Abstract
Sensory hair cell loss is a major contributor to disabling hearing and balance deficits that affect >250 million people worldwide. Sound exposures, infections, drug toxicity, genetic disorders, and aging all can cause hair cell loss and lead to permanent sensory deficits. Progress toward treatments for these deficits has been limited, in part because hair cells have only been obtainable via microdissection of the anatomically complex internal ear. Attempts to produce hair cells in vitro have resulted in reports of some success but have required transplantation into embryonic ears or coculturing with other tissues. Here, we show that avian inner ear cells can be cultured and passaged for months, frozen, and expanded to large numbers without other tissues. At any point from passage 6 up to at least passage 23, these cultures can be fully dissociated and then aggregated in suspension to induce a mesenchymal-to-epithelial transition that reliably yields new polarized sensory epithelia. Those epithelia develop numerous hair cells that are crowned by hair bundles, composed of a single kinocilium and an asymmetric array of stereocilia. These hair cells exhibit rapid permeance to FM1-43, a dye that passes through open mechanotransducing channels. Because a vial of frozen cells can now provide the capacity to produce bona fide hair cells completely in vitro, these discoveries should open new avenues of research that may ultimately contribute to better treatments for hearing loss and other inner ear disorders.
Keywords: balance, culture, hearing, regeneration, stem cell
Sensory hair cells, which are named for mechanotranducing hair bundles that extend into the endolymph of the internal ear, are essential for hearing and balance. When hair cells are lost through trauma, toxicity, infection, genetic disorders, or aging, that can lead to impaired hearing or disruption of vestibular reflexes that normally stabilize gaze, posture, and locomotion (1, 2). Such deficits affect humans and other mammals permanently, but birds, fish, and amphibians are able to regenerate hair cells and recover sensory functions within weeks. In those species, supporting cells that reside between neighboring hair cells respond to the loss by dedifferentiating, dividing, and giving rise to progeny that can differentiate as self-renewing supporting cells and replacement hair cells (3–6).
Although the deficits caused by hair cell loss remain clinically irreversible, supporting cells in the balance organs of humans and rodents appear to hold the potential for regeneration because some are able to respond to trauma by dividing (7). The incidence of vestibular supporting cell division is low in adult mammals (7, 8), but the majority of supporting cells in the balance organs of young mammals enter S-phase and proliferate when cultured with appropriate pharmacological agents (9–13).
In mammals, vestibular supporting cell responses to mitogens decline dramatically during the first postnatal weeks, and that decline appears to be responsible for the mammalian ear's unique vulnerability to permanent hair cell loss (13). In vitro pharmacological treatments that help to restore proliferation in mature mammalian vestibular epithelia have recently been identified (13–15), but the achievement of effective regeneration in mammalian ears is likely to depend in part on discovering how hair cell differentiation is controlled.
The progeny of supporting cell divisions and of a recently discovered stem cell are important sources of newly differentiated hair cells in nonmammalian regeneration (16, 17), and there is evidence that supporting cells sometimes convert directly into hair cells without an immediately preceding cell division (18). The basic helix–loop–helix gene Atoh1 is required for the embryonic differentiation of hair cells, and forced expression of Atoh1 induces the formation of ectopic hair cells in organ culture (19–21). In fact, functional auditory recovery in adult guinea pigs has been attributed to viral delivery of Atoh1 (22), but much remains to be learned about how cells are guided to differentiate as hair cells.
A number of cell types have been investigated for their potential to begin differentiating toward a hair cell phenotype. Cultures of conditionally immortalized cells (23, 24), primary cells dissected from the ears of mice (25, 26), murine ES cells (27), and olfactory precursor cells (28) all have been found to contain some cells that express hair cell proteins. However, the formation of hair bundles, characteristic hair cell bodies, and other more convincing indications of hair cell differentiation are reported to require transplantation of cultured cells into the ears of embryos (27) or coculturing with other tissues from the developing head (26).
Because avian vestibular organs produce hair cells throughout life (29–31), we investigated the differentiation of hair cells by culturing cells from the utricular sensory epithelium of chicken embryos, greatly expanding their numbers, and freezing, thawing, and passaging them during several weeks and months of culture. Then, we experimented with advanced-passage cells taken from these cultures at various times and discovered that a mesenchymal-to-epithelial transition can lead to the differentiation of bona fide hair cells completely in vitro.
Results
Production of Pure Utricular Cell Cultures.
To avoid potential contamination by inadvertent inclusion of cells from the nonsensory epithelium and the underlying stroma, we followed three procedural steps when isolating pure sheets of hair cell epithelium and only cultured the cells from the central part of the sensory epithelium (see Methods). We pooled those cells from 16 ears and used trypsin and gentle trituration to produce partially dissociated primary sensory epithelium cultures (Fig. 1A). On different days, we repeated all of the steps to establish four independently derived lines of cells for use in parallel testing to make certain that the results could be consistently replicated.
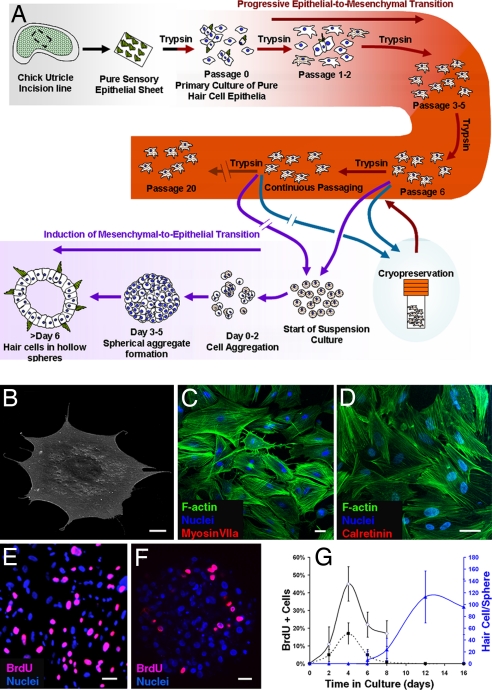
Fig. 1.
Advanced-passage cultures from pure hair cell epithelia. (A) A schematic diagram showing how inner-ear hair cells were produced in vitro. (B) Cells cultured from embryonic day 14 chicken embryo hair cell epithelia changed from columnar to flattened polygonal shapes as illustrated in this scanning electron micrograph of a cell from passage 3. (C and D) By passage 3, preexisting hair cells and the hair cell markers myosin VIIa and calretinin were no longer detectable in the 2D cultures, and phalloidin-labeled F-actin became organized in stress fibers in the cultured cells. (E–G) Cells in 2D cultures proliferated and continued to incorporate BrdU during 24-h labeling periods that preceded fixations at different stages (E and solid dark line in G), but the incidence of proliferation decreased after cells were suspended and formed spheres (F and dashed dark line in G, mean ± standard deviation). Hair cells differentiated as early as day 6 after suspension and increased in number for at least 6 more days (blue line in G). (Scale bars: 20 μm.)
To expand the number of proliferating cells and eliminate preexisting postmitotic hair cells, we passaged the cells for several weeks and monitored the progressive loss of hair cells. The primary cultures [designated passage (P)0] contained many solitary cells but also small epithelial islands composed of preexisting hair cells and supporting cells, which were visibly linked by epithelial junctions. The supporting cells that grew in isolation as individual cells and those that were near the edges of the epithelial islands were the first to flatten and spread as thin polygonal shaped cells (13–15), followed in turn by the remaining supporting cells within those islands. In contrast to the supporting cells, the hair cells remained essentially cylindrical and were readily identifiable by their circular apical surfaces and hair bundles. Each week, we passaged the cells, counting and progressively expanding the cultures into larger wells and then into flasks in subsequent passages, identified here as P1 through P23 [supporting information (SI) Fig. 5]. We froze all of the cells at P5 through P7 and periodically thawed and cultured cells from those stored vials, continuously passaging some cells from each of the four original cultures and freezing samples at later passages.
By the end of the first three passages, the cells in the epithelial islands had become completely disaggregated, and >95% grew as solitary cells (Fig. 1B and SI Fig. 5), with the remaining cells growing in small clumps that lacked epithelial junctions (SI Fig. 5). After 3–4 weeks in 2D culture, preexisting hair cells and the hair cell markers myosin VIIa and calretinin were no longer detectable in the cultures (Fig. 1 C and D). These cultured cell populations doubled every 118 ± 18 h (mean ± standard deviation) during at least 19 passages in which cell numbers were quantified. The cells in these cultures incorporated BrdU as they entered S-phase and divided to produce millions more cells than were in the primary cultures (Fig. 1 E–G and SI Fig. 5).
An Epithelial-to-Mesenchymal Transition Occurred in the 2D Cultures.
The disaggregation of the epithelial islands coincided with the progressive loss of epithelial junctions and the loss of E- and N-cadherin expression (Fig. 2 A and B). Like other dissociated epithelial cells, these transitioned to a mesenchymal phenotype and grew as solitary cells when cultured on a solid substrate (32). F-actin was organized in stress fibers in these cells (Figs. 1D and 2), and they expressed the mesenchymal intermediate filament, vimentin (Fig. 2C), and the mesenchymal transcription factor, slug (Fig. 2D). Vimentin and slug were not expressed in the hair cell epithelium in vivo (Fig. 2 C and D).
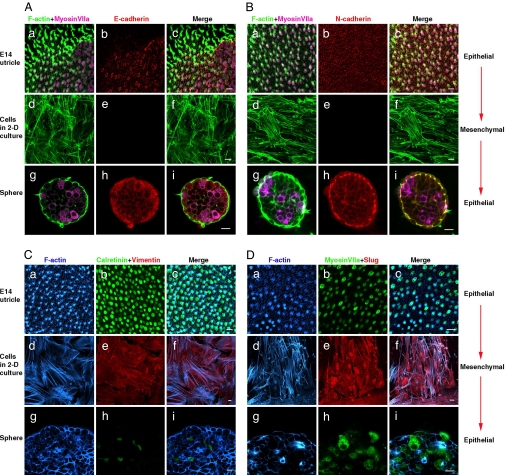
Fig. 2.
A mesenchymal-to-epithelial transition leads to hair cell differentiation. (a–c) The utricle sensory epithelium fixed in situ. (d–f) Fixed 2D cell cultures. (g–j) Spheres of sensory epithelium produced by dissociation and aggregation of cells from 2D cultures under conditions that prevented adhesion to a substrate. Phalloidin labeled F-actin that was in cortical bands and hair bundles, and the hair cells expressed myosin VIIa in the epithelia of the utricle and the spheres (a and g in A, B, and D). F-actin was organized as stress fibers, and there was no expression of myosin VIIa in the 2D cultures (d in A, B, and D). Likewise, E- and N-cadherin were expressed in the epithelia (b and h in A and B) but were absent from the cells cultured on 2D substrates. The sensory epithelia also expressed the hair cell marker calretinin (b and h in C) but not the mesenchymal intermediate filament, vimentin, or the mesenchymal transcriptional factor, slug (b and h in D). Both vimentin (e in C) and slug (e in D) were expressed in the cells cultured and passaged in 2D culture, but those mesenchymal proteins were not detectable in the epithelia that formed via aggregation (h in C and D). (Scale bars: 10 μm.)
Suspension Culture Led to a Mesenchymal-to-Epithelial Transition.
Reasoning that differentiation and the establishment of cell polarity would depend on the formation of epithelial junctions, we attempted to induce junction formation by growing cells to 100% confluence, but that failed to produce any detectable signs of E-cadherin, N-cadherin, myosin VIIa, or calretinin expression, and the F-actin did not form cortical bands (Fig. 2). We suspected that adhesion to the rigid 2D substrate was preventing epithelial junction formation by supplying signals that overwhelmingly promoted cell spreading and the retention of the mesenchymal phenotype (Fig. 3A). To test this hypothesis, we dissociated cells that had been passaged at least six times (≈6 weeks) and cultured them in suspension with gentle agitation in serum-free medium containing N2, B27, and FGF2. Later, we achieved similar but more consistent results by suspending the cells in hanging drops of that medium. At the start, all these suspension cultures contained >95% individual, fully dissociated cells (Fig. 3B). Within 8 h, both suspension methods resulted in the progressive aggregation of the cells into small clusters (Fig. 3C). After 1–2 days, 85–95% of the suspensions contained larger aggregates that formed when the small clusters adhered to each other and coalesced (Fig. 3 D–F). By 4–6 days, >50% of the now spherical aggregates developed lumens (Fig. 3G), and by day 8, these hollow spheres averaged 238 ± 51 μm in diameter, and their irregular surfaces had become smooth as they passed through changes that resembled embryonic compaction (Fig. 3 H and I).
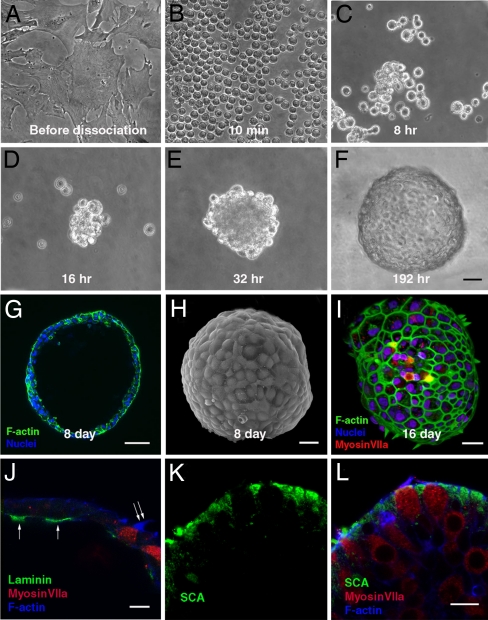
Fig. 3.
Aggregation of late-passage cells led to formation of hollow smooth-surfaced spheres with apical structures pointing outward. (A) Advanced passage cells growing in 2D culture. (B) A fully dissociated sample of cells 10 min after it was trypsinized from a 2D culture such as that in A and placed in suspension. (C–E) Cells adhered to each other and progressively formed larger aggregates at 8 (C), 16 (D), and 32 (E) h after dissociation from the 2D cultures and suspension in the serum-free medium. (F) The aggregates formed smooth spheres by day 8 after suspension. (G and H) An optical section showing the hollow lumen of such a sphere (G) and a scanning electron micrograph showing the characteristic smooth surface of a sphere 8 days after suspension of the cells from an advanced-passage 2D culture (H). (I) Phalloidin-labeled F-actin in hair bundles projecting outward from cells that are also labeled by anti-myosin VIIa. F-actin juxtaposed to epithelial junctions is also visible in this 3D reconstruction from confocal microscopy. (J) Laminin (arrows) is expressed in patches along the inner basal epithelial surface of the sphere shown and was found in similar patches in four other spheres. Note the hair bundle extending from the sphere's outer surface (double arrow). Other cells in the epithelia of the spheres express the supporting cell marker, SCA (in K and the merged image in L). (Scale bars: 20 μm in A–F; 50 μm in G; 30 μm in H and I; and 10 μm in J–L.)
The aggregation of the cells in suspension culture led to a decline in the rate of proliferation from that measured in parallel 2D cultures (Fig. 1 E–G), and it induced the mesenchymal-to-epithelial transition we sought (Figs. 2 and 3 A–I). After the solitary cells aggregated in suspension, they formed cell–cell adhesions and developed the epithelial junctions required for apical-basal polarity and the development of asymmetric distributions of proteins into apical and basolateral domains (33). After 6–8 days, the cells in these spherical aggregates expressed E- and N-cadherin (Fig. 2 A and B) and no longer expressed detectable levels of vimentin and slug (Fig. 2 C and D). Their actin filaments became organized in cortical bands juxtaposed to apical epithelial junctions that were readily identifiable in light and EM (Figs. 2, 3 H and I, and 4 A and G), and laminin was expressed at the inner basal surface of the spherical epithelia (Fig. 3J).
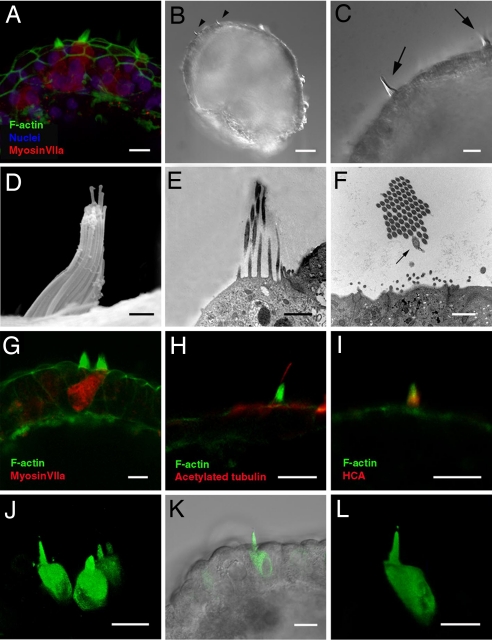
Fig. 4.
Bona fide hair cells form sensory hair bundles that project outward from the spheres. (A) Hair bundles on the apical surfaces of myosin VIIa-positive hair cells surrounded by epithelial junctions. (B and C) Hair bundles (arrowheads and arrows) at the surface of a sphere visualized by differential interference contrast microscopy. (D) Another hair bundle visualized by scanning EM. The spheres in B–D were produced from cells that had been cultured through 19 passages. (E and F) Longitudinal (E) and transverse (F) sections through hair bundles at the surfaces of spheres viewed by transmission EM. Note the characteristic eccentrically positioned kinocilium (arrow in F, which was observed in a fortuitous transverse section through one of the 18 hair bundles examined in the five spheres that were processed for transmission EM). (G) Phalloidin-labeled stereocilia on the surface of a myosin VIIa-positive hair cell and its neighbor, and F-actin outlined the surrounding columnar cells of the sphere's wall. (H) Anti-acetylated tubulin labeled a single kinocilium extending above the phalloidin-labeled hair bundles on at least 8 of the 21 myosin VIIa-positive hair cells observed in limited examinations of the five spheres immunostained with that antibody. (I) Hair bundle double-labeled with phalloidin and antibody to the 275-kDa hair cell antigen (HCA). (J–L) In optical assays for open mechanotransduction channels, FM1-43 permeated hair cells after 10-sec exposures to the dye that were followed by thorough rinsing, but no other cells in the spheres were labeled. The spheres and hair cells in this figure were all derived from cultures that had been frozen at P5, and then thawed and continuously cultured until P19, when they were dissociated and aggregated in suspension. (Scale bars: 10 μm in A, C, and G–L; 50 μm in B; and 1 μm in D–F).
Hair Cell Differentiation Followed the Mesenchymal-to-Epithelial Transition.
As early as day 6 after suspension, some of the spheres began to develop cells that expressed the hair cell markers myosin VIIa (Figs. 3I and 4 A and G and SI Fig. 6), calretinin, parvalbumin 3, and otoferlin (HCS-1) (SI Fig. 6). Two days later, clearly recognizable hair cell bundles crowned the apical surfaces of 24 ± 19 cells per sphere (mean ± standard deviation, representing 3 ± 2% of the cells in each sphere), which also expressed pairs of the hair cell markers. Four days later, the spheres averaged 113 ± 44 hair cells (mean ± standard deviation, or 15 ± 6% of the cells in each sphere) with those characteristics (Fig. 1E, n = 6 spheres per time point). In each case, the hair bundles and apical surfaces of the hair cells projected outward toward the medium surrounding the sphere, rather than into the sphere's lumen (Figs. 3I and 4 B and C). Confocal microscopy (Fig. 3I) and both scanning (Fig. 4D) and transmission (Fig. 4 E and F) electronic microscopy showed individual hair cells and regions that contained small groups of hair cells that alternated with the other epithelial cells in mosaic patterns (Figs. 3I and 4 A and G). The supporting cell marker SCA was expressed in other epithelial cells of the spheres (Fig. 3 K and L) (34).
The hair bundles that developed on these polarized hair cells were composed of actin-filled stereocilia and a single eccentrically positioned kinocilium that labeled with anti-acetylated tubulin (Fig. 4 F and H). They also labeled with hair cell antigen, an antibody to a 275-kDa antigen specific to hair cell bundles (Fig. 4I) (35). Hair cell bundles developed in spheres made from cell cultures maintained to at least P23.
N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl)pyridiniumdibromide (FM1-43) Permeates the Hair Cells Rapidly and Selectively.
By virtue of its rapid permeation through mechanosensory transduction channels that are in the open state (36, 37), the cationic, styryl pyridinium dye FM1-43 provides a visual assay for ion channel function in hair cells and has been used to identify the onset of transduction channel function in developing hair cells (38, 39). To test for the possibility that the hair cells might express functional mechanotransduction channels, we briefly incubated spheres produced from the P15 passage of one cell line and others from the P19 of another line with a fixable form of FM1-43. Ten seconds of incubation in 5 μM FM1-43 that was followed by 5 min of thorough rinsing in DMEM/F12 labeled just the hair cells in those spheres (Fig. 4 J–L), and incubation in 1 mM gentamicin blocked the permeation of FM1-43 reversibly. Using differential interference contrast microscopy after a brief exposure to FM1-43, we identified 25 hair bundles in optical sections of five living spheres. All 25 of those hair cells were labeled by FM1-43 (Fig. 4 J–L). Although dye permeation experiments cannot take the place of electrophysiological investigations for establishing the functional status of the hair cells, the evidence from these FM1-43 permeation experiments is consistent with the possibility that the new hair cells contain open and potentially functional mechanotransduction channels.
Discussion
We have isolated and investigated cells from the utricular hair cell epithelium of embryonic day 14 chicken embryos. In 2D culture, these cells grew for months, with ≈5-day population doublings. By the end of the third passage, the cells had lost their original epithelial characteristics as they completed an epithelial-to-mesenchymal transition. After the epithelial-to-mesenchymal transition, fully dissociated cell suspensions were readily produced by trypsinization, and samples taken from P6 to P23 were aggregated under suspension culture conditions, which eliminated opportunities for adhesion to a substrate and thereby promoted cell–cell adhesion. Within days, small aggregates formed, adhered to each other, and coalesced as larger aggregates whose cells underwent a mesenchymal-to-epithelial transition that reliably yielded hollow spheres of polarized epithelium. By 6 days after the change to suspension culture, the spheres began to develop hair cells that grew in number over the subsequent days. These cells expressed multiple hair cell proteins, developed normal hair cell shapes, and most significantly developed bona fide hair bundles and exhibited rapid permeance to a dye that enters open mechanotransduction channels.
The results of this study differ in several important ways from prior attempts to produce hair cells in vitro. The hair cells formed in our experiments were produced entirely in vitro from homogeneous passaged lines of cells that were frozen, thawed, and greatly expanded during weeks and months of culture. The formation of these hair cells did not require transplantation into the ears of embryos (27) or coculturing with other tissues from the head (26). These hair cells also were crowned by bona fide hair bundles, as confirmed by immunocytochemistry and ultrastructural examination. Our experiments also differed in regard to the procedures that we used.
The spheres described here were produced by methods that are distinctly different from those described in two prior investigations that sought to produce hair cells by suspension culture (25, 40). The cells that we used to produce spheres were from completely dissociated suspensions of cells that originated from cultures that had completed 8 to >20 population doublings while passaged on progressively larger 2D substrates for 6 to >20 weeks after the primary cultures were established. Rigorous procedural controls for distinguishing between preexisting hair cells and the newly generated hair cells sought in this form of investigation are important, so we did not produce spheres directly from freshly dissected and dissociated sensory epithelia to avoid the potential for carrying fragments of preexisting hair cell epithelia over into such cultures where they would form spheres by adhesive resealing.
We confirmed that the epithelial junctions between the cells disappeared before P6. After that, when grown on 2D substrates, new junctions failed to develop even at sites where the individual cells, which now exhibited mesenchymal phenotypes, eventually grew into contact with each other. In each of the experiments, other cells from the sampled suspension were returned to culture flasks for continuing expansion. Those cells and their progeny expressed mesenchymal phenotypes while they grew on the 2D substrates of the flasks. When those cells and cells from passages to at least P23 were sampled and tested for their capacity to undergo a mesenchymal-to-epithelial transition and form sensory epithelia in suspension, they reliably produced sensory epithelia and hair cells.
These results have revealed that it is possible to produce bona fide hair cells completely in vitro without the need for transplantation of cells into embryos or coculturing with other tissues from the developing head. They also have shown that supporting cells from the avian vestibular epithelium transition to a mesenchymal phenotype when passaged in cultures that provide a 2D substrate for adhesion. Under those conditions, the supporting cells quickly spread, changed from columnar to squamous, proliferated, progressively lost epithelial junctions, lost apical-basal polarity, and exhibited a mesenchymal phenotype. When those cells were fully dissociated and then cultured in suspension so as to prevent adhesion to a substrate, cell–cell adhesion was promoted. The aggregated cells then transitioned from a relatively homogeneous mesenchymal phenotype to an epithelial phenotype, and they formed spheres of sensory epithelia that developed hair cells that alternated in a mosaic pattern with cells that expressed a supporting cell marker.
It appears likely that a mesenchymal-to-epithelial transition may be fundamental to the in vitro generation of neuroepithelia from other organs, independent of whether the 2D cultures are from mammalian or nonmammalian sources. The presence of a mesenchymal feeder layer has been shown to influence the differentiation of hair cell characteristics in mammalian experiments (26, 41), and we cannot rule out the possibility of species differences in hair cell differentiation requirements. Yet, our experiments raise the possibility that such mesenchymal coculturing may have stimulated differentiation by virtue of the promotion of a mesenchymal-to-epithelial transition. In fact, we expect that cells cultured from the ears of embryonic mammals may be induced to generate hair cells by methods similar to those we have described above. In preliminary experiments, we have observed that immortalized cells from the ears of wild-type and H-2Kb-tsA58 transgenic mice will form spheres when aggregated after 2D culture, but we have not observed hair bundle formation as yet.
In summary, lines of epithelial cells that undergo an epithelial-to-mesenchymal transition can be expanded in culture for months, will survive cryogenic storage, and can be used to produce hair cells through culture procedures that promote a mesenchymal-to-epithelial transition. The production of bona fide hair cells that develop hair bundles and normal characteristics while completely in vitro, without the need for specialized and time-consuming microdissections of animal ears and also without cell transplantation into embryonic ears or coculturing with other tissues, should accelerate research and allow more effective screening of drug candidates for the treatment of hearing impairment, tinnitus, motion sickness, and other highly prevalent hearing and balance disorders.
Methods
Generation of the Advanced-Passage Cell Cultures.
Generation of the primary cultures.
We dissected utricles from embryonic day 14 white leghorn chicken embryos and used microdissection, followed by thermolysin digestion, followed by a second microdissection to isolate the pure central part of the hair cell epithelia. We pooled the pure sensory epithelia from 16 utricles and dissociated them in 0.05% trypsin-EDTA for 10 min at 37°C followed by gentle trituation. After centrifugation, we produced a suspension of single cells with a few cell clumps. We plated ≈8,000 cells per cm2 in 24-well plates in DMEM/F-12 with 10% FBS at 37°C in a 5% CO2 atmosphere. Four independent primary (P0) cell cultures were generated from 64 ears following these procedures.
Cell expansion.
Cells were passaged when they reached ≈70% confluence. We gradually expanded the cells into larger wells and flasks during subsequent passages up to P23.
Sphere Generation.
We used two methods to culture cells in liquid suspension beginning at P6. First, we cultured 500-μl suspensions of ≈32,000 cells per ml in 24-well plates with gentle agitation in a serum-free medium containing DMEM/F-12, B27, N2, FGF2, and heparan sulfate proteoglycan. More recently, we have cultured suspensions of 4,000 cells in 20-μl hanging drops.
Proliferation Assays.
The expansion of cultures passaged in serum-containing medium was evaluated by counting cells, using a hemocytometer. We determined the incidence of S-phase entry in serum-free cultures by adding 3 μg/ml BrdU and compared the incidence of S-phase entry in 2D cultures and suspension cultures. BrdU incorporation was detected by using anti-BrdU immunocytochemistry, and unlabeled DNA was counterstained with DAPI. The BrdU-positive and DAPI-stained nuclei were counted by using MetaMorph software or Volocity software after complete 3D reconstruction from the confocal images of the spheres.
Assays for Mesenchymal and Epithelial Phenotypes.
To assay for and localize cells that had adopted a mesenchymal phenotype, we used antibodies against the mesenchymal intermediate filament, vimentin, and the mesenchymal zinc finger transcriptional factor, slug, and fluorescent phalloidin labeling of F-actin organized in stress fibers. To assay for cells that had adopted an epithelial phenotype, we used antibodies against E-cadherin and N-cadherin proteins localized to adherens junctions and fluorescent phalloidin labeling of F-actin organized in cortical bands juxtaposed to epithelial cell–cell junctions.
Hair Cell Differentiation Assay.
We used the following hair cell markers in immunocytochemical assays for hair cell differentiation: anti-myosin VIIa, anti-calretinin, anti-parvalbumin 3, and anti-HCS-1/otoferlin.
Hair Cell Bundle Identification.
We used scanning EM, transmission EM, immunocytochemical labeling of the kinocilium (anti-acetylated tubulin) and hair cell antigen (antibody specific to a 275-kDa antigen of the avian hair bundle), and fluorescent phalloidin labeling of stereocilia F-actin to identify the newly formed hair bundles.
Assay for Potential Mechanotransduction Channel Function.
We used FM1-43FX permeation and gentamicin block and recovery to investigate the potential for mechanotransduction channel function in the newly generated hair cells.
Further Details.
Further details of the methods may be found in SI Methods.
Supplementary Material
Acknowledgments
We thank Drs. Kevin S. Lee, Jeffrey S. Holt, and Scott O. Zeitlin for careful reading and comments on the manuscript; Ryan Susa for artwork and technical assistance; Stefan Heller (Stanford University, Stanford, CA) for anti-parvalbumin 3; Guy Richardson (University of Sussex, Brighton, England) for HCA and SCA antibodies; Robert Goldman (Northwestern University Medical School, Chicago, IL) for anti-vimentin; and the Developmental Studies Hybridoma Bank (Iowa City, IA) for anti-slug, anti-E-cadherin, anti-N-cadherin and anti-laminin antibodies. This work was supported by National Institutes of Health Grants DC00200 and DC006182 (to J.T.C.) and by the Grass Foundation.
Abbreviations
- FM1-43
N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino)styryl) pyridiniumdibromide
- Pn
passage n.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 16400.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704576104/DC1.
References
- 1.Nadol JB., Jr N Engl J Med. 1993;329:1092–1102. doi: 10.1056/NEJM199310073291507. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy R, Clemis JD. Otolaryngol Clin North Am. 1990;23:1075–1082. [PubMed] [Google Scholar]
- 3.Corwin JT, Oberholtzer JC. Neuron. 1997;19:951–954. doi: 10.1016/s0896-6273(00)80386-4. [DOI] [PubMed] [Google Scholar]
- 4.Bryant J, Goodyear RJ, Richardson GP. Br Med Bull. 2002;63:39–57. doi: 10.1093/bmb/63.1.39. [DOI] [PubMed] [Google Scholar]
- 5.Holley MC. Br Med Bull. 2002;63:157–169. doi: 10.1093/bmb/63.1.157. [DOI] [PubMed] [Google Scholar]
- 6.Morest DK, Cotanche DA. J Neurosci Res. 2004;78:455–460. doi: 10.1002/jnr.20283. [DOI] [PubMed] [Google Scholar]
- 7.Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- 8.Lambert PR. Laryngoscope. 1994;104:701–718. doi: 10.1288/00005537-199406000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Zheng JL, Helbig C, Gao WQ. J Neurosci. 1997;17:216–226. doi: 10.1523/JNEUROSCI.17-01-00216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng JL, Keller G, Gao WQ. J Neurosci. 1999;19:2161–2170. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montcouquiol M, Corwin JT. J Neurosci. 2001;21:570–580. doi: 10.1523/JNEUROSCI.21-02-00570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montcouquiol M, Corwin JT. J Neurosci. 2001;21:974–982. doi: 10.1523/JNEUROSCI.21-03-00974.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu R, Montcouquiol M, Marchionni M, Corwin JT. Eur J Neurosci. 2007;25:1363–1372. doi: 10.1111/j.1460-9568.2007.05414.x. [DOI] [PubMed] [Google Scholar]
- 14.Davies D, Magnus C, Corwin JT. Eur J Neurosci. 2007;25:985–998. doi: 10.1111/j.1460-9568.2007.05355.x. [DOI] [PubMed] [Google Scholar]
- 15.Meyers JR, Corwin JT. J Neurosci. 2007;27:4313–4325. doi: 10.1523/JNEUROSCI.5023-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JE, Corwin JT. J Neurosci. 1996;16:649–662. doi: 10.1523/JNEUROSCI.16-02-00649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Schier H, Hudspeth AJ. Proc Natl Acad Sci USA. 2006;103:18615–18620. doi: 10.1073/pnas.0608536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor RR, Forge A. J Comp Neurol. 2005;484:105–120. doi: 10.1002/cne.20450. [DOI] [PubMed] [Google Scholar]
- 19.Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 20.Zheng JL, Gao WQ. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 21.Woods C, Montcouquiol M, Kelley MW. Nat Neurosci. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- 22.Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 23.Lawlor P, Marcotti W, Rivolta MN, Kros CJ, Holley MC. J Neurosci. 1999;19:9445–9458. doi: 10.1523/JNEUROSCI.19-21-09445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Germiller JA, Smiley EC, Ellis AD, Hoff JS, Deshmukh I, Allen SJ, Barald KF. Dev Dyn. 2004;231:815–827. doi: 10.1002/dvdy.20186. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Liu H, Heller S. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- 26.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Roblin G, Liu H, Heller S. Proc Natl Acad Sci USA. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle KL, Kazda A, Hort Y, McKay SM, Oleskevich S. Stem Cell. 2007;25:621–627. doi: 10.1634/stemcells.2006-0390. [DOI] [PubMed] [Google Scholar]
- 29.Jorgensen JM, Mathiesen C. Naturwissenschaften. 1988;75:319–320. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- 30.Goodyear RJ, Gates R, Lukashkin AN, Richardson GP. J Neurocytol. 1999;28:851–861. doi: 10.1023/a:1007070121751. [DOI] [PubMed] [Google Scholar]
- 31.Matsui JI, Haque A, Huss D, Messana EP, Alosi JA, Roberson DW, Cotanche DA, Dickman JD, Warchol ME. J Neurosci. 2003;23:6111–6122. doi: 10.1523/JNEUROSCI.23-14-06111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 33.Yeaman C, Grindstaff KK, Nelson WJ. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 34.Kruger RP, Goodyear RJ, Legan PK, Warchol ME, Raphael Y, Cotanche DA, Richardson GP. J Neurosci. 1999;19:4815–4827. doi: 10.1523/JNEUROSCI.19-12-04815.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartolami S, Goodyear R, Richardson G. J Comp Neurol. 1991;314:777–788. doi: 10.1002/cne.903140410. [DOI] [PubMed] [Google Scholar]
- 36.Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geleoc GS, Holt JR. Nat Neurosci. 2003;6:1019–1020. doi: 10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Si F, Brodie H, Gillespie PG, Vazquez AE, Yamoah EN. J Neurosci. 2003;23:10815–10826. doi: 10.1523/JNEUROSCI.23-34-10815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doetzlhofer A, White PM, Johnson JE, Segil N, Groves AK. Dev Biol. 2004;272:432–447. doi: 10.1016/j.ydbio.2004.05.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.