Abstract
Recent technical advances allow detection of several hundred volatile organic compounds (VOCs) in human exhaled air, many of which reflect unidentified endogenous pathways. Our group has previously estimated plasma glucose levels in healthy adults during a standard oral glucose tolerance test via exhaled VOC analysis. As a result of the metabolic characteristics of hyperglycemia in the diabetic (low insulin and increased free fatty acids and ketones), we hypothesized that different exhaled VOC profiles may be present in children with type 1 diabetes mellitus (T1DM) during spontaneous hyperglycemia. Exhaled methyl nitrate strongly correlated specifically with the acute, spontaneous hyperglycemia of T1DM children. Eighteen experiments were conducted among 10 T1DM children. Plasma glucose and exhaled gases were monitored during either constant euglycemia (n = 5) or initial hyperglycemia with gradual correction (n = 13); all subjects received i.v. insulin and glucose as needed. Gas analysis was performed on 1.9-liter breath samples via gas chromatography using electron capture, flame ionization, and mass selective detection. Among the ≈100 measured exhaled gases, the kinetic profile of exhaled methyl nitrate, commonly present in room air in the range of 5–10 parts per trillion, was most strongly statistically correlated with that of plasma glucose (P = 0.003–0.001). Indeed, the kinetic profiles of the two variables paralleled each other in 16 of 18 experiments, including repeat subjects who at different times displayed either euglycemia or hyperglycemia.
Keywords: exhaled gases, volatile organic compounds, gas chromatography, plasma glucose
The analysis of volatile organic compounds (VOCs) has been recognized for decades as a diagnostic tool with great potential for application to human breath, and several attempts have been made to use this technique for metabolic monitoring. However, intrinsic difficulties in measurement and analysis have resulted in inconsistent results, severely limiting its practical applicability.
Recent advances in VOC analytical technology may have reduced the impact of these technical issues, lowering detection limit of measurable gas concentrations, and increasing the repeatability and stability of measurements. Indeed, in recent years a rising number of studies centered on exhaled VOC clinical applications have been generated (1–5). Most studies, however, are focused on the detection of single disease markers, i.e., exhaled gas profiles constantly present in definite groups of patients, independent of their moment-by-moment metabolic changes. We believe that this approach, while having the potential of detecting important diagnostic markers, greatly underutilizes exhaled gas analysis. Exhaled gas profiles are likely involved in endogenous metabolic processes and are, therefore, constantly changing in response to the extremely complex human endogenous biochemical milieu. The extreme versatility of exhaled gas analysis (combining simultaneous measurements of 100 or more exhaled gases in each breath with easy, noninvasive, and painless collection methods) therefore appears especially suitable for the definition and monitoring of the time course of evolving, complex metabolic conditions, including, among others, inflammation, dyslipidemia, and diabetes. In recent years, our laboratory has concentrated on this approach for the use of exhaled VOC analysis in pathological conditions (4, 5). In this study, we describe the potential applications of VOC analysis to the monitoring of hyperglycemia in type 1 diabetes mellitus (T1DM). Blood glucose testing is the very base of diabetes management and can currently be accurately performed only through a blood sample. Attempts to develop alternative, noninvasive monitoring methods have been pursued for decades and, if successfully developed, are likely to have an immense global impact on diabetes screening, diagnosis, monitoring, and prevention.
We have recently demonstrated that it is possible to monitor plasma blood glucose during the transient hyperglycemia of a standard oral glucose tolerance test (OGTT) in healthy subjects through multilinear regression analysis of the combined exhaled profiles of ethanol and acetone (5). It is unlikely, however, that a similar exhaled gas profile would be present in all hyperglycemic situations. In T1DM, for instance, spontaneous (not postprandial) hyperglycemia is metabolically very different from the postprandial hyperglycemia in the healthy subject. Whereas circulating insulin increases rapidly in response to hyperglycemia in the healthy subject, hypoinsulinemia is present in the diabetic (indeed, it is actually causing hyperglycemia). Differing insulin concentrations affect lipolysis and, therefore, circulating lipid and ketone concentrations, which in turn acutely influence oxidative and inflammatory status, resulting in altered concentrations of oxidative markers and pro- and antiinflammatory cytokines. Because each of these correlated metabolic processes can conceptually generate one or a series of discrete exhaled VOCs, it is possible that each metabolic condition, in which hyperglycemia is one of the measurable components, may result in a distinct, characteristic pattern of exhaled VOCs.
In the present study, therefore, we analyzed the exhaled gas profiles of children with T1DM during either a sustained euglycemic state or spontaneous morning hyperglycemia and its gradual correction via i.v. insulin infusion.
Results and Discussion
Glucose and Insulin.
Five of the participants were euglycemic (80–130 mg/dl) at the start of the study; euglycemia was maintained for 2 h (average plasma glucose during last 60 min, 98 ± 3 mg/dl), and the experiment was then concluded. In the remaining 13 experiments, subjects had initial hyperglycemia (range, 160–410 mg/dl) that was gradually corrected via tapered insulin infusion, so that when euglycemia was achieved a basal rate of insulin infusion was present; this condition was maintained for at least 60 min, during which plasma glucose was not different from the euglycemic group (102 ± 4 mg/dl) (Fig. 1).
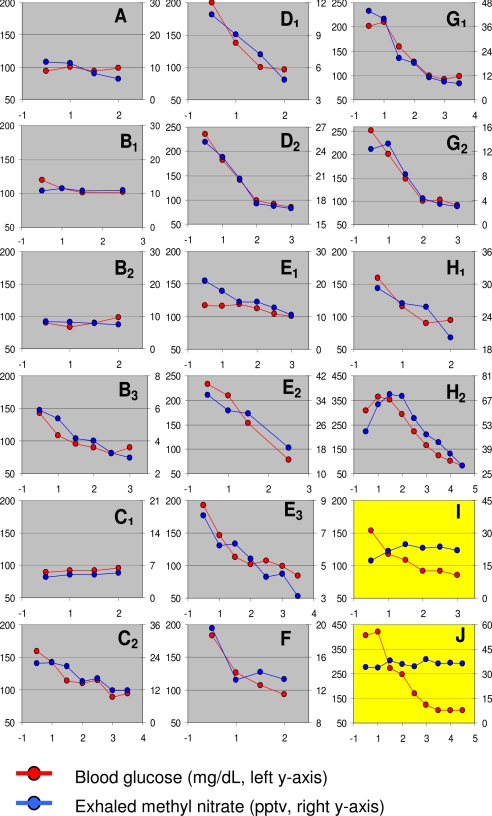
Fig. 1.
Plasma glucose and exhaled methyl nitrate profiles in 18 experiments performed in 10 children with T1DM. Children started experiments in either hyperglycemic or euglycemic conditions; if hyperglycemic, euglycemia was gradually restored by i.v. insulin infusion; if euglycemic, euglycemia was maintained for the duration of the study. In 16 of 18 experiments, exhaled methyl nitrate profiles closely paralleled plasma glucose. The left y axis shows blood glucose levels in milligrams per deciliter, the right y axis is exhaled (Δ) methyl nitrate in pptv, and the x axis is time in hours. Each subject is denoted by a letter, and multiple visits by a single subject are denoted by a subscripted number following the letter.
At the end of the study, all participating subjects displayed similar circulating insulin concentrations (14 ± 3 microunits/ml). Insulin infusion rates were similar in all subjects during the last 60 min of the experiments (euglycemic group, 1.1 ± 0.2 units/h; hyperglycemic group, 1.1 ± 0.3 units/h); in the hyperglycemic group, initial infusion rates were higher proportional to the levels of hyperglycemia (mean 3.1 ± 0.2 units/h, range 2.5–5.0 units/h).
Exhaled Gases (Methyl Nitrate).
The exhaled gas profiles of ≈100 gas species (List 1) were defined and compared with plasma glucose concentrations. The VOC displaying the greatest correlation with glucose was methyl nitrate (CH3ONO2), a gas ubiquitously present in both urban and rural air in the range of 3–15 parts per trillion by volume (pptv), with natural oceanic sources, minor industrial use as an explosive, and present in situ in urban atmospheric environments and in the University of California at Irvine (UCI) General Clinical Research Center (GCRC). For this reason, the exposure of lungs to inhaled trace amounts of methyl nitrate is a commonplace in everyday life. Its level in the UCI GCRC air varied from 5 to 10 pptv; however, within each experiment, its room air concentration never varied by >2 pptv. None of the other six alkyl nitrates quantified (see List 1) followed the trend with glucose of methyl nitrate.
List 1.
Gases quantified for the T1DM study
| Methane (CH4) | Cyclohexane (C6H12) | 1,2,4-Trimethylbenzene (C9H12) | CFC-12 (CCl2F2) |
| Carbon monoxide (CO) | 2,2-Dimethylbutane (C6H14) | 1,3,5-Trimethylbenzene (C9H12) | CFC-11 (CCl3F) |
| Carbon dioxide (CO2) | 2,3-Dimethylbutane (C6H14) | m-Ethyltoluene (C9H12) | CFC-113 (C2Cl3F3) |
| Ethane (C2H6) | 2-Methylpentane (C6H14) | p-Ethyltoluene (C9H12) | Chloromethane (CH3Cl) |
| Ethene (C2H4) | 3-Methylpentane (C6H14) | n-Decane (C10H22) | Bromomethane (CH3Br) |
| Ethyne (C2H2) | Methylcyclopentane (C6H12) | α-Pinene (C10H16) | Iodomethane (CH3I) |
| Propene (C3H6) | n-Heptane (C7H16) | β-Pinene (C10H16) | Trichloromethane (CHCl3) |
| Propane (C3H8) | Methylcylohexane (C7H14) | d-Limonene (C10H16) | Tribromomethane (CHBr3) |
| i-Butane (C4H10) | 2-Methylhexane (C7H16) | 1,3-Diethylbenzene (C10H14) | Carbon tetrachloride (CCl4) |
| n-Butane (C4H10) | 3-Methylhexane (C7H16) | 1,4- Diethylbenzene (C10H14) | Tetrachloroethylene (C2Cl4) |
| 1-Butene (C4H8) | 2,3-Dimethylpentane (C7H16) | 1,2-Diethylbenzene (C10H14) | 1,2-Dichloroethene (C2H2Cl2) |
| i-Butene (C4H8) | 2,4-Dimethylpentane (C7H16) | n-Undecane (C11H24) | Methyl chloroform (CH3CCl3) |
| trans-2-Butene (C4H8) | Toluene (C7H8) | Acetaldehyde (CH3CHO) | Ethyl chloride (C2H5Cl) |
| cis-2-Butene (C4H8) | n-Octane (C8H18) | Methanol (CH3OH) | Trichloroethylene (C2HCl3) |
| 1,3-Butadiene (C4H6) | Ethylbenzene (C8H10) | Ethanol (CH3CH2OH) | Bromodichloromethane (CHBrCl2) |
| i-Pentane (C5H12) | m-Xylene (C8H10) | Acetone (CH3COCH3) | Dichloromethane (CH2Cl2) |
| n-Pentane (C5H12) | p-Xylene (C8H10) | Propan-1-ol (C3H8O) | Dibromomethane (CH2Br2) |
| 1-Pentene (C5H10) | o-Xylene (C8H10) | Propan-2-ol (C3H8O) | Methyl nitrate (CH3ONO2) |
| Isoprene (C5H8) | 2-Methylheptane (C8H18) | Butanone (C4H8O) | Ethylnitrate (C2H5ONO2) |
| cis-2-Pentene (C5H10) | 3-Methylheptane (C8H18) | 2-Pentanone (C5H10O) | i-Propylnitrate (C3H7ONO2) |
| trans-2-Pentene (C5H10) | 2,2,4-Trimethylpentane (C8H18) | 3-Pentanone (C5H10O) | n-Propylnitrate (C3H7ONO2) |
| 2-Methyl-2-butene (C5H10) | 2,3,4-Trimethylpentane (C8H18) | Methyl isobutyl ketone (C6H12O) | 2-Butylnitrate (C4H9ONO2) |
| Cyclopentane (C5H10) | n-Nonane (C9H20) | Carbonyl sulfide (OCS) | 2-Pentylnitrate (C5H11ONO2) |
| n-Hexane (C6H14) | i-Propylbenzene (C9H12) | Carbon disulfide (CS2) | 3-Pentylnitrate (C5H11ONO2) |
| cis-3-Hexene (C6H12) | n-Propylbenzene (C9H12) | Dimethyl disulfide (C2H6S2) | |
| Benzene (C6H6) | 1,2,3-Trimethylbenzene (C9H12) | Dimethyl selenide (C2H6Se) |
In most cases, these compounds were quantitatively present in the room. Levels in the exhaled breath depended largely on production or absorption by the particular patient(s). CFC, chlorofluorocarbon.
Exhaled methyl nitrate profiles closely paralleled plasma glucose profiles in 16 of 18 experiments (Fig. 1). The methyl nitrate data in Fig. 1 are shown using a linear scale for each subject with adjusted minima (not necessarily zero) and maxima to show high correlation with the glucose data. Individual differences in methyl nitrate absolute concentrations were observed both from subject to subject but also for repeat measurements with the same subject (e.g., Fig. 1, H1 and H2). In the euglycemic group, methyl nitrate concentrations averaged 11 ± 3 pptv at the beginning of the study; after euglycemia was maintained for 2 h, methyl nitrate concentrations were not significantly different (8 ± 1 pptv; P = 0.32). These data are consistent with our previous findings of the measurable amount of 5 pptv of methyl nitrate in the breath of healthy subjects (Δ: room, 5 ± 2 pptv; breath, 10 ± 2 pptv) (4, 5). Conversely, in the hyperglycemic group, initial methyl nitrate concentrations were significantly greater (27 ± 6 pptv) and decreased significantly after hyperglycemia was corrected (15 ± 2 pptv; P = 0.01). Once stable euglycemia was achieved in the hyperglycemic group, the change in exhaled methyl nitrate for the remaining duration of the study was −3.7 ± 1.2 pptv, similar to the change observed in the euglycemic group (−3.1 ± 2.4 pptv; P = 0.080).
In Fig. 1, subject C displayed lower and constant methyl nitrate levels in her breath when she was euglycemic upon study initiation, whereas, when she arrived hyperglycemic, her methyl nitrate was significantly elevated and then decreased as she approached euglycemia. These data indicate that the same person may react differently depending on their blood glucose levels. Subject B arrived twice euglycemic and once hyperglycemic, and again exhaled methyl nitrate followed the plasma glucose profile.
Data analysis with a mixed model for repeated measurement was performed, and all of the terms of the interaction between methyl nitrate and glucose over time displayed high statistical significance (P = 0.003–0.001). The levels of significance were further enhanced (P < 0.001) by introduction of a 30-min lag time for methyl nitrate (this procedure was suggested by the visual detection of a “rightward shift” of the two curves in at least some subjects) (Fig. 2). Finally, because the different degrees of hyperglycemia determined a longer duration of the study for some subjects, resulting in different clock times at which the actual euglycemic period started, the analysis was repeated incorporating the different study starting point, again confirming the highly significant correlation between glucose and exhaled methyl nitrate.
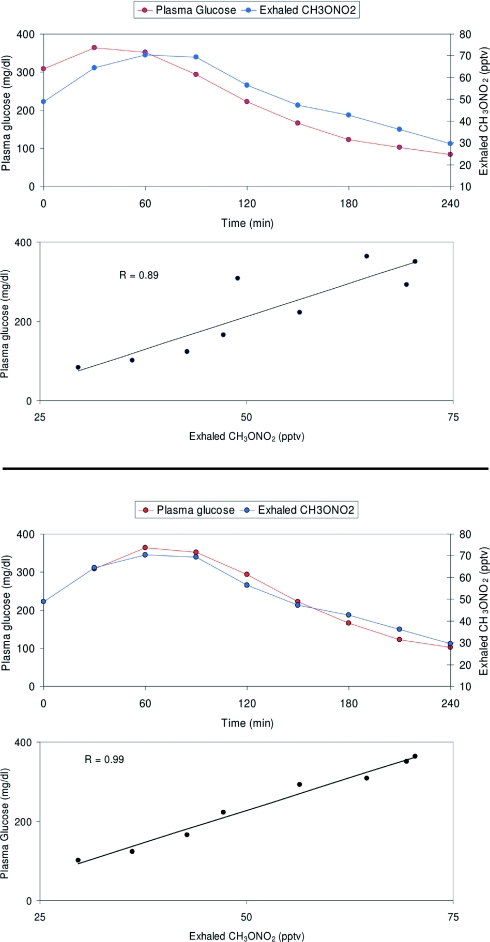
Fig. 2.
Plasma glucose and exhaled methyl nitrate profiles in one child (Fig. 1, H2) with T1DM who started the experiment in hyperglycemic conditions, with gradual correction of hyperglycemia via i.v. insulin infusion. The two upper graphs show data as they were initially recorded, already displaying a strong correlation between the two variables; when corrected for a 30-min time lag (the lower two graphs), the two curves appear to overlap almost exactly, with further strengthening of their correlation, suggesting that at least in some subjects a certain delay may exist between changes in plasma glucose and corresponding changes in exhaled VOCs.
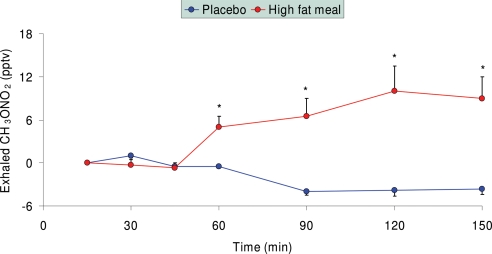
In this separate study of healthy subjects, after ingestion of noncaloric placebo, exhaled methyl nitrate concentration moderately decreased by the end of the study (Fig. 3). Conversely, after ingestion of a fat-rich meal, exhaled methyl nitrate markedly increased over time by ≈10 pptv.
Fig. 3.
Exhaled methyl nitrate profiles in healthy children after ingestion of either a noncaloric placebo (n = 8) or a high-fat semiliquid meal (n = 11). Exhaled methyl nitrate levels were significantly elevated after fat ingestion, probably correlating with increased circulating lipid concentrations.
The main finding of our study is that, among the ≈100 gases detected in the exhaled breath of children with T1DM, methyl nitrate displayed a kinetic profile closely paralleling that of plasma glucose in 16 of 18 experiments, during which plasma glucose was either constantly euglycemic or initially hyperglycemic (160–410 mg/dl) with gradual correction and reestablishment of euglycemia. Our data confirm the potential use of exhaled gas analysis as a noninvasive tool to monitor metabolic alterations, including hyperglycemia, in diabetic patients and expands prior findings from ours and other laboratories.
In a prior study (5) we were able to estimate with good accuracy plasma glucose profiles during a standard OGTT in 10 healthy young adults via multilinear regression analysis of exhaled ethanol and acetone, which in that experiment were the two exhaled gases with the highest individual correlations with circulating glucose. In the present study, ethanol and acetone did not correlate closely with plasma glucose, whereas methyl nitrate, a gas that did not correlate in our previous OGTT study, did. Although this lack of consistency may appear disconcerting after a superficial analysis of our results, we believe this is actually a strong supportive factor for the remarkable flexibility of exhaled gas analysis as a metabolic monitoring tool. Although it is, in fact, possible that no single exhaled gas or fixed “set” of exhaled gases may consistently correlate with circulating glucose concentrations per se, multiple gases or sets of gases may each correlate with hyperglycemia in a different metabolic context. By identifying these multiple sets of gases, our technique is therefore likely to allow not only an accurate estimate of plasma glucose but also of a series of additional related variables.
Hyperglycemia may occur in the context of very different metabolic conditions generated by multiple simultaneous biochemical processes, each potentially capable of independently altering the exhaled gas profile. The physiological, transient postprandial hyperglycemia occurring in healthy subjects, for instance, is paralleled by a rapid insulin response. In turn, in addition to its glucoregulatory effect, insulin will suppress lipolysis, transiently reducing circulating free fatty acids and their oxidation, thereby also reducing ketone bodies (6). Furthermore, gut bacteria may add to the circulation gaseous by-products of their own metabolism of ingested food, such as ethanol from carbohydrate fermentation (7–9), which may explain why a reduction in exhaled acetone and a transient increase in exhaled ethanol paralleled hyperglycemia in our previous OGTT study. A very different situation, on the other hand, is present during spontaneous hyperglycemia in T1DM. Because no gut absorption of nutrients is occurring, the concentrations of bacterial byproducts are unlikely to change. Furthermore, insulin is not increased; in fact, hypoinsulinemia is most likely the very cause of the hyperglycemic episode, with a reverse effect on lipid metabolism, i.e., increased lipolysis, increased free fatty acids and ketone concentrations, all factors favoring a proinflammatory, prooxidative status (10, 11). In this context, it is not surprising that the exhaled gas pattern in the present study was markedly different from that observed during the OGTT study.
Although we had not specifically predicted the observed strong correlation of exhaled methyl nitrate with plasma glucose, this correlation did not come as a surprise. The known chemical characteristics of this gas, in fact, place it as one of the possible candidates to track hyperglycemia indirectly via the simultaneous presence of oxidative stress. Indeed, when healthy children were studied after ingestion of either a noncaloric placebo meal or a fat-rich meal, exhaled methyl nitrate concentrations were significantly greater after lipid ingestion (Fig. 3) (¶), a finding consistent with the effect of acutely elevated free fatty acid concentrations and the consequent oxidative stress. Although free fatty acids could not be measured in our T1DM children, it is likely that they were similarly elevated as a consequence of the relative hypoinsulinemia that must have caused hyperglycemia.
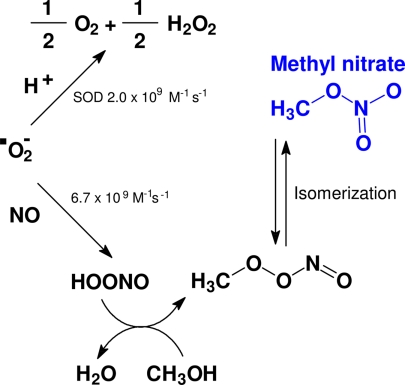
Methyl nitrate is the least reactive of all alkyl nitrates that are commonly observed in the atmosphere. Their main sources are marine emissions, biomass burning, and photochemical production in the atmosphere (13–15). In healthy subjects, exhaled methyl nitrate concentrations are slightly greater than room air concentration (normally by <10 pptv), indicating a small net output of this gas by the human body. The biochemical production of this gas has not been postulated; however, from our results it is clear that oxidative processes play a major role in its production. As a byproduct of physiological, energy-generating oxidative reactions, a small fraction of oxygen flowing through the mitochondria is converted to superoxide ion (O2•−), a free radical potentially capable of damaging cells and tissues (Fig. 4). These deleterious effects are prevented by multiple antioxidant mechanisms, such as the action of the enzyme superoxide dismutase, which converts superoxide to the less reactive oxygen (O2) and hydrogen peroxide (H2O2), via addition of protons (16). However, superoxide can also react very rapidly with nitric oxide (NO) (17, 18), forming a nitrate molecule that can be protonated (19), the speed of this reaction is likely accelerated at lower pH. This protonated nitrate species can react with methanol, yielding a water molecule and a molecule similar to methyl nitrate, which can isomerize to methyl nitrate. Within the human body, some colonizing bacteria could produce methanol directly or oxidize methane (CH4) to methanol (CH3OH) (20, 21), providing the methyl radical (22, 23), or other metabolic sources may be responsible for it. When hyperglycemia occurs in diabetics, an accelerated metabolic flux through the mitochondria may lead to increased superoxide formation, possibly directly linking blood glucose levels with systemic oxidation; in the extreme case of severe hyperketonemia, the pH shift toward acidosis may further accelerate this chain of reactions.
Fig. 4.
Schematic representation of methyl nitrate formation in vivo. In vivo, a small but relatively constant fraction of superoxide ion (O2•−) is diverted from its interaction with superoxide dismutase (SOD) and reacts with nitric oxide (NO) to eventually form methyl nitrate (CH3ONO2).
Although the large majority of the experiments included in this study displayed the reported close correlation between plasma glucose and exhaled methyl nitrate, two of 18 did not. Interestingly, one of the two was the subject who started the study with the highest plasma glucose, 410 mg/dl (>70 mg/dl above any other participant). His exhaled methyl nitrate levels were also among the highest but remained elevated after correction of hyperglycemia (Fig. 1, subject J). We can speculate that if blood glucose had remained elevated for a prolonged period before the study (possibly even at concentrations higher than those recorded at admission), this may have overstimulated prooxidative mechanisms, preventing a prompt correction of the acute inflammatory/oxidative milieu shortly after euglycemia was resumed. Separate studies in a similar group of patients have indicated that concentrations of proinflammatory cytokines, which closely parallel oxidative stress markers, increase acutely during hyperglycemia and may remain elevated for several hours after hyperglycemia is corrected.∥ Even in subjects with good concordance between plasma glucose and methyl nitrate, the latter appears to lag the former, as shown by the statistical improvement of correlation introducing a 30-min lag. Finally, this subject was undergoing growth hormone supplementation therapy. Despite its beneficial effects, growth hormone is known to exert a prooxidative effect (24) and may have contributed to the sustained exhaled methyl nitrate levels. The second subject with discordant glucose and methyl nitrate profiles (Fig. 1, subject I) also was atypical in that although her prestudy glucose concentration was not exceptionally elevated, although her methyl nitrate concentrations were, and she displayed markedly greater levels of discomfort during study procedures than the rest of the study group. Analysis of the data from these two atypical subjects suggests that exhaled methyl nitrate may indeed reflect acute increases in systemic oxidative stress, but data interpretation may be confounded when oxidative processes are simultaneously stimulated by mechanisms independent of hyperglycemia and its related metabolic alterations.
The above considerations are relevant to the overall practical applicability of exhaled methyl nitrate as a hyperglycemic marker in diabetes. The ultimate goal of our line of experiments is to produce predictive algorithms that will allow conversion of exhaled gas concentrations into blood glucose readings. Our present results allow estimation of glucose curves from exhaled gas only on an individual basis, because a general algorithm applicable to the whole study population will require identification and quantification of the relative contribution of all pertinent covariates, which can only be obtained through additional, more complete studies. As stated above, a time-lag effect is probably one of the interfering factors, but even this effect could only be incompletely defined because of the relatively long intervals (30 min) between time points (incidentally, a lag-time effect may probably explain why localized discordance was present at the first or last time points in some experiments, such as E1 and E3, despite an overall good concordance between exhaled methyl nitrate and plasma glucose values). Importantly, because prevention and reversal of hypoglycemia, in addition to hyperglycemia, has recently become a major area of concern in the management of T1DM, future studies must also include hypoglycemic conditions (for obvious ethical reasons experimental hypoglycemia could not be established in our pediatric-age population).
In conclusion, analysis of exhaled breath in children with T1DM during euglycemia or spontaneous hyperglycemia revealed a strong correlation between the kinetic profiles of plasma glucose and exhaled methyl nitrate. The characteristics of methyl nitrate formation suggest that this gas may reflect, rather than hyperglycemia per se, the specific and complex pattern of metabolic alteration accompanying hyperglycemia in T1DM. This pattern includes not only hypoinsulinemia and increased lipids and ketones but also alterations in inflammatory and oxidative markers, now considered main determinants of diabetic vascular complications. Optimization of exhaled gas analysis in diabetes may prove invaluable not only in monitoring glycemic control but also in determining the actual pathogenic potential of a given glycemic state in terms of onset/progression of diabetic complications.
Materials and Methods
All protocols were approved by the UCI Institutional Review Board; subjects and parents/guardians signed informed assent and consent forms. Studies were performed at the UCI GCRC, and gas analysis was performed in the Laboratory of F.S.R. and D.R.B. in the UCI Department of Chemistry.
Eighteen individual studies were performed among 10 children (seven males and three females) with T1DM (diagnosed >2 years before enrollment). The mean age of the participants was 13.8 ± 0.5 yr (range 11–15 yr). The mean HbA1c level was 8.0 ± 0.8% (range 6.7–9.2%). The participants took no medications other than insulin replacement (with the single exception of patient J, who was on growth hormone replacement). In addition there were no tissue complications, autonomic neuropathy, or other chronic pathology.
Participants were admitted at the UCI GCRC at 7 a.m. To reproduce a real-life scenario, participants had been asked to eat a light breakfast at approximately 6 a.m. Patients on insulin pumps followed their usual regimen; those on multiple insulin injections had their last slow-acting insulin (glargine) injection no later than the night before and injected fast-acting insulin only in the morning. Upon admission, breath and room air samples were collected and i.v. lines were placed in both arms for blood drawing and study infusions (insulin and 20% dextrose). A continuous insulin infusion was started (target: at least 90 min of glycemia between 90 and 110 mg/dl). If the participant was hyperglycemic at admission (and it should be noted that, because hyperglycemia was spontaneous, its magnitude varied across subjects), insulin was infused i.v. at a rate of 1.0 unit/h for every 50 mg/dl above euglycemia and then gradually tapered down as blood glucose approached euglycemia. Once euglycemia was achieved, or if the patient's blood glucose was already on target at admission, i.v. insulin infusion was continued at the minimum level that allowed maintenance of euglycemia. For patients on insulin pumps, this level corresponded to the their normal basal rate (between 0.9 and 1.4 units/h), whereas in patients on multiple injections, in which the last glargine injection had a residual effect estimated as equivalent to the infusion of 0.7–1.0 units/h, the additional i.v. infusion average was 0.35 ± 0.1 units/h. Small amounts of i.v. glucose were infused if necessary to prevent hypoglycemia, based on glucose readings taken at 10- to 15-min intervals. Every 30 min, blood samples were matched by the collection of exhaled gas samples. To collect the gas samples, participants exhaled for 10–15 s into specially designed, electropolished, 1.9-liter, stainless-steel canisters that were sterilized before use by baking at 150°C for 12 h and evacuated to <10−5 atm (1 atm = 101.3 kPa). Subjects took a deep inspiration to total lung capacity and then slowly exhaled until near residual volume through a mouthpiece connected to the canister via a three-way valve. Gas from the first 2–3 s of the exhalation maneuver was vented to the room to clear the system of anatomic dead space. A single practice was normally sufficient to train subjects for this technique. Measurement of the CO2 concentrations in the exhaled breath (≈5%) demonstrated that the captured sample was almost entirely alveolar air. A room air sample was simultaneously collected in an identical canister for each exhaled gas sample. Canisters containing breath and room air samples were then stored at room temperature for later analysis. All exhaled methyl nitrate data reported are in Δ values (breath minus room).
In a separate set of experiments, 12 healthy children (six males and six females, ages 11–15 yr), were studied after an overnight fast. Children ingested either a noncaloric placebo or a fat-rich shake (1.5 grams of fat per kilogram of body weight), and repeated matched blood and exhaled breath samples were collected over 150 min. Circulating variables from this study have been previously published.¶
Laboratory Techniques.
Immediately after blood draws, 0.4 ml of blood was spun on a microcentrifuge for rapid glucose content determination on a Beckman Glucose Analyzer II (Beckman Coulter, Fullerton, CA), using the glucose oxidase method.
VOCs were cryogenically trapped and injected into a multicolumn/detector gas chromatography system. The detectors included two flame ionization detectors (FID), two electron capture detectors (ECD), and a quadruple mass spectrometer (MSD). Five different columns were used for separations and combined with the detectors as follows: PLOT/FID, DB-1/FID, DB-5/ECD, RTX-1701/ECD, and DB-5ms/MSD. This analysis allows accurate quantification of a large variety of different gas species. For a more detailed discussion of gas analysis, see Colman et al. (12).
Acknowledgments
We thank the UCI GCRC nurses and staff for excellent research support and Brent Love for the chromatographic analysis. This research was supported by National Institutes of Health Grants M01-RR00827-28 and K-23 RR018661-01 and Juvenile Diabetes Research Foundation Grant 11-2003-332.
Abbreviations
- GCRC
General Clinical Research Center
- OGTT
oral glucose tolerance test
- pptv
parts per trillion by volume
- T1DM
type 1 diabetes mellitus
- VOC
volatile organic compound
- UCI
University of California at Irvine.
Footnotes
The authors declare no conflict of interest.
Blake, D. R., Iwanaga, K., Novak, B. J., Meinardi, S. Pescatello, A., Cooper, D. M., Galessetti, P. R., American Diabetes Association 64th Annual Scientific Sessions, June 4–8, 2004, Orlando, FL, abstr. 1549-P.
Galassetti, P., Flores, R., Larson, J., Zaldivar, F., Barnett, M., Rosa, J., American Diabetes Association 66th Annual Scientific Sessions, June 9–13, 2006, Washington, DC, abstr. 235-OR.
References
- 1.Moser B, Bodrogi F, Eibl G, Lechner M, Rieder J, Lirk P. Respirat Physiol Neurobiol. 2005;145:295–300. doi: 10.1016/j.resp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. J Chromatogr B. 1999;729:75–88. doi: 10.1016/s0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 3.Rieder J, Lirk P, Ebenbichler C, Gruber G, Prazeller P, Lindinger W, Amann A. Wien Klin Wochenschr. 2001;113:181–185. [PubMed] [Google Scholar]
- 4.Kamboures MA, Blake DR, Cooper DM, Newcomb RL, Barker M, Larson JK, Meinardi S, Nussbaum E, Rowland FS. Proc Natl Acad Sci USA. 2002;102:15762–15767. doi: 10.1073/pnas.0507263102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galassetti P, Novak B, Nemet D, Rose-Gottron C, Cooper DM, Meinardi S, Newcomb R, Zaldivar F, Blake DR. Diabetes Technol Ther. 2005;7(1):115–123. doi: 10.1089/dia.2005.7.115. [DOI] [PubMed] [Google Scholar]
- 6.Galassetti P, Mann S, Tate D, Neill RA, Costa F, Wasserman DH, Davis SN. Am J Physiol. 2001;280:E908–E917. doi: 10.1152/ajpendo.2001.280.6.E908. [DOI] [PubMed] [Google Scholar]
- 7.Kaji H, Asanuma Y, Yahara O, Shibue H, Hisamura M, Saito N, Kawakami Y, Murao M. J Forensic Sci. 1984;24:461–471. doi: 10.1016/s0015-7368(84)72325-5. [DOI] [PubMed] [Google Scholar]
- 8.Baraona E, Julkunen R, Tannenbaum L, Lieber CS. Gastroenterology. 1986;90:103–110. doi: 10.1016/0016-5085(86)90081-8. [DOI] [PubMed] [Google Scholar]
- 9.Bivin WS, Heinen BN. J Bacteriol. 1985;58:355–357. doi: 10.1111/j.1365-2672.1985.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopes HF, Morrow JD, Stoijiljkovic MP, Goodfriend TL, Egan BM. Am J Hypertens. 2003;16:331–336. doi: 10.1016/s0895-7061(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 11.Stojiljkovic MP, Lopes HF, Zhang D, Morrow JD, Goodfriend TL, Egan BM. J Hypertens. 2002;20:1215–1221. doi: 10.1097/00004872-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 12.Colman JJ, Swanson AL, Meinardi S, Sive BC, Blake DR, Rowland FS. Anal Chem. 2001;73:3723–3731. doi: 10.1021/ac010027g. [DOI] [PubMed] [Google Scholar]
- 13.Blake NJ, Blake DR, Swanson AL, Atlas E, Flocke F, Rowland FS. J Geophys Res. 2003;108:D2. [Google Scholar]
- 14.Moore RM, Blough NV. Geophys Res Lett. 2002;29:27/1–27/4. [Google Scholar]
- 15.Simpson IJ, Meinardi S, Blake DR, Blake NJ, Rowland FS, Atlas E, Flocke F. Geophys Res Lett. 2002;29:1–4. [Google Scholar]
- 16.Voet D, Voet J. Biochemistry. 2nd Ed. New York: Wiley; 1995. p. 1360. [Google Scholar]
- 17.Hurst JK, Lymar SV. Acc Chem Res. 1999;32:520–528. [Google Scholar]
- 18.Beckman JS, Koppenol WH. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 19.Pryor WA, Squadrito GL. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 20.Xin J-YC, Jun-Ru N, Jian-Zhong H, Shao-Feng X, Chun-Gu L, Shu-Ben Z. Biocatal Biotransform. 2004;22(3):225–229. [Google Scholar]
- 21.Choi HS, Kim HG, Kim SU, Lee SG, Ryu GH. 2002-73376. Korean Patent Appl KR. 2004 20021125.
- 22.de Assis M, Saliba AM, Vidipo LA, de Salles JB, Plotkowski M. Immunol Cell Biol. 2004;82(4):383–392. doi: 10.1111/j.0818-9641.2004.01249.x. [DOI] [PubMed] [Google Scholar]
- 23.Takoudes TG, Haddad J. Laryngoscope. 2001;111(2):283–289. doi: 10.1097/00005537-200102000-00018. [DOI] [PubMed] [Google Scholar]
- 24.Brown-Borg HM, Bode AM, Bartke A. Endocrine. 1999;11(1):41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]