003) BayGenomics: a resource of insertional mutations in mouse embryonic stem cells. Nucleic Acids Res 31: 278–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SC, Ganchi PA, Ballard DW, Greene WC (1993) NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science 259: 1912–1915 [DOI] [PubMed] [Google Scholar]
- Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, Hashimoto H, Mak TW, Yagita H, Okumura K, Yeh WC, Nakano H (2001) Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-κB activation and protection from cell death. J Biol Chem 276: 36530–36534 [DOI] [PubMed] [Google Scholar]
- Takaesu G, Surabhi RM, Park KJ, Ninomiya-Tsuji J, Matsumoto K, Gaynor RB (2003) TAK1 is critical for IκB kinase-mediated activation of the NF-κB pathway. J Mol Biol 326: 105–115 [DOI] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G (2003) CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature 424: 793–796 [DOI] [PubMed] [Google Scholar]
- Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM (2004) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430: 694–699 [DOI] [PubMed] [Google Scholar]
- Xiao G, Harhaj EW, Sun SC (2001) NF-κB-inducing kinase regulates the processing of NF-κB2 p100. Mol Cell 7: 401–409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1