Abstract
Von Willebrand factor (VWF) dimerizes through C-terminal CK domains, and VWF dimers assemble into multimers in the Golgi by forming intersubunit disulfide bonds between D3 domains. This unusual oxidoreductase reaction requires the VWF propeptide (domains D1D2), which acts as an endogenous pH-dependent chaperone. The cysteines involved in multimer assembly were characterized by using a VWF construct that encodes the N-terminal D1D2D′D3 domains. Modification with thiol-specific reagents demonstrated that secreted D′D3 monomer contained reduced Cys, whereas D′D3 dimer and propeptide did not. Reduced Cys in the D′D3 monomer were alkylated with N-ethylmaleimide and analyzed by mass spectrometry. All 52 Cys within the D′D3 region were observed, and only Cys1099 and Cys1142 were modified by N-ethylmaleimide. When introduced into the D1D2D′D3 construct, the mutation C1099A or C1142A markedly impaired the formation of D′D3 dimers, and the double mutation prevented dimerization. In full-length VWF, the mutations C1099A and C1099A/C1142A prevented multimer assembly; the mutation C1142A allowed the formation of almost exclusively dimers, with few tetramers and no multimers larger than hexamers. Therefore, Cys1099 and Cys1142 are essential for the oxidoreductase mechanism of VWF multimerization. Cys1142 is reported to form a Cys1142–Cys1142 intersubunit bond, suggesting that Cys1099 also participates in a Cys1099–Cys1099 disulfide bond between D3 domains. This arrangement of intersubunit disulfide bonds implies that the dimeric N-terminal D′D3 domains of VWF subunits align in a parallel orientation within VWF multimers.
Keywords: disulfide bond, mass spectrometry, oxidoreductase
Von Willebrand factor (VWF) is an essential hemostatic protein that initiates the formation of a platelet plug by binding to connective tissue exposed at sites of vascular injury and to receptors on the surface of platelets. These functions depend on binding sites for many ligands within the repeated multidomain structure of the VWF subunit: D1-D2-D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK. In addition, normal function requires the assembly of VWF into large multimers, and defects in VWF multimerization cause the inherited bleeding disorder, von Willebrand disease (VWD) type 2A (1).
VWF assembly begins in the endoplasmic reticulum (ER) of endothelial cells and megakaryocytes where VWF is synthesized as a precursor that consists of a 22-aa signal peptide, a 741-aa propeptide (domains D1D2), and a 2,050-aa mature subunit. Also in the ER, proVWF monomers form homodimers “tail-to-tail” through interchain disulfide bonds between their CK domains (2–5). The proVWF dimers are transported to the Golgi where they assemble into large multimers “head-to-head” through interchain disulfide bonds between two D3 domains, and the propeptide is cleaved by furin (2, 6).
VWF multimerization in the Golgi involves a unique oxidoreductase mechanism that requires the VWF propeptide, which functions as an endogenous chaperone to promote disulfide-bond formation specifically under acidic conditions (2, 7–9). The reaction appears to proceed through a transient, disulfide-linked intermediate between the propeptide and the D3 region of VWF that forms in the ER and resolves in the Golgi to yield disulfide-linked VWF multimers (10). Partially purified proVWF dimers will form proVWF multimers in vitro at acidic pH, and the reaction is prevented by N-ethylmaleimide (NEM) (11), which indicates that reduced cysteines are required.
VWF multimers purified from blood contain little or no reduced cysteine, suggesting that successful multimer assembly engages all VWF cysteine residues in intrasubunit or intersubunit disulfide bonds (12, 13). However, the subunits at the ends of VWF multimer have not formed intersubunit disulfide bonds and are likely to have reduced Cys residues within their D3 domains. These Cys may have transiently paired with the VWF propeptide (10) and may be key participants in the oxidoreductase mechanism of VWF multimer assembly.
Using a model system that simplifies the analysis of VWF multimer assembly (10, 14), we now have shown that Cys1099 and Cys1142 are oxidized when VWF multimerization succeeds and reduced when it does not. In addition, mutation of these Cys residues prevents the assembly of VWF multimers. Thus, the integrity of VWF multimers may depend on just two disulfide bonds between the D3 domains of VWF subunits.
Results
Secreted D′D3 Monomer Contains Reduced Cys.
Reduced thiols, presumably in Cys residues, are essential for VWF multimerization (11), but whether the propeptide, D′D3 region, or another factor contains mechanistically significant reduced Cys is unknown. The N-terminal D1D2D′D3 region of the proVWF subunit reproduces essential features of multimer assembly, forming intersubunit disulfide bonds between D3 domains in the Golgi apparatus when expressed in mammalian cells (10), and this model system was used to identify reduced Cys that participate in multimer assembly.
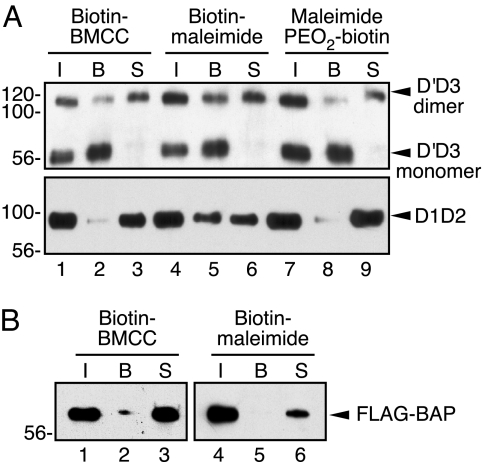
VWF FLAG-D1D2D′D3-c-Myc was expressed in BHK cells, and secreted proteins were reacted with biotin-containing maleimide reagents [supporting information (SI) Fig. 5] to alkylate free thiols (15, 16). Species with alkylated Cys were adsorbed on neutravidin-agarose and identified by Western blotting (Fig. 1). FLAG-tagged bacterial alkaline phosphatase (FLAG-BAP) has two intrachain disulfide bonds and no reduced Cys (17), and FLAG-BAP treated with biotin-maleimide reagents did not bind to neutravidin agarose (Fig. 1B), confirming the specificity of the maleimide reagents for reduced Cys.
Fig. 1.
Secreted D′D3-c-Myc monomer contains reduced Cys, but D′D3-c-Myc dimer and FLAG-D1D2 do not. (A) Media from BHK-fur4-D1D2D′D3-FLAGNT-c-MycCT cells were reacted with 850 μM biotin-BMCC (Pierce, Rockford, IL), biotin-maleimide (Sigma, St. Louis, MO), or maleimide PEO2-biotin (Pierce) for 1 h at room temperature, followed by addition of 40 mM NEM. Biotinylated and nonbiotinylated D′D3-c-Myc and FLAG-D1D2 were isolated by immunoprecipitation, separated on neutravidin-agarose, and detected by SDS/PAGE and Western blotting with anti-VWF (Upper) or anti-FLAG (Lower) as described in Materials and Methods. The lanes are: total immunoprecipitate (I), fraction bound to neutravidin-agarose (B), and unbound supernatant (S). (B) Bacterial alkaline phosphatase (FLAG-BAP) (2 μg/ml) in media from BHK-fur4-D1D2D′D3-FLAGNT-c-MycCT cells was reacted with biotin-BMCC or biotin-maleimide and analyzed by using anti-FLAG as described for A. Similar results were obtained with maleimide PEO2-biotin (data not shown).
These BHK cells secrete VWF propeptide (FLAG-D1D2) and a mixture of D′D3-c-Myc monomer and dimer (10). Most of the D′D3-c-Myc dimers and FLAG-D1D2 did not bind to neutravidin-agarose (Fig. 1A), indicating that the majority of these proteins contain only oxidized (disulfide-bonded) Cys. In contrast, all of the D′D3 monomer bound to neutravidin-agarose (Fig. 1A), suggesting that all secreted D′D3 monomer contained reduced Cys that was biotinylated. None of these proteins bound to neutravidin-agarose when alkylated with NEM (data not shown). Similar results were obtained for BHK cells transiently expressing FLAG-propeptide on one plasmid and D′D3-c-Myc on a separate plasmid (data not shown). Thus, D′D3 monomer contains reduced Cys that are not present in D′D3 dimer, and these residues may normally form intersubunit disulfide bonds.
Only Two Cys Are Reduced in D′D3 Monomer.
The oxidation state of Cys residues in secreted D′D3 monomer was established by differential alkylation, proteolytic digestion, and nano-LC-linear quadrupole ion trap Fourier transform cyclotron resonance mass spectrometry (nano-LC-FTMS) (18). The D′D3 monomer was treated with NEM in conditioned medium, to alkylate any reduced Cys and prevent disulfide rearrangement. The modified product was purified and then reduced completely and alkylated with 4-vinylpyridine (4VP) or iodoacetamide (IAA). N-linked oligosaccharides at Asn857, Asn1147, and Asn1231 (19) were removed with protein N-glycosidase F to simplify MS analysis. Samples were digested with various proteases including trypsin, thermolysin, Asp-N, pepsin, Arg-C, chymotrypsin, and Glu-C. Chymotrypsin sometimes cleaved after 4VP-modified Cys, which was useful for identifying Cys1225 and Cys1227. Double digestion with Glu-C/Asp-N was performed to isolate a peptide containing Cys1157 and Cys1165 (Table 1).
Table 1.
Cys-containing peptides in protease digestions of D′D3 by using nano-LC-FTMS
| Sequence | Start–End | Modification | Theoretical m/z | Observed m/z | Enzyme |
|---|---|---|---|---|---|
| SCRPPMVKL | 766–774 | 1 4VP | 568.3100 | 568.3116 | Pepsin |
| LVCPADNLR | 774–782 | 1 IAA | 529.2772 | 529.2772 | Trypsin |
| DNLRAEGLECTKTCQNY | 779–795 | 2 4VP | 1,084.4992 | 1,084.5011 | Asp-n |
| CMSMGCVSGCLCPPGMVR | 799–816 | 4 4VP | 1,125.9860 | 1,125.9957 | Arg-c |
| CVSGCLCPPGM | 804–814 | 3 4VP | 691.3004 | 691.3028 | Pepsin |
| CVALER | 821–826 | 1 4VP | 398.2131 | 398.2129 | Trypsin |
| CPCFHQGK | 827–834 | 2 IAA | 517.2214 | 517.2219 | Trypsin |
| IGCNTCVCR | 844–852 | 3 IAA | 570.2420 | 570.2420 | Trypsin |
| WNCTDHVCDATCS | 856–868 | 3 IAA | 813.2931 | 813.2961 | Trypsin |
| VCDATCSTI | 862–870 | 2 4VP | 561.7522 | 561.7537 | Chymotrypsin |
| DATCSTIGMAHYLTF | 864–878 | 1 4VP | 868.3952 | 868.3973 | Asp-n |
| DGLKYLFPGECQYVLVQ | 879–895 | 1 4VP | 1,039.0274 | 1,039.0302 | Asp-n |
| CQYVLVQDYCGSNPGTFR | 889–906 | 2 4VP | 1,130.5200 | 1,130.5277 | Arg-c |
| ILVGNKGCSHPSVKCKKR | 907–924 | 2 4VP, 4 acetyl-K | 1,166.6248 | 1,166.6248 | Trypsin |
| GCSHPSVK | 913–920 | 1 4VP | 460.2270 | 460.3322 | Trypsin |
| KQTYQEKVCG | 985–994 | 1 4VP | 644.8220 | 644.8241 | Pepsin |
| LCGNFDGIQNND | 995–1006 | 1 4VP | 707.8070 | 707.8094 | Thermolysin |
| DFGNSWKVSSQCA | 1020–1032 | 1 4VP | 767.3438 | 767.3448 | Asp-n |
| VSSQCADTR | 1027–1035 | 1 4VP | 536.2486 | 536.2485 | Trypsin |
| VPLDSSPATCHNNIMK | 1037–1052 | 1 4VP | 916.4457 | 916.4449 | Trypsin |
| DSSCRILTS | 1057–1065 | 1 4VP | 1,086.5254 | 1,086.5264 | Asp-n |
| ILTSDVFQDCNK | 1062–1073 | 1 IAA | 720.3460 | 720.3469 | Trypsin |
| DVCIY | 1082–1086 | 1 4VP | 717.3282 | 717.3298 | Asp-n |
| SIGDCACF | 1093–1100 | 2 4VP | 513.2149 | 513.2166 | Pepsin |
| DTCSCESIGDCACF | 1087–1100 | 3 4VP, 1 NEM | 947.3515 | 947.3586 | Chymotrypsin |
| DCACFC | 1096–1101 | 3 4VP, 1 NEM | 996.3412 | 996.3452 | Asp-n |
| YAHVCAQHGKVVT | 1107–1119 | 1 4VP | 759.3883 | 759.3909 | Pepsin |
| TATLCPQSCEER | 1122–1133 | 2 IAA | 726.3169 | 726.3170 | Trypsin |
| ENGYECEWR | 1137–1145 | 1 NEM | 655.7596 | 655.7599 | Trypsin |
| ENGYECEWR | 1137–1145 | 1 4VP | 645.7646 | 645.7658 | Arg-c |
| WRYNSC | 1144–1149 | 1 4VP | 467.2060 | 467.2081 | Pepsin |
| YNSCAPACQVTCQHPEPL | 1146–1163 | 3 IAA | 1,066.4540 | 1,066.4550 | Trypsin |
| VTCQHPEPLAC | 1155–1165 | 2 4VP | 704.3316 | 704.3361 | Glu-c/asp-n |
| ACPVQCVEGCH | 1164–1174 | 3 4VP | 730.8178 | 730.8217 | Chymotrypsin |
| CPPGKILDELL | 1177–1187 | 1 4VP | 651.8606 | 651.8631 | Chymotrypsin |
| DELLQTCV | 1184–1191 | 1 4VP | 1,025.4978 | 1,025.5004 | Asp-n |
| DELLQTCVDPEDCPVC | 1184–1199 | 3 4VP | 1,047.4551 | 1,047.4573 | Asp-n |
| NPSDPEHCQIC | 1215–1225 | 2 4VP | 726.8060 | 726.8082 | Chymotrypsin |
| HCDVVNL | 1226–1232 | 1 4VP | 904.4351 | 904.4401 | Chymotrypsin |
| DVVNLTCEACQ | 1228–1238 | 2 4VP | 702.8186 | 702.8207 | Asp-n |
All sequences were confirmed from manual interpretation of the MS/MS spectra. Observed m/z were obtained by selected ion extraction by using Xcalibur software to search for m/z values of singly, doubly, or triply charged species.
Cys modified by NEM, 4VP, and IAA have distinct masses, and the specific modifications of all Cys except for Cys921 were identified in these digests by nano-LC-FTMS (Table 1). Several Lys residues flank Cys921, which prevented its identification in tryptic digests; 4VP-modified Cys921 was observed in a sample reacted with sulfosuccinimidyl acetate to acetylate Lys residues and prevent their recognition by trypsin. Thus, all 52 Cys in D′D3 were characterized: 50 were alkylated only by 4VP or IAA and therefore involved in intrachain disulfide bonds; Cys1099 and Cys1142 were alkylated by NEM, indicating that they are reduced in D′D3 monomer and potentially form intersubunit disulfide bonds in VWF multimers.
Cys1099.
A singly charged ion with m/z = 996.3462 was observed in an Asp-N digest, corresponding to the predicted m/z = 996.3418 for the peptide 1096DCACFC1101 with two 4VP modifications and one NEM modification (Fig. 2A). The MS/MS spectrum for this ion showed that Cys1097 was modified by 4VP because of the presence of the b2 ion at the expected m/z = 324.09 and the b3 ion at m/z = 395.18 (Fig. 2B). Similarly, the y2 ion at m/z = 374.15 indicated that Cys1101 was 4VP-modified. However, Cys1099 was modified by NEM, as shown by the y3 ion at m/z = 602.18, the y4 ion at m/z = 673.18, and the y5 ion at m/z = 881.27. NEM modification on Cys1099 was confirmed by the b4 and b5 ions at m/z = 623.09 and 770.18, respectively (Fig. 2B). This peptide also was observed as a doubly charged ion with m/z = 498.6753 (data not shown). Further evidence that Cys1099 was NEM-modified was provided from a doubly charged (m/z = 947.3586) chymotryptic peptide (1087DTCSCESIGDCACF1100), whose MS/MS spectrum identified Cys1089, Cys1091, and Cys1097 as 4VP-modified and Cys1099 as NEM-modified (data not shown). Therefore, Cys1099 is reduced in the D′D3 monomer.
Fig. 2.
Nano-LC-FTMS of D′D3 monomer peptides containing NEM-modified Cys1099 and Cys1142. (A) MS spectrum of the DC[4VP]AC[NEM]FC[4VP] singly charged ion at m/z = 996.3452. The peptide corresponds to Asp1096–Cys1101 of VWF. (B) MS/MS spectrum acquired from the DC[4VP]AC[NEM]FC[4VP] ion. The asterisk indicates a DCACFC ion with neutral loss of one 4VP modification. (C) MS spectrum of the ENGYEC[NEM]EWR doubly charged ion at m/z = 655.7599, corresponding to Glu1137-Arg1145 of VWF. (D) MS/MS spectrum acquired from the ENGYEC[NEM]EWR ion. Complete lists of assigned ions are given in SI Tables 2 and 3.
Cys1142.
A doubly charged ion with m/z = 655.7599 was detected in a trypsin digest corresponding to the predicted m/z = 655.7596 for the peptide 1137ENGYEC[NEM]EWR1145 (Fig. 2C). The identity of the peptide was confirmed by its MS/MS spectrum (Fig. 2D), which contains the expected y4 (1142C[NEM]EWR1145), y5 (1141EC[NEM]EWR1145), y6 (1140YEC[NEM]EWR1145), and y7 (1139GYEC[NEM]EWR1145) ions at m/z = 718.36, 847.36, 1010.45, and 1067.45, respectively. The b7 ion also was present at the predicted m/z = 950.73 for NEM modification of Cys1142. These data show that Cys1142 is reduced in the D′D3 monomer, which is consistent with the identification of a Cys1142-Cys1142 intersubunit disulfide bond in plasma VWF multimers by using direct methods (20).
The corresponding 4VP-modified doubly charged ion has a predicted m/z = 645.7646, which corresponds to the minor tryptic peptide, 1137ENGYEC[4VP]EWR1145 (SI Fig. 6), that was observed at m/z = 645.7658 (Table 1). Detection of both NEM- and 4VP-modified peptides confirms that the nano-LC-FTMS method identifies both species, when present. The occurrence of some 4VP-modified Cys1142 may reflect incomplete modification of reduced Cys when NEM is added directly to conditioned medium.
Cys1099 and Cys1142 Are Required for VWF Multimer Assembly.
When BHK-fur4 cells were transfected with FLAG-D1D2D′D3-c-Myc containing the mutations C1099A or C1142A, D′D3 monomer was secreted efficiently, and little D′D3 dimer was detected (Fig. 3A). Cells expressing the double mutant (C1009A/C1142A) secreted only D′D3 monomer. Thus, Cys1099 and Cys1142 are essential for intersubunit disulfide-bond formation between D′D3 dimers. Reaction with biotin-maleimide reagents as described in Fig. 1 showed that all of these mutant D′D3 monomers contained reduced Cys residues (data not shown).
Fig. 3.
Mutation of Cys1099 or Cys1142 impairs intersubunit disulfide bond formation. (A) BHK cells were transiently transfected with FLAG-D1D2D′D3-c-Myc (WT, lane 1), FLAG-D1D2D′D3-c-Myc (C1099A) (lane 2), FLAG-D1D2D′D3-c-Myc (C1142A) (lane 3), or FLAG-D1D2D′D3-c-Myc (C1099A/C1142A) (lane 4). Conditioned media were analyzed by SDS/PAGE under nonreducing conditions and Western blotting with antI–VWF. (B) BHK cells were transiently transfected with full-length VWF (WT, lane 1), VWF (C1099A) (lane 2), VWF (C1142A) (lane 3), or VWF (C1099A/C1142A) (lane 4). VWF multimers in conditioned media were analyzed by SDS-agarose gel electrophoresis and Western blotting with anti-VWF (32).
The same mutations were introduced into full-length VWF for expression in BHK cells (Fig. 3B). VWF C1099A and VWF C1099A/C1142A did not form multimers and were secreted only as dimers. VWF C1142A was secreted mainly as dimers but did form some tetramers and a trace of hexamers, in agreement with our previous studies (20). Thus, both of the Cys residues that are reduced in monomeric D′D3 appear to be necessary for the efficient assembly of full-length VWF multimers.
Discussion
When VWF subunits assemble into multimers, intersubunit disulfide bonds form between D3 domains except possibly at the extreme ends of the multimer, where Cys residues that normally make intersubunit bonds may remain unpaired. These cysteines were identified in a truncated VWF construct that permits the preparative isolation of D′D3 monomers (Fig. 1), which may correspond to the terminal subunits of VWF multimers.
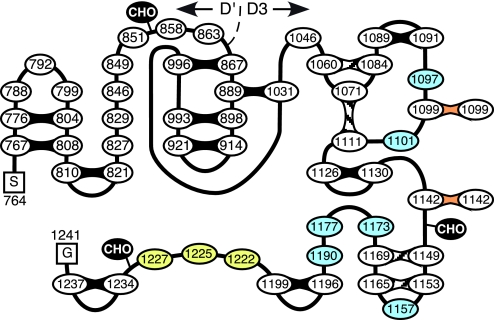
The D′D3 region of VWF contains 52 Cys residues, but a relatively small number of reduced Cys was expected. Proteolytic digestion of plasma VWF indicated that intersubunit disulfide bonds must be confined to the 28 cysteines in the D3 domain from Cys1046 through Cys1237; 8 of these are reported to form intrachain disulfide bonds (12), and we had shown previously that Cys1142 makes a Cys1142–Cys1142 intersubunit bond (20), leaving 17 Cys that might be reduced in monomeric D′D3. In fact, only two reduced Cys were found, Cys1099 and Cys1142 (Fig. 2). Interestingly, mutation of either Cys1099 or Cys1142 prevented multimer assembly (Fig. 3), and a simple explanation for this finding would be that Cys1099 pairs directly with Cys1142 on another subunit. However, our previous direct isolation of a Cys1142–Cys1142 intersubunit bond suggests instead that VWF multimers probably contain just two disulfide bonds, Cys1099–Cys1099 and Cys1142–Cys1142, between the N termini of the subunits (Fig. 4). This arrangement implies that the N termini of VWF subunits align in a parallel orientation during multimer assembly and within finished multimers.
Fig. 4.
Disulfide bond structure of the D′D3 region of VWF. The 52 Cys residues in the D′D3 region of VWF (Ser741-Gly1241) are shown as numbered ovals. Locations of N-linked oligosaccharides (CHO) are shown. Intrasubunit disulfide bonds that have been characterized (12) are shaded black [based on protein sequencing of plasma VWF (12)] or hatched (based on ref.12 and Table 1). Intersubunit disulfide bonds are indicated in orange. At least one of Cys1222, Cys1225, and Cys1227 (yellow) makes at least one intrachain disulfide bond with a subset of Cys1097, Cys1101, Cys1157, Cys1173, Cys1177, and Cys1190 (blue).
A mutation affecting one of these Cys residues has been observed in an unusual form of VWD type 2A, initially named “type IIC Miami”, that is characterized by dominant inheritance and a markedly increased plasma concentration of VWF with very small multimers (21). In two families, the causative mutation was C1099Y (22). Thus, mutation of Cys1099 prevents multimer assembly (Fig. 3) (22) but is compatible with efficient VWF secretion and normal intravascular survival in vivo (22).
The N-terminal region of VWF (domains D1D2D′D3) profoundly affects the pattern of intersubunit disulfide bonds in recombinant VWF. For example, VWF residues Arg1204–Asn1479 contains seven Cys residues: Cys1222, Cys1225, Cys1227, Cys1234, and Cys1237 from the C terminus of the D3 domain, and Cys1272 and Cys1458 from the adjacent domain A1. Plasma VWF contains intrachain disulfide bonds linking Cys1234–Cys1237 and Cys1272–Cys1458, and the pairing of the remaining Cys residues is unknown (12). When the Arg1204–Asn1479 fragment is expressed in mammalian cells with a signal peptide, it is secreted efficiently as a disulfide-linked homodimer (23) with interchain disulfide bonds involving one or more of Cys1222, Cys1225, and Cys1227; mutation of any one of these three Cys prevents dimerization (24). However, larger constructs containing the rest of domains D′D3 (e.g., D′D3A1) do not dimerize unless the propeptide (D1D2) also is included (7, 8, 10). Furthermore, Cys1222, Cys1225, and Cys1227 make only intrachain disulfide bonds in monomeric D′D3 (Table 1 and SI Fig. 7), and mutation of all three of them does not impair multimer assembly by full-length VWF (20). Therefore, Cys1222, Cys1225, and Cys1227 probably do not form interchain disulfide bonds in native VWF but can do so when the D1D2D′D3 regions, which are required for normal pH-dependent multimerization in the Golgi, are absent.
This conclusion is supported by structural studies of plasma VWF. Trypsin digestion yields a major ≈116-kDa product that contains disulfide-linked ≈50-kDa and ≈13-kDa polypeptides (25). Some reports describe this ≈116-kDa species as a homodimer of ≈50-kDa polypeptides, but the data included in these reports confirm the presence of a disulfide-linked ≈10- to 13-kDa component (26, 27). The ≈50-kDa fragment consists of Val1212–Lys1491 (28). The ≈13-kDa fragment begins with Gln1053 (29) and could extend to Arg1136 (9.4 kDa) or Arg1145 (10.5 kDa) but probably not to the next trypsin site at Lys1181 (≈18 kDa). Therefore, Cys1099–Cys1099 and/or Cys1142–Cys1142 disulfide bonds between two ≈13-kDa fragments would produce a ≈116-kDa tetramer with the composition (≈13 kDa + ≈50 kDa)2. This structure implies that at least one of Cys1222, Cys1225, and Cys1227 forms an intrachain disulfide bond with Cys residues in the D3 domain whose disulfide pairing is not yet known (Fig. 4). Candidates include Cys1097 or Cys1101, which are within the ≈13-kDa component of the 116-kDa trypsin-digestion product.
How Cys1099 and Cys1142 actually form intersubunit disulfide bonds remains to be determined. A transient disulfide-linked complex between the VWF propeptide (D1D2) and D′D3 has been identified in the ER during VWF biosynthesis, and this complex appears to rearrange in the Golgi to yield VWF multimers (10). Cys1099 or Cys1142 might reasonably participate in this oxidoreductase reaction intermediate, in which case, rearrangement would yield intersubunit disulfide bonds between D3 domains with transfer of electrons to yield reduced Cys residues on D1D2. However, secreted D1D2 does not contain reduced Cys residues (Fig. 1), which suggests that unidentified electron acceptors in the Golgi apparatus can rapidly oxidize them. Alternatively, D1D2 may not contain Cys residues that participate directly in VWF multimer assembly. Instead, mechanistically important thiols may be confined to the D3 domains, and D1D2 may promote multimer assembly mainly by approximating them.
Materials and Methods
Plasmid Constructs and Protein Expression.
Plasmids encoding human VWF fragments were derived from pSVHVWF1.1 (30): pSVH-D3-FLAGNT/c-MycCT encodes VWF Met1–Gly1241 with a FLAG tag (DYKDDDDK) after the signal peptide between Cys22 and Ala23 and a c-Myc tag (EQKLISEEDL) after Gly1241; pSVH-propeptide-FLAGNT is similar but has a stop codon after Arg763; pSVH-D′D3Δpro-c-Myc encodes VWF Met1–Ala33, followed by Ala764–Gly1241, c-Myc tag, and stop codon; pSVH-D′D3Δpro encodes VWF Met1–Ala33, followed by Ala764–Gly1241 (10).
Plasmids pSVH-D3-FLAGNT/c-MycCT (C1099A), pSVH-D3-FLAGNT/c-MycCT (C1142A), and pSVH-D3-FLAGNT/c-MycCT (C1099A/C1142A) were made by using a QuikChange XL kit (Stratagene, La Jolla, CA) to mutate plasmid pSVH-D3-FLAGNT/c-MycCT. A similar strategy was used to make mutations in full-length VWF to yield plasmids pSVH-VWF (C1099A), pSVH-VWF (C1142A), and pSVH-VWF (C1099A/C1142A).
BHK cells were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) plus 10% heat-inactivated FBS and 2 mM glutamine. BHK cells were transiently transfected by using Lipofectamine PLUS in Opti-MEM (Invitrogen). Stably transfected BHK cell lines were prepared as described for BHK-fur4-D1D2D′D3-FLAGNT-c-MycCT cells (10, 31).
Detection of Reduced Cys.
After reaction with biotin-maleimide reagents (Fig. 1 and SI Fig. 5), media from BHK-fur4-D1D2D′D3-FLAGNT-c-MycCT cells (10) were incubated overnight at 4°C with 1:100 volume of anti-c-Myc antibody or anti-FLAG M2 antibody (Sigma), respectively, and protein G-Sepharose (1:10 volume) (GE Healthcare, Piscataway, NJ). Beads were washed three times with 50 mM Tris·HCl (pH 7.4)/300 mM NaCl/5 mM EDTA/0.02% NaN3/0.1% Triton X-100/1 mM NEM, and proteins were eluted in 1% SDS/1 mM NEM at 100°C for 5 min. The supernatant (80 μl) was diluted with 400 μl of 50 mM Tris·HCl (pH 7.4)/300 mM NaCl/5 mM EDTA/0.02% NaN3/0.2% SDS/0.5% Triton X-100/1 mM NEM and incubated with 100 μl of neutravidin-agarose (Pierce) overnight at 4°C. Unbound protein in the supernatant was immunoadsorbed and eluted as described above. After washing the neutravidin-agarose beads, bound proteins were eluted by heating to 100°C in 100 μl of 1% SDS/1 mM NEM for 5 min.
Samples of total immunoprecipitate and neutravidin-bound and unbound material were heated to 100°C in SDS sample buffer with or without 10% 2-mercaptoethanol for 5 min, electrophoresed on 4–15% gradient SDS/PAGE (Bio-Rad, Hercules, CA) or 4% SDS/PAGE (Invitrogen) gels, and transferred to PVDF membranes (Bio-Rad). Blots were incubated at room temperature for 1 h in 50 mM Tris·HCl (pH 7.4)/150 mM NaCl/0.1% Tween 20/0.5% casein and incubated overnight at 4°C in either a 1:2,000 dilution of FLAG M2-HRP antibody (Sigma) or a 1:1,000 dilution of HRP-conjugated rabbit polyclonal anti-VWF (P226; DAKO, Carpenteria, CA). Blots were developed by using the ECL-Plus kit (GE Healthcare).
D′D3 Monomer Production.
BHK cells stably transfected with pSVH-D′D3Δpro were grown in FreeStyle 293 Expression Medium (Invitrogen). Conditioned medium was collected after 72 h, and 144 μM PMSF and 10 mM NEM were added. The medium was applied onto a HiTrap Q column (5 ml; GE Healthcare) in 20 mM Hepes (pH 7.4)/20 mM NaCl, developed with an NaCl gradient (0.02–1 M). Fractions containing D′D3 were concentrated by ultrafiltration (Vivaspin 20; Sartorius, Goettingen, Germany) and chromatographed on a C4 column (10 × 150 mm; Vydac, Hesperia, CA) developed with an acetonitrile gradient (0–100%) in 0.1% TFA. Fractions containing D′D3 were dialyzed against 20 mM Hepes (pH 7.4)/150 mM NaCl, concentrated, and chromatographed on Superdex 200 by using an AKTA system (GE Healthcare). Purified D′D3 was stored at −80°C.
Enzymatic Digestions.
D′D3 (1 μg/ml) in TBS containing 1% RapiGest SF (Millipore, Billerica, MA) was reduced with 25 mM Tris(2-carboxyethyl)phosphine for 2 h at room temperature and alkylated with 25 mM 4VP or iodoacetamide (IAA) for 2 h at room temperature. Samples were desalted and buffer exchanged by ultrafiltration (YM-3 Microcon; Millipore).
Protein N-glycosidase F digestions were done in 100 mM NH4HCO3 (pH 8.0) overnight at 37°C with 50 units of enzyme per microgram of substrate. Trypsin or thermolysin (Sigma) digestions were performed in 50 mM NH4HCO3 (pH 8.0) at an enzyme-to-substrate ratio of 1:50 (wt/wt) at 37°C overnight. Aspartic-N (Asp-N; Princeton Separations, Freehold, NJ) digestions were performed in 50 mM NH4HCO3 (pH 8.0) at an enzyme-to-substrate ratio of 1:100 (wt/wt) at 37°C overnight. Pepsin (Princeton Separations) digestions were done in 50 mM ammonium acetate (pH 4.0) at an enzyme-to-substrate ratio of 1:50 (wt/wt) at 37°C overnight. Arginine-C (Arg-C; Princeton Separations) digestions were done at 37°C overnight in 50 mM NH4HCO3 (pH 8.0)/7.7 mM DTT/1 mM calcium acetate, at an enzyme-to-substrate ratio of 1:50 (wt/wt). Chymotrypsin (Princeton Separations) digestions were carried out at 37°C overnight in 50 mM NH4HCO3 (pH 8.0)/1 mM CaCl2 at an enzyme-to-substrate ratio of 1:50 (wt/wt). Double digests with Glutamic-C (Glu-C; Princeton Separations) and Asp-N (Glu-C/Asp-N) were done sequentially with initial addition of Glu-C at an enzyme-to-substrate ratio of 1:20 (wt/wt) at 37°C overnight in 50 mM NH4HCO3 (pH 8.0), followed by digestion with Asp-N as described above.
To limit trypsin digestion to Arg residues, Lys residues of reduced and alkylated D′D3 were modified with a 25-fold excess of sulfosuccinimidyl acetate (Pierce) in 0.1 M NaHCO3 (pH 8.5) for 2.5 h at room temperature. The reaction was quenched by adding 1 M glycine (pH 8.0) for 1 h at room temperature.
Peptides were desalted by adsorption on NuTip Hypercarbon cartridges (Glygen, Columbia, MD) equilibrated with 0.1% formic acid and elution with 0.1% formic acid, 60% acetonitrile. Peptides were lyophilized and dissolved in 0.1% formic acid for analysis.
Mass Spectrometry.
Mass spectrometry was performed by using a linear quadrupole ion trap Fourier transform cyclotron resonance mass spectrometer (LTQ-FTMS; Thermo Scientific, Waltham, MA) interfaced to a nanoliquid chromatograph (Eksigent nano-LC; Eksigent, Livermore, CA) as described (18). Theoretical lists of peptide masses were generated for each enzymatic digest considering 0–3 missed cleavages and including modification of all Cys by NEM and/or 4VP or by NEM and/or IAA, also including acetylated Lys where appropriate. Modification of Cys (residue mass = 103.0092 Da) by NEM yields S-ethylsuccinimidocysteine (residue mass = 228.0569 Da), by 4VP yields S-pyridylethylcysteine (residue mass = 208.0670 Da), and by IAA yields carboxamidomethylcysteine (residue mass = 160.0306 Da). Acetylation of Lys increases the residue mass from 128.0950 Da to 170.1055 Da). By using selected ion extraction and Xcalibur software (Thermo Scientific), MS spectra were analyzed for m/z signals corresponding to the singly, doubly, and triply protonated species. MS/MS spectra were searched against a database of D′D3 sequence y by using MASCOT (Matrix Science, Oxford, U.K.). For each Cys residue, searches were performed for all permutations of NEM-modified Cys and 4VP-modified Cys or NEM-modified Cys and IAA-modified Cys, as appropriate. Except for Cys1099 and Cys1142, when an observed peptide ion indicated modification of any Cys by 4VP or IAA, the corresponding ion (or ions) with Cys modified by NEM was searched for but was not detected.
Supplementary Material
Acknowledgments
We thank Elodee A. Tuley (Washington University) for help with VWF multimer gel electrophoresis. This work was supported in part by National Institutes of Health Grant R01 HL72917 (to J.E.S.) and National Cancer Institute Cancer Center Support Grant P30 CA91842 (to R.R.T.).
Abbreviations
- 4VP
4-vinylpyridine
- C[NEM]
Cys modified by N-ethylmaleimide, or S-ethylsuccinimidocysteine
- C[4VP]
Cys modified by 4-vinylpyridine, or S-pyridylethylcysteine
- ER
endoplasmic reticulum
- IAA
iodoacetamide
- NEM
N-ethylmaleimide
- VWF
von Willebrand factor.
Footnotes
Conflict of interest statement: J.E.S. is a consultant for Baxter BioSciences.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705175104/DC1.
References
- 1.Sadler JE, Budde U, Eikenboom JC, Favaloro EJ, Hill FG, Holmberg L, Ingerslev J, Lee CA, Lillicrap D, Mannucci PM, et al. J Thromb Haemost. 2006;4:2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagner DD, Marder VJ. J Cell Biol. 1984;99:2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner DD, Lawrence SO, Ohlsson-Wilhelm BM, Fay PJ, Marder VJ. Blood. 1987;69:27–32. [PubMed] [Google Scholar]
- 4.Voorberg J, Fontijn R, Calafat J, Janssen H, van Mourik JA, Pannekoek H. J Cell Biol. 1991;113:195–205. doi: 10.1083/jcb.113.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsumi A, Tuley EA, Bodo I, Sadler JE. J Biol Chem. 2000;275:25585–25594. doi: 10.1074/jbc.M002654200. [DOI] [PubMed] [Google Scholar]
- 6.Wagner DD, Fay PJ, Sporn LA, Sinha S, Lawrence SO, Marder VJ. Proc Natl Acad Sci USA. 1987;84:1955–1959. doi: 10.1073/pnas.84.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verweij CL, Hart M, Pannekoek H. EMBO J. 1987;6:2885–2890. doi: 10.1002/j.1460-2075.1987.tb02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise RJ, Pittman DD, Handin RI, Kaufman RJ, Orkin SH. Cell. 1988;52:229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- 9.Vischer UM, Wagner DD. Blood. 1994;83:3536–3544. [PubMed] [Google Scholar]
- 10.Purvis AR, Sadler JE. J Biol Chem. 2004;279:49982–49988. doi: 10.1074/jbc.M408727200. [DOI] [PubMed] [Google Scholar]
- 11.Mayadas T, Wagner DD. J Biol Chem. 1989;264:13497–13503. [PubMed] [Google Scholar]
- 12.Marti T, Rosselet SJ, Titani K, Walsh KA. Biochemistry. 1987;26:8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- 13.Legaz ME, Schmer G, Counts RB, Davie EW. J Biol Chem. 1973;248:3946–3955. [PubMed] [Google Scholar]
- 14.Voorberg J, Fontijn R, van Mourik JA, Pannekoek H. EMBO J. 1990;9:797–903. doi: 10.1002/j.1460-2075.1990.tb08176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locker JK, Griffiths G. J Cell Biol. 1999;144:267–279. doi: 10.1083/jcb.144.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braakman I, Helenius J, Helenius A. Nature. 1992;356:260–262. doi: 10.1038/356260a0. [DOI] [PubMed] [Google Scholar]
- 17.Kim EE, Wyckoff HW. Clin Chim Acta. 1990;186:175–187. doi: 10.1016/0009-8981(90)90035-q. [DOI] [PubMed] [Google Scholar]
- 18.King JB, Gross J, Lovly CM, Rohrs H, Piwnica-Worms H, Townsend RR. Anal Chem. 2006;78:2171–2181. doi: 10.1021/ac051520l. [DOI] [PubMed] [Google Scholar]
- 19.Titani K, Kumar S, Takio K, Ericsson LH, Wade RD, Ashida K, Walsh KA, Chopek MW, Sadler JE, Fujikawa K. Biochemistry. 1986;25:3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- 20.Dong Z, Thoma RS, Crimmins DL, McCourt DW, Tuley EA, Sadler JE. J Biol Chem. 1994;269:6753–6758. [PubMed] [Google Scholar]
- 21.Ledford MR, Rabinowitz I, Sadler JE, Kent JW, Civantos F. Blood. 1993;82:169–175. [PubMed] [Google Scholar]
- 22.Schneppenheim R, Obser T, Drewke E, Ledford MR, Lavergen JM, Meyer D, Plendl H, Wieding JU, Budde U. Thromb Haemost. 2001;(Suppl):P1805. [Google Scholar]
- 23.Azuma H, Dent JA, Sugimoto M, Ruggeri ZM, Ware J. J Biol Chem. 1991;266:12342–12347. [PubMed] [Google Scholar]
- 24.Azuma H, Hayashi T, Dent JA, Ruggeri ZM, Ware J. J Biol Chem. 1993;268:2821–2827. [PubMed] [Google Scholar]
- 25.Sixma JJ, Sakariassen KS, Stel HV, Houdijk WP, In der Maur DW, Hamer RJ, de Groot PG, van Mourik JA. J Clin Invest. 1984;74:736–744. doi: 10.1172/JCI111489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pareti FI, Niiya K, McPherson JM, Ruggeri ZM. J Biol Chem. 1987;262:13835–13841. [PubMed] [Google Scholar]
- 27.Fujimura Y, Usami Y, Titani K, Niinomi K, Nishio K, Takase T, Yoshioka A, Fukui H. Blood. 1991;77:113–120. [PubMed] [Google Scholar]
- 28.Fujimura Y, Titani K, Holland LZ, Roberts JR, Kostel P, Ruggeri ZM, Zimmerman TS. J Biol Chem. 1987;262:1734–1739. [PubMed] [Google Scholar]
- 29.Pareti FI, Fujimura Y, Dent JA, Holland LZ, Zimmerman TS, Ruggeri ZM. J Biol Chem. 1986;261:15310–15315. [PubMed] [Google Scholar]
- 30.Matsushita T, Sadler JE. J Biol Chem. 1995;270:13406–13414. doi: 10.1074/jbc.270.22.13406. [DOI] [PubMed] [Google Scholar]
- 31.Bodo I, Katsumi A, Tuley EA, Eikenboom JCJ, Dong Z, Sadler JE. Blood. 2001;98:2973–2979. doi: 10.1182/blood.v98.10.2973. [DOI] [PubMed] [Google Scholar]
- 32.Raines G, Aumann H, Sykes S, Street A. Thromb Res. 1990;60:201–212. doi: 10.1016/0049-3848(90)90181-b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.