Abstract
High sensitivity C-reactive protein (hsCRP) has previously been shown to be an independent predictor for the development of cardiovascular disease. However, little is known about the association between hsCRP and the severity of cardiovascular events that occur. This study compared the characteristics of incident myocardial infarctions (MIs) in 40 initially healthy women with very high baseline hsCRP (> 7.5 mg/L) to infarctions in 40 women with very low hsCRP (≤1 mg/L). At baseline, very high hsCRP was associated with a clinical diagnosis of hypertension, higher body-mass index, lower high-density lipoprotein, and higher triglycerides. Our analysis found that the two study groups had similar proportions of ST-segment elevations on electrocardiograms, peak cardiac enzyme levels, post-infarct left ventricular function, and burden of coronary atherosclerosis seen on angiography. However, subjects with very high hsCRP suffered infarctions significantly earlier than those with very low hsCRP (median time-to-event 4.45 years vs. 6.64 years respectively, p < 0.0001). In addition, higher baseline levels of hsCRP were associated with significantly more fatal MIs (0% vs. 4.6% vs. 9.6% in subjects with hsCRP <1 mg/L, 1-3 mg/L, and ≥3 mg/L, respectively, p for trend = 0.02). In conclusion, this study demonstrates that in initially healthy women, high levels of hsCRP predict earlier occurrence of myocardial infarctions as well as a greater likelihood that infarctions will be fatal.
Keywords: C-reactive protein, inflammation, myocardial infarction
Introduction
Numerous large scale studies have demonstrated that high sensitivity C-reactive protein (hsCRP) is an independent risk factor for the development of myocardial infarction, stroke, and cardiovascular death.1-6 However, little is known about the association between initial hsCRP levels and the clinical characteristics of myocardial infarctions (MIs) that later occur. Low hsCRP levels have been associated with slower progression of atherosclerosis7,8 and thus may be associated with delayed onset of clinical events as well as less extensive vascular disease at the time of infarction. In addition, lower markers of systemic inflammation have been associated with lower propensity for coronary artery plaque rupture.9,10 These vascular characteristics corresponding to low hsCRP might therefore be associated with milder infarctions. By contrast, elevated levels of hsCRP may be associated with greater burden of disease, more extensive vulnerable plaque, and more severe infarctions. To address this question, we compared the clinical characteristics of initially healthy women with very high baseline levels of hsCRP (>7.5 mg/L) to women with very low baseline levels of hsCRP (≤1 mg/L), all of whom went on to have a first ever MI during a median 10 year follow-up period.
Methods
All study participants were enrolled in the Women's Health Study, a prospective cohort of 39,876 initially healthy American women age ≥45 years. The details of the study design and primary outcomes have been reported previously.11-13 Within this cohort, 27,939 participants provided baseline demographic data, health history, and blood samples that could be assayed for lipid levels and hsCRP. Study participants were followed for a median of 10 years for incident cardiovascular events, and all reported endpoints were confirmed by a blinded endpoints committee. Myocardial infarction was defined by the World Health Organization criteria of ischemic symptoms accompanied by elevated cardiac enzymes or diagnostic electrocardiograms.
For the purpose of this analysis, we identified all women participating in the Women's Health Study who went on to have a confirmed incident MI during follow-up. We then selected the 40 subjects with the highest baseline hsCRP levels (> 7.5 mg/L), and for comparison the 40 subjects with the lowest baseline hsCRP (< 1 mg/L). We reviewed physician reports, hospital discharge summaries, cardiac catheterization reports, echocardiography reports, electrocardiograms, and laboratory data to ascertain clinical characteristics of the infarctions in each of these subjects.
Severity of MI was determined by 30-day mortality, presence of ST-segment elevations on electrocardiogram, peak cardiac enzyme levels (creatine kinase, creatine kinase-MB isoenzyme, and cardiac troponin), and post-infarct left ventricular ejection fraction as determined by echocardiography or left ventriculography at cardiac catheterization. Burden of coronary artery disease was evaluated from cardiac catheterization reports. Stenoses were considered clinically significant if ≥70% obstructed in an epicardial artery or ≥50% in the left main coronary artery. Time to event was defined as time from randomization until MI. All records were reviewed by a researcher (S.B.) blinded to baseline values of hsCRP.
Subjects with very low baseline hsCRP were compared to subjects with very high hsCRP in terms of baseline characteristics and infarction characteristics. Differences in mean values were analyzed for significance using the Student's t-test if the distributions approximated normality, and using the Wilcoxon Rank Sum test if non-normally distributed. Differences in proportions were analyzed for significance using a χ2 analysis for nominal measures and the Cochran-Armitage test for trend for ordinal measures. Differences in event rates were analyzed for significance with the Log Rank test, and hazard ratios were calculated using the Cox Proportional Hazards Model. All p-values are two-tailed, and p≤0.05 was considered statistically significant. All statistical analyses were run using the SAS statistical software version 9.0 (SAS Institute Inc., Cary, NC) and S-Plus software version 6.2 (Insightful Corp., Seattle, WA).
Results
Baseline characteristics of the two study groups at the time of enrollment are reported in Table 1. No significant differences were noted in age, race, total and low-density lipoprotein (LDL) cholesterol, smoking status, presence of diabetes mellitus, serum creatinine, family history of premature atherosclerosis, exercise frequency, hormone replacement therapy use, and random treatment assignment to aspirin between the 2 groups at study entry. As might be expected given known modest correlations in this cohort between components of the metabolic syndrome and hsCRP,14 those with very high levels of hsCRP had somewhat lower levels of high-density lipoprotein (HDL) cholesterol, higher levels of triglycerides, and greater mean body mass index compared to those with very low hsCRP levels at study entry. In addition, the study group with very high hsCRP levels was more likely to carry a diagnosis of hypertension and be treated for hypertension at study entry.
Table 1.
Baseline Characteristics of Subjects According to High-Sensitivity C-Reactive Protein Levels at Randomization
| High-Sensitivity C-Reactive Protein | |||
|---|---|---|---|
| Variable | ≤ 1.0 mg/L (n = 40) | > 7.5 mg/L (n = 40) | p-value |
| Median hsCRP (range), mg/L | 0.69 (0.22 – 0.98) | 14.01 (7.53 – 92.0) | |
| Age (years) | 60.0 ± 9.4 | 61.2 ± 8.1 | 0.6 |
| White Race | 40 (100%) | 36 (90%) | 0.12 |
| Hypertension | 15 (38%) | 26 (65%) | 0.01 |
| Hypertension therapy | 5 (13%) | 19 (48%) | 0.001 |
| Lipids (mg/dL) | |||
| Total Cholesterol | 217 ± 36 | 230 ± 41 | 0.14 |
| LDL-C | 129 ± 33 | 137 ± 33 | 0.3 |
| HDL-C | 53.1 ± 19.6 | 41.8 ± 10.5 | 0.002 |
| Triglycerides | 151 ± 103 | 236 ± 150 | 0.004 |
| Cholesterol-lowering therapy | 4 (10%) | 5 (13%) | >0.9 |
| Smoking | |||
| Current | 7 (18%) | 11 (28%) | 0.5 |
| Past | 17 (43%) | 13 (33%) | |
| Never | 16 (40%) | 16 (40%) | |
| Diabetes mellitus | 4 (10%) | 10 (25%) | 0.08 |
| Body-mass index (kg/m2) | 25.1 ± 3.3 | 29.8 ± 6.1 | <0.0001 |
| < 25 | 19 (50%) | 11 (28%) | |
| 25 - 30 | 17 (45%) | 12 (31%) | 0.001 (trend) |
| ≥ 30 | 2 (5%) | 16 (41%) | |
| Creatinine (mg/dL) | 0.76 ± 0.13 | 0.74 ± 0.16 | 0.6 |
| Exercise (times/week) | |||
| Rarely/Never | 21 (53%) | 22 (55%) | 0.5 |
| < 1 | 6 (15%) | 7 (18%) | |
| 1 - 3 | 9 (23%) | 10 (25%) | |
| ≥ 4 | 4 (10%) | 1 (3%) | |
| Hormone therapy use | |||
| Never | 21 (53%) | 14 (35%) | 0.11 |
| Past | 9 (23%) | 7 (18%) | |
| Current | 10 (25%) | 19 (48%) | |
| Randomized to aspirin | 17 (43%) | 23 (58%) | 0.18 |
Values are number of subjects (percentage) or mean ± SD unless otherwise specified.
Table 2 presents data comparing the clinical characteristics of incident MIs in the two study groups. As shown, no significant differences were found between those with very low and very high baseline hsCRP levels in terms of the proportion of MIs with ST-elevations or post-infarct left ventricular function. We also observed no differences in the location of coronary stenoses or the number of vessels with significant stenoses. With respect to peak cardiac enzyme levels, there was a higher peak creatine kinase level in subjects with very high hsCRP (median 747 IU vs. 393 IU in high vs. low hsCRP groups, respectively; p = 0.04). However, since creatine kinase-MB isoenzyme and cardiac troponin levels were not significantly different, this may represent a false positive due to multiple testing rather than a true difference in myocardial necrosis.
Table 2.
Characteristics of Incident Myocardial Infarctions According to Baseline High-Sensitivity C-Reactive Protein
| High-Sensitivity C-Reactive Protein | |||
|---|---|---|---|
| Variable | ≤ 1.0 mg/L (n = 40) | > 7.5 mg/L (n = 40) | p-value |
| Time to event (median) | 6.64 years | 4.45 years | <0.0001 |
| Fatal within 30 days | 0 (0%) | 5 (12.5%) | 0.055 |
| ST-segment elevations | 24 (62%) | 21 (58%) | 0.8 |
| Peak cardiac enzymes | |||
| Creatine kinase (IU) | 393 (279 – 854) | 747 (428 – 1381) | 0.04 |
| Creatine kinase-MB (IU) | 43.9 (23.2 – 113) | 52.0 (19.9 – 169) | 0.9 |
| Troponin (ng/mL) | 8.9 (6.6 – 32.0) | 17.8 (4.2 – 38.3) | 0.7 |
| Left ventricular ejection fraction (%) | 55% (40% - 60%) | 57% (50% - 60%) | 0.3 |
| Coronary artery narrowed | |||
| Left main ≥ 50% | 1 (3%) | 2 (5%) | >0.9 |
| Left anterior descending ≥ 70% | 18 (50%) | 18 (47%) | >0.9 |
| Left circumflex ≥ 70% | 13 (36%) | 13 (34%) | >0.9 |
| Right coronary ≥ 70% | 14 (39%) | 18 (47%) | 0.5 |
| Number of coronary arteries narrowed ≥ 70% | |||
| 0 | 6 (17%) | 3 (8%) | 0.6 (trend) |
| 1 | 19 (53%) | 24 (63%) | |
| 2 | 7 (19%) | 5 (13%) | |
| 3 | 4 (11%) | 6 (16%) | |
Values are number of subjects (percentages) or median (interquartile range).
There appeared to be a trend towards increased fatalities within 30 days of MI in the high hsCRP group (12.5% vs. 0% in high vs. low hsCRP groups, respectively; p = 0.055). To further explore this result, we retrospectively analyzed the full cohort of Women's Health Study participants with baseline blood samples who suffered incident MIs during follow up (257 subjects) and compared 30-day mortality based on initial hsCRP levels. In this analysis, there were 0 fatal events among 45 women with hsCRP < 1 mg/L, 3 fatal events among 66 women (4.6%) with hsCRP ≥ 1.0 mg/L and < 3.0 mg/L, and 14 fatal events among 146 women (9.6%) with hsCRP ≥ 3.0 mg/L (p for trend = 0.02). Other characteristics of women who went on to have fatal MIs, including hypertension, lipid levels and body-mass index, were not significantly different from those who had non-fatal MIs.
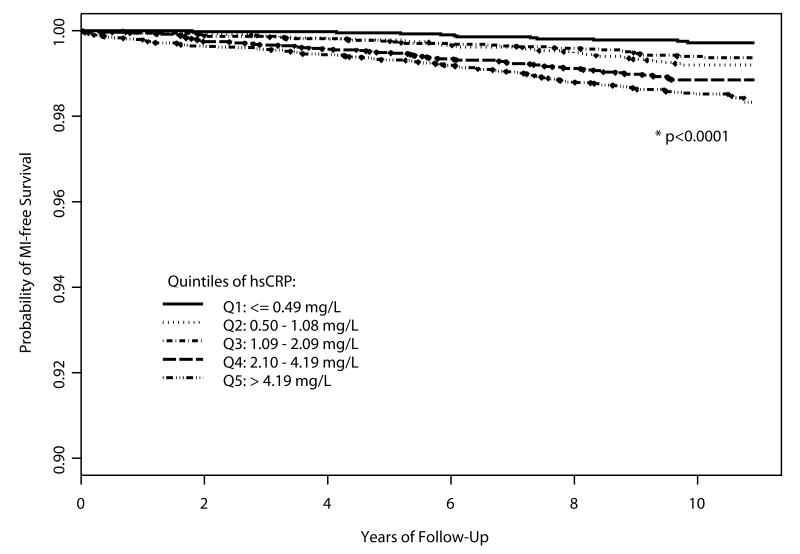
Subjects with very high hsCRP levels at study entry also suffered MIs significantly earlier than those with very low hsCRP (median time-to-event 4.45 vs. 6.64 years, respectively, p < 0.0001). To further pursue this observation, we divided the full cohort of Women's Health Study subjects who provided baseline blood samples into quintiles of baseline hsCRP based on its distribution among women not taking hormone therapy, following guidelines from the Department of Health and Human Services for lipid standardization.15 Kaplan-Meier MI-free survival curves were constructed according to quintiles of hsCRP (Figure). Higher quintiles of hsCRP were associated with earlier infarctions across the full 10-year period of exposure (p < 0.0001). In Cox Proportional Hazards models, unadjusted hazard ratios for MI were higher for increasing quintiles of hsCRP (p for trend < 0.0001) (Table 3). As expected based on previously published results,16 these effects were attenuated but persisted after adjustment for traditional cardiac risk factors.
Figure.
MI-free Survival According to Quintile of Baseline hsCRP
Table 3.
Cox Hazard Ratio for Incident Myocardial Infarction by Quintile of High-Sensitivity C-Reactive Protein
| Quintiles of hsCRP (Range, mg/L) | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| ≤ 0.49 | 0.50 – 1.08 | 1.09 – 2.09 | 2.10 – 4.19 | > 4.19 | p for trend | |
| Number of events | 11 | 36 | 32 | 70 | 108 | |
| Event rate (per 1000 person-years of follow-up) | 0.26 | 0.76 | 0.60 | 1.12 | 1.49 | |
| Crude Model | 1.0 (ref) | 2.9 (1.5 – 5.8) | 2.3 (1.2 – 4.5) | 4.3 (2.3 – 8.1) | 5.7 (3.1 – 10.6) | <0.0001 |
| Age-adjusted Model | 1.0 (ref) | 2.5 (1.3 – 5.0) | 1.8 (0.9 – 3.7) | 3.3 (1.8 – 6.3) | 4.5 (2.4 – 8.4) | <0.0001 |
| Risk Factor-adjusted Model* | 1.0 (ref) | 2.1 (1.1 – 4.2) | 1.5 (0.7 – 2.9) | 2.2 (1.2 – 4.3) | 2.3 (1.2 – 4.5) | 0.04 |
Values are hazard ratio (95% CI)
Adjusted for age, smoking, diabetes mellitus, blood pressure, LDL-C, body-mass index, and use of hormone replacement therapy
Discussion
In this prospective evaluation of initially healthy women who subsequently had an MI, we observed that women with the highest baseline level of hsCRP had earlier MIs, and were more likely to have fatal events, as compared to women with very low baseline hsCRP levels. No other differences in MI characteristics were found, including proportion of infarctions with ST-segment elevations, peak cardiac enzyme levels, residual cardiac function, and burden of coronary atherosclerosis on angiography.
One hypothesis of this study was that higher hsCRP levels would be associated with a propensity for plaque instability and atherothrombosis. While transmural infarcts and extent of myocardial damage appeared to be similar in the two study groups, the finding of increased propensity for fatal MIs with increasing hsCRP was intriguing. While CRP may play a biologic role in leading to fatal infarctions, causality cannot be assumed from the data in this study. As a marker of systemic inflammation, hsCRP may simply represent a downstream summation of risk factors that predispose an individual to infarctions that are fatal. Alternatively, hsCRP may represent a subclinical health state that portends poor tolerance of myocardial damage. While this association was based on a small total number of fatal MIs, the trend achieved statistical significance and warrants exploration in future studies.
A second hypothesis of this study was that high circulating levels of hsCRP, signifying systemic inflammation, would lead to more extensive atherosclerosis in the coronary tree. In our data, hsCRP did not have any apparent association with the extent of plaque burden seen on coronary angiography in subjects at the time of their infarctions, a finding consistent with previous studies.17-22 However, mirroring what has been demonstrated in previous studies of hsCRP and cardiovascular events, this study found that baseline hsCRP values are associated with earlier myocardial infarctions, even after adjusting for traditional cardiac risk factors.1,3-5,16,23 Despite earlier MIs (and therefore earlier angiography) in the high hsCRP group, these subjects had the same atherosclerotic plaque build-up and myocardial damage as subjects in the low hsCRP group. Thus, it is possible that elevated hsCRP is associated with an acceleration of the atherosclerotic process, findings that are consistent with prior studies.7,8
Limitations of this study merit consideration. All hsCRP samples were drawn at study enrollment, and levels were not re-measured at the time of MI. While we cannot rule out changes in hsCRP immediately preceding infarction, hsCRP has been shown to be stable over time in asymptomatic individuals.5,24 Furthermore, this study was designed specifically to ascertain the predictive ability of baseline hsCRP on characteristics of future infarctions. In addition, our conclusions are derived from data on incident MIs in women; similar studies would be needed to extend these results to men, recurrent infarctions, and other cardiovascular events.
Acknowledgments
Supported by grants from the Donald W. Reynolds Foundation, Las Vegas, NV and grant T32 HL007575-21 from the National Heart, Lung, and Blood Institute, Bethesda, MD.
Footnotes
Conflicts of Interest: Dr. Ridker is listed as a coinventor on patents held by Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes mellitus.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 2.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 3.Tracy RP, Lemaitre RN, Psaty BM, Ives DG, Evans RW, Cushman M, Meilahn EN, Kuller LH. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 4.Koenig W, Sund M, Frohlich M, Fischer HG, Lowel H, Doring A, Hutchinson WL, Pepys MB. C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 5.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Bmj. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation. 2005;112:900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- 8.Schillinger M, Exner M, Mlekusch W, Sabeti S, Amighi J, Nikowitsch R, Timmel E, Kickinger B, Minar C, Pones M, Lalouschek W, Rumpold H, Maurer G, Wagner O, Minar E. Inflammation and Carotid Artery--Risk for Atherosclerosis Study (ICARAS) Circulation. 2005;111:2203–2209. doi: 10.1161/01.CIR.0000163569.97918.C0. [DOI] [PubMed] [Google Scholar]
- 9.Ishikawa T, Hatakeyama K, Imamura T, Date H, Shibata Y, Hikichi Y, Asada Y, Eto T. Involvement of C-reactive protein obtained by directional coronary atherectomy in plaque instability and developing restenosis in patients with stable or unstable angina pectoris. Am J Cardiol. 2003;91:287–292. doi: 10.1016/s0002-9149(02)03156-9. [DOI] [PubMed] [Google Scholar]
- 10.Berk BC, Weintraub WS, Alexander RW. Elevation of C-reactive protein in “active” coronary artery disease. Am J Cardiol. 1990;65:168–172. doi: 10.1016/0002-9149(90)90079-g. [DOI] [PubMed] [Google Scholar]
- 11.Buring JE, Hennekens CH. The Women's Health Study: summary of the study design. J Myocardial Ischemia. 1992;4:27–29. [Google Scholar]
- 12.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 13.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 14.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25:2016–2021. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]
- 15.Hainline A, Karon J, Lippel K. Manual of Laboratory Operations: Lipid Research Clinics Program and Lipid and Lipoprotein Analysis. Bethesda, MD: Department of Health and Human Services; 1982. [Google Scholar]
- 16.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 17.Redberg RF, Rifai N, Gee L, Ridker PM. Lack of association of C-reactive protein and coronary calcium by electron beam computed tomography in postmenopausal women: implications for coronary artery disease screening. J Am Coll Cardiol. 2000;36:39–43. doi: 10.1016/s0735-1097(00)00680-x. [DOI] [PubMed] [Google Scholar]
- 18.Hunt ME, O'Malley PG, Vernalis MN, Feuerstein IM, Taylor AJ. C-reactive protein is not associated with the presence or extent of calcified subclinical atherosclerosis. Am Heart J. 2001;141:206–210. doi: 10.1067/mhj.2001.112488. [DOI] [PubMed] [Google Scholar]
- 19.Wang TJ, Larson MG, Levy D, Benjamin EJ, Kupka MJ, Manning WJ, Clouse ME, D'Agostino RB, Wilson PW, O'Donnell CJ. C-reactive protein is associated with subclinical epicardial coronary calcification in men and women: the Framingham Heart Study. Circulation. 2002;106:1189–1191. doi: 10.1161/01.cir.0000032135.98011.c4. [DOI] [PubMed] [Google Scholar]
- 20.Wang TJ, Nam BH, Wilson PW, Wolf PA, Levy D, Polak JF, D'Agostino RB, O'Donnell CJ. Association of C-reactive protein with carotid atherosclerosis in men and women: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2002;22:1662–1667. doi: 10.1161/01.atv.0000034543.78801.69. [DOI] [PubMed] [Google Scholar]
- 21.Park R, Detrano R, Xiang M, Fu P, Ibrahim Y, LaBree L, Azen S. Combined use of computed tomography coronary calcium scores and C-reactive protein levels in predicting cardiovascular events in nondiabetic individuals. Circulation. 2002;106:2073–2077. doi: 10.1161/01.cir.0000033819.29662.09. [DOI] [PubMed] [Google Scholar]
- 22.Khera A, de Lemos JA, Peshock RM, Lo HS, Stanek HG, Murphy SA, Wians FH, Jr, Grundy SM, McGuire DK. Relationship between C-reactive protein and subclinical atherosclerosis: the Dallas Heart Study. Circulation. 2006;113:38–43. doi: 10.1161/CIRCULATIONAHA.105.575241. [DOI] [PubMed] [Google Scholar]
- 23.Mendall MA, Strachan DP, Butland BK, Ballam L, Morris J, Sweetnam PM, Elwood PC. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J. 2000;21:1584–1590. doi: 10.1053/euhj.1999.1982. [DOI] [PubMed] [Google Scholar]
- 24.Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem. 2001;47:444–450. [PubMed] [Google Scholar]