Abstract
PKA type I and type II are activated in Aplysia neurons by stimulation with serotonin (5-HT), which causes long-term facilitation (LTF). The proteolysis of the regulatory subunit (R) is thought important for the persistent activation of PKA, which is necessary to produce LTF. In this study, we report that the type I regulatory subunit (RI) and type II regulatory subunit (RII) are differentially regulated by proteolytic cleavage. RI, but not RII, was selectively cleaved after 5-HT treatment for 2 h in Aplysia neurons. Interestingly, the proteasome inhibitor MG132 inhibited the cleavage of RI caused by 5-HT treatment in Aplysia neuron. Besides extracts from Aplysia ganglia treated with 5-HT cleaved 35S-labeled RI synthesized in vitro, but not 35S-labeled RII. This suggests that 5-HT induces the activation state of RI-specific proteolytic cleavage.
Key: PKA, cAMP, long-term facilitation, long-term memory, proteolysis, cleavage, Aplysia
Introduction
The cAMP second-messenger pathway is critical for learning and synaptic plasticity. [1]. The cAMP-dependent protein kinase (PKA) plays a major role in this pathway. Animals have two isotypes of PKA, which differ in R subunits [2]. Both types of R subunits from yeast to humans are conservatively built. Although the two types of PKA share a similar structure, they differ in their intracellular distribution: type I is mainly cytosolic, whereas most of type II is bound to membranes through the association of RII with A kinase-anchored proteins (AKAPs) [3].
During the formation of long-term facilitation (LTF) by application of the neurotransmitter serotonin (5-HT), PKA is persistently active for at least 24 hr [4],[5],[6]. This persistent activation leads to persistent phosphorylation of the transcription factor cAMP response element-binding protein (CREB), which regulates the expression of genes to produce LTF [7]. The biochemical properties of PKA R subunits in Aplysia nervous tissue were first characterized by Eppler [8]. Bergold et al. [9] cloned and characterized Aplysia RI, and Hegde, et al. [10] demonstrated that Aplysia R subunits and mammalian R subunits are degraded through the ubiquitin proteasome pathway. The degradation of R subunits produces the persistent activity of R subunit to activate transcriptional factors, which are necessary for LTF. Chely, et al. On the other hand, [11] showed that Aplysia RII is autophosphorylated and bind AKAPs. Furthermore, 5-HT increases RII protein levels at Aplysia nerve endings to at the late stages of LTF [12]. Presumably, it is to strengthen synaptic connections. Thus, PKA type I and type II have distinct roles in the synaptic facilitation produced by 5-HT. PKA type I most likely functions early in the formation of LTF by providing persistent kinase activity. PKA type II may support LTF at a late stage by acting with AKAP to strengthen synaptic connections by phosphorylating proteins at sensory neuron synapses to enhance the release of neurotransmitters [12]. However, it remains unclear how neurons differentially regulate each type of PKAs. In this study, we found that treatment of Aplysia ganglia with 5-HT induced cleavage of RI, but not RII. Besides extracts from Aplysia ganglia treated with 5-HT induced cleavage of 35S-labeled RI synthesized in vitro, but not 35S-labeled RII. We propose that selective cleavage of RI is one of the mechanisms to produce the persistent activity of PKA type I and to protect RII from proteolysis.
Materials and methods
Cerebral, buccal, pleural, pedal and abdominal ganglia were obtained from Aplysia as described previously [13]. Ganglia were treated in the presence/absence of 500 μM of 5-HT (Sigma, St. Louis, MO) for 2 h at 18°C. For time course experiments, ganglia were cultured in L15 medium at 18°C until recovery. Then, ganglia were homogenized in 1% NP-40, 10mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM EDTA, and 0.1 % protease inhibitor cocktail (Sigma, St Louis, MO) on ice. To remove cell debris, the homogenate was centrifuged for 2 min at 3,000 × g. The recovered supernatant was used as extract of Aplysia ganglia. For the MG132 treatment, ganglia were treated with 10 μM of MG132 (Boston Biochem, Cambridge, MA)) 10 min before treatment with 5-HT and continuously treated with MG132 during the 5-HT treatment.
Immunoblotting
Proteins extracted from ganglia were dissolved in Laemmli and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting with anti-RI, anti-RII, or anti-α-tubulin antibodies. The anti-α-tublin antibody (T5168) was from Sigma and the polyclonal antibodies to Aplysia RI and RII were described previously [12], [14].
Plasmid construction
Total RNA was isolated using Trizol reagent (Life Technologies, Rockville, MD). Reverse transcription was performed with random primers and the resulting cDNA was used as a template for PCR. Gene-specific primer sets were used to amplify specific genes. These primer sets were: (1) RI, 5′-GGCGAATTCATGGCGGCCAACACCGACGAGG -3′ and 5′-GGCGATATCCTACACCGACAGGGATACAAAGC -3′. (2) RII, 5′-GGCGAATTCATGAATTTCGAGATCCCACCGG -3′ and 5′-GGCGATATCTCACCGCAGGTCCGAGATGTTGG -3′. The amplified sequences were inserted into the EcoRI/XhoI sites of pcDNA3.1(+) (Invitrogen, Carlsbad, CA).
Site-directed mutagenesis
Single amino acid substitutions in RI subunits were introduced by PCR. Complementary sense and antisense oligonucleotide primers were synthesized for PCR amplification of recombinant RI or RII subunits encoded in pcDNA3ApRI or pcDNA3ApRII. The primer set used to generate pcDNA3ApRI(S98A) and pcDNA3ApRI(S98E) was 5′-CATGAGGAGAGGGGCGGTGGCGGCGGAGGTATACAGGG -3′ and 5′-CCCTGTATACCTCCGCCGCCACCGCCCCTCTCCTCATG -3′ or 5′-CAT GAG GAG AGG GGC GGT GGA GGC GGA GGT ATA CAG GG -3′ and 5′-CCC TGT ATA CCT CCG CCT CCA CCG CCC CTC TCC TCA TG-3′, respectively.
Kinase assay
The recombinant gluthathion S-transferase (GST) Ap-CREB1a fusion protein was expressed in E. coli BL21 and purified with Gluthatione Sepharose 4B (GE Healthcare, Piscataway, NJ). Aplysia ganglia treated with or without 5-HT were immediately frozen on dry ice and homogenized in potassium phosphate buffer (10 mM potassium phosphate, 2 mM EDTA, and 150 mM NaCl). The extracts were incubated with GST-fused proteins in kinase buffer (50 mM Tris-HCl pH 7.5, 5 mM MgCl2, 10 μCi γ-32ATP, 50 μM ATP, and 1 mM Na3VO4) at 30°C for 30 min. Proteins from the reaction mixture were separated by SDS-PAGE, and analyzed by autoradiography.
Preparation of 35S-labeled R subunits
cDNAs were transcribed and translated from pcDNA3ApRI, pcDNA3ApRII, pcDNA3ApRI(S98A), and pcDNA3ApRI(S98E) in vitro using a TnT T7 coupled reticulocyte lysate system (Promega, Madison, Wis) as instructed by the manufacturer in the presence of 35S-labeled methionine (MP Biomedical, Solon, OH).
In vitro protein cleavage assay
35S-labeled R subunits were incubated with extracts of Aplysia ganglia in 10 mM Tris-HCl [pH 7.4], 150 mM NaCl in the presence/absence of 10 μM of cAMP for 30 min at 30 °C. The reactions were stopped by addition of 3 × Laemmli buffer and heated for 10 min at 98 °C. The proteins were subjected to SDS-PAGE and analyzed by autoradiography.
Results
5-HT induces the cleavage of PKA RI, but not RII
A protocol producing long-term sensitization and the stimulation with 5-HT producing LTF change the level of R subunits [4],[12],[13]. We examined the protein levels of RI and RII in Aplysia ganglia after 5-HT treatment. Ganglia were treated with 5-HT for 2 h, then desheathed and homogenized. The protein levels of RI and RII were analyzed by Western blotting with specific anti-RI or anti-RII antibodies [12]. After the incubation with 5-HT for 2 h, the protein level of RI (52 kDa band) was decreased (Fig. 1. lane 2) compared with that in Aplysia ganglia cultured without 5-HT (Fig. 1. upper left panel, lane 1). On the other hand, the protein level of RII was not changed after 5-HT treatment for 2 h (Fig. 1. lane 5, compared with lane 4). Interestingly, a band showing mobility at 37 kDa was stronger after 5-HT treatment for 2 h (Fig. 1. lane 2). We suspected the 37-kDa fragment to be a cleaved product of RI as previously reported [15],[16]. It has been reported that 5-HT induces polyubiquitylation of R subunits [10] and produce the long-lasting activity of PKA [10]. Besides proteasome inhibitor MG132 blocks LTF [14]. We suspected that there might be a relation between MG132 treatment and cleavage of RI. When ganglia were treated with MG132 and 5-HT together, cleavage of RI was inhibited (Fig. 1. lane 3). We concluded that the degradation of RI is regulated by cleavage as well as by the ubiquitin-proteasome pathway. A question is why the proteasome inhibitor blocked cleavage of RI since it inhibits the degradation of polyubiquitylated proteins. Lee et al,.[17] reported that MG132 inhibits the activity of calpains as well as the 20S protease. However, neither calpain inhibitor I and II, nor EGTA inhibited the cleavage (data not shown). Presumably, the ubiquitin-proteasome pathway is indirectly involved in cleavage of RI. The treatment of ganglia with 5-HT and MG132 also increase the level of RII (Fig. 1. lane 6). Interestingly, we observed the ladder bands between 58 kDa and 75 kDa. They may be polyubiquitylated RII. The protein level of α-tubulin was not affected by 5-HT and/or MG132.
Fig. 1.
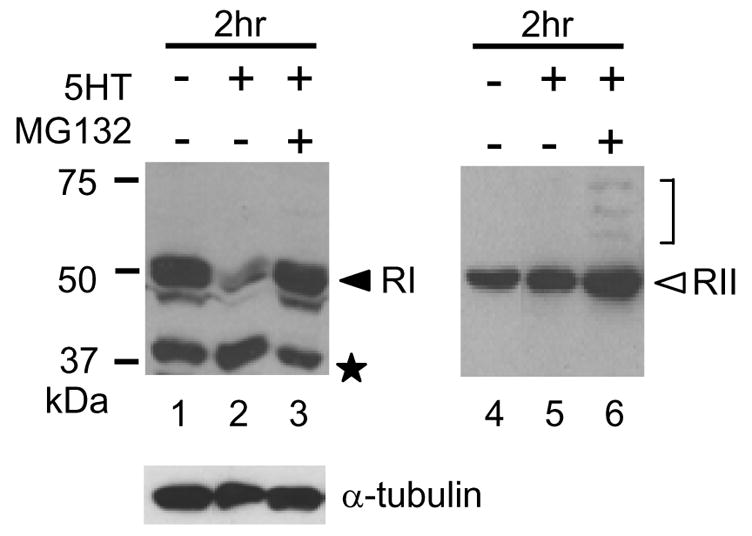
Western blotting of RI, RII and α-tubulin in Aplysia ganglia. Aplysia ganglia were dissected and treated with/without 500 μM 5-HT. Ganglia were homogenized and dissolved in Laemmli. The proteins were analyzed by SDS-PAGE and detected with polyclonal Ab for RI, polyclonal Ab for RII, and monoclonal Ab for α-tubulin. MG132 was applied 10 min before 5-HT and continuously applied during the 5-HT treatment. Lanes 1, RI and RII after treatment without 5-HT for 2 h. Lanes 2 and 5, RI and RII after treatment with 5-HT for 2 h. Lanes 3 and 6, RI and RII after treatment with 5-HT and MG132 for 2 h. Protein levels of α-tubulin are shown in the lower panel. Molecular mass markers are shown on the left (in KiloDaltons). The closed arrowhead and open arrowhead indicate RI and RII, respectively. An asterisk indicates the 37-kDa cleaved fragment. The bracket indicates the putative polyubiquitylated RII.
Extracts from Aplysia ganglia treated with 5-HT persistently phosphorylate Aplysia CREB1a
Persistently activated PKA targets ApCREB1a, which is the Aplysia homologue of CREB, for the formation of LTF [7]. To examine the persistent activity of PKA during the formation of LTF, we performed an in vitro kinase assay using GST-CREB1a produced in bacteria. Aplysia ganglia were treated with 5-HT, immediately frozen on dry ice, and homogenized. Extracts from Aplysia ganglia were incubated with GST-CREB1a in the presence of γ32-ATP and phosphorylated GST-CREB1a was analyzed by autoradiography. Extracts from ganglia treated with 5-HT for 2 h efficiently phosphorylated CREB1a (Fig. 2B. lane 2 compare with lane 1) and a high level of phosphorylation lasted for at least 8 h (Fig. 2B. lane 4). Extracts from ganglia prepared 24 h after 5-HT incubation phosphorylated CREB1a although the level was decreased compared with that prepared 8 h after 5-HT incubation (Fig. 2 lane 6). We confirmed that 5-HT treatment induces persistent kinase activity and ability of Aplysia ganglia to phosphorylate CREB1a. This observation corresponds to previous reports [6], [18].
Fig. 2.
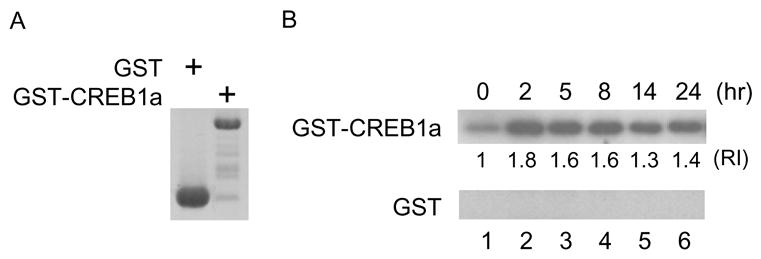
Extracts from Aplysia ganglia treated with 5-HT phosphorylate Ap-CREB1a and this ability lasts 24 h. (A) GST alone and GST-RI were expressed in bacteria. The products were analyzed by SDS-PAGE and visualized by Coomassie Brilliant Blue staining. (B) GST or GST-CREB1a incubated with extracts from Aplysia ganglia and γ-32ATP at 30°C for 30 min. The products were analyzed by SDS-PAGE and assessed by autoradiography. Extracts from Aplysia ganglia without 5-HT treatment weakly phosphorylated GST-CREB1a (upper panel, lane 1) but not GST alone (lower panel, lane 1). Aplysia ganglia treated with 5-HT for 2 h phosphorylated GST-CREB1a efficiently (upper panel, lane 2) and persistently phosphorylated GST-CREB1a 2 to 24 h after (upper panel, lane 2 to 6). “RI” indicates the relative intensity of phosphorylated GST-CREB1a.
The extract from Aplysia ganglia treated with 5-HT cleaves 35S-labeled RI synthesized in vitro, but not 35S-labeled RII
The difference in proteolysis of RI vs. RII may be explained by the different cellular distribution of two subunits forms [12]. If cleavage occurs only in the cytoplasm and not at the synapses where RII is located, then extract from Aplysia ganglia treated with 5-HTshould cleave both exogenous RI and RII. To test this hypothesis, 35S-labeled R subunits synthesized in vitro were incubated with extracts from Aplysia ganglia treated with/without 5-HT in the presence of 10 μM of cAMP. The extracts from ganglia cultured without 5-HT did not proteolyze RI (Fig. 3A. lane 1). On the other hand, the extracts from Aplysia ganglia treated with 5-HT for 2 h cleaved 35S-labeled RI (Fig. 3A. lane 2) and produced a 37kDa-fragment (Fig. 3A. closed star) but did not cleave35S-labeled RII (Fig. 3A. lane 4, compare with lane 3). This result suggests that the difference in proteolysis between RI and RII is due to the difference in their molecular characteristics, not to the difference in their localizations.
Fig. 3.
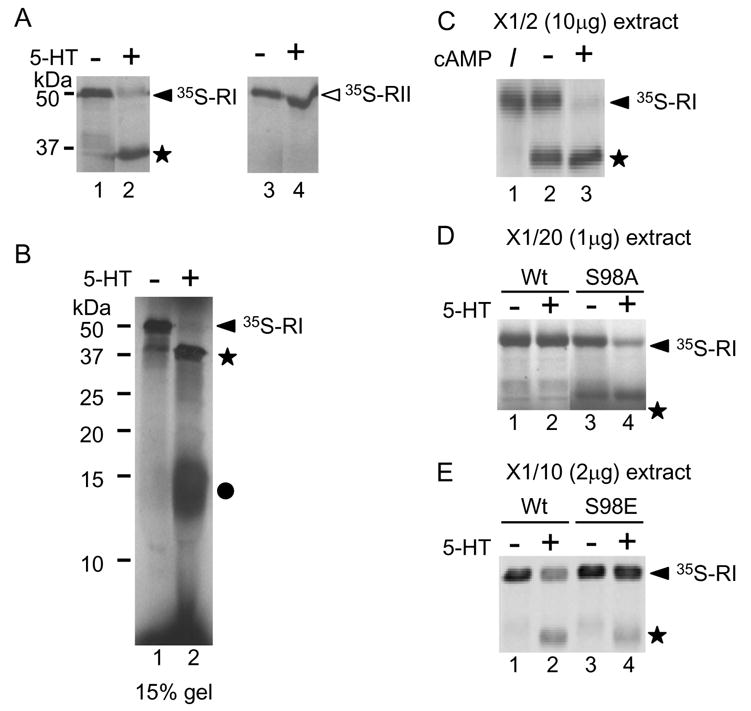
Autoradiography of 35S-labeled RI and RII. 35S-labeled proteins were produced in vitro and incubated with extracts from Aplysia ganglia treated with or without 5-HT. (A) 35S-labeled RIs were incubated with extracts from ganglia not treated with 5-HT (lane 1) and treated with 5-HT (lane 2). 35S-labeled RIIs were incubated with extracts from ganglia cultured without 5-HT (lane 3) or with 5-HT (lane 4). (B) Extracts from ganglia treated with 5-HT cleave RI and produce two fragments, 15 kDa and 37 kDa. Lane 1 shows RI incubated with extract from ganglia treated without 5-HT. Lane 2 shows RI incubated with extract from ganglia treated with 5-HT. A closed circle indicates the 15-kDa fragment. (C) cAMP enhances cleavage of RI. 35S-labeled RI (shown in lane 1) were incubated with ganglia treated with 5-HT in the absence of cAMP (lane 2) or in the presence of 100 mM of cAMP (lane 3). I indicates input 35S-labeled RI before incubation with extract. (D) The S98A mutation in RI decreases its sensitivity to cleavage. Radiolabeled proteins were incubated with twenty-fold diluted extract from ganglia treated without 5-HT (lane 1 and 3) or with 5-HT (lane 2 and 4). The wild type RIs with ganglia not treated or treated with 5-HT are shown in lane 1 and 2. The S98A mutants are shown in lanes 3 and 4. (E) 35S-labeled Rs were incubated with ten-fold diluted extract from ganglia treated without 5-HT (lane and 3) or with 5-HT (lane 2 and 4). The wild-type RIs are shown in lane 1 and 2. The S98E RI mutant is shown in lanes 3 and 4.
We observed at 37-kDa fragment but not the other fragments (Fig. 1). We performed SDS-PAGE using 15 % gel to obtain a higher resolution of the cleaved products. 35S-labeled RI incubated with extracts from ganglia treated with 5-HT produced a 37kDa fragment (Fig. 3B. star) and a 15-kDa fragment (Fig. 3B. closed circle). This suggests that cleavage of RI after 5-HT treatment occurs at a protease-sensitive region as previously shown [19],[20]. Both RI and RII subunits, have a protease-sensitive region in the hinge region. The hinge region contains a catalytic subunit-binding domain. Therefore, once cleaved by proteases, R subunits loose the ability to hold C subunits for inhibition of kinase activity, and PKA is activated [20]. The hinge region of both RI and RII are sensitive to proteolysis. Why RI is selectively cleaved? We further studied to answer this question.
When the concentration of cAMP increases, cleavage of R subunits efficiently occurs [19]. When the concentration of cAMP increases, C subunits are released and the hinge region is exposed to the outside of the molecule. Then, R subunits become more sensitive to the protease [20]. We performed the above experiments in the presence of 10 μM of cAMP and used 20 μg of extracts. We questioned if cleavage of RI is cAMP-dependent. 10 μg of extracts from ganglia treated with 5-HT completely cleaved 35S-labeled RI (Fig. 3C. lane 3) but cleaved about 50% of 35S-labeled RI in the absence of cAMP (Fig. 3C lane 2). This suggests that cAMP enhances cleavage of RI.
Next, we suspected that the modification of R subunits by phosphorylation might paly a role in cleavage of R subunits. Functionally, RI and RII can be distinguished on the basis of their potential for autophosphorylation [19]. RII, but not RI, contains an autophosphorylation site at the 96th amino acid residue where is located in the hinge region. We suspected that autophosphorylation may prevent cleavage of RII. However, 35S-labeled RII S96A mutant was still resistant to cleavage (data not shown). Then, we examined the phosphorylation site of RI located around the hinge region. Hashimoto, et al. [21] reported that a serine residue at position 98, where is located in the hinge region, is phosphorylated by a cGMP-dependent protein kinase. 35S-labeled RI S98A mutant was incubated with 1 μg of extract from Aplysia ganglia treated with 5-HT. The extract did not cleave wild-type RI (Fig. 3D. compare lanes 1 and 2) but partially cleaved the RI S98A mutant (Fig. 3D. compare lanes 3 and 4). Next, we examined RI S98E mutant, which mimics phosphorylation. The wild-type RI was cleaved after incubation with 2 μg of extract from Aplysia ganglia treated with 5-HT (Fig. 3E compare lane 2 and 3) but the S98E mutant was relatively resistant (Fig. 3E compare lane 3 and 4). We concluded that the phosphorylation of 98S in RI partially protected RI from cleavage.
Discussion
We found that the treatment of Aplysia ganglia with 5-HT causes cleavage of RI but not RII and extracts from Aplysia ganglia treated with 5-HT for 2 h have an ability to cleave 35S-labeled RI selectively in vitro. Our observation suggests that Aplysia neurons have a mechanism whereby only RI is cleaved in response to 5-HT. Bergold, et al. [22] found that loss of the regulatory subunit of PKA depends on protein synthesis during exposure to 5-HT, and then Hegde, et al. [18] provided evidence that a ubiquitin carboxy-terminal hydrolase (Uch) is induced as an immediate early gene (IEG) product only in neurons in response to 5-HT. Presumably, up-regulation of Uch does not directly act on cleavage of RI since it appears to associate with the proteasome and to increase the rate of release of ubiquitin from intermediates of proteolytic degradation. However, Uch may indirectly contribute cleavage since MG132 inhibited cleavage of RI (Fig. 1). Proteolytic regulation of R subunits by cleavage has been reported in rat alveolary type II cells where it is caused by calpain [23]. We propose that cleavage of RI is one of the mechanisms to produce persistent kinase activity of PKA in neuron.
In our study, the phosphorylation of RI at 98S partially protected against cleavage. The serine residue of RI at position 98 is phosphorylated by a cGMP-dependent protein kinase (PKG) [21]. Activation of PKG and inhibition of PKA is sufficient to induce long-term depression (LTD) of synaptic transmission at Schaffer collateral-CA1 synapses in the rat [24],[25]. In contrast, the cGMP-PKG pathway produces long-term potentiation (LTP) in the rat hippocampus [26]. Furthermore, the cGMP-PKG pathway triggered by nitric oxide (NO) produces transcription-dependent, long-term hyperexcitability (LTH) of nociceptive sensory neurons in Aplysia [27]. For these regulation through cGMP-PKG pathway, cleavage of RI may have a role.
Persistent activation of PKA by proteolysis is thought necessary to produce long-lasting memories [4],[13],[18], [28]. However, it appeared to be difficult to explain how neurons regulate the level of two different PKAs at the same time if both R subunits are susceptible to proteolysis. Here, we proposet that selective cleavage of RI provides the persistent kinase activity of PKA type I in the nucleus to phosphorylate transcription factors for initiating and maintaining the gene expression cascade required for long-term facilitation, whereas PKA type II is protected from cleavage and is provided to synaptic endings in order to strengthen synaptic connections.
Acknowledgments
This work was supported by grants from the National Institute of Neurological Disorders and Stroke MH048850 and NS29255 (J.H.S.). Animals were provided by the National Center for Research Resources, National Resource for Aplysia at the University of Miami under National Institutes of Health Grant RR1029.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- 2.Corbin JD, Keely SL, Park CR. The distribution and dissociation of cyclic adenosine 3′:5′-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975;250:218–225. [PubMed] [Google Scholar]
- 3.Michel JJ, Scott JD. AKAP mediated signal transduction. Annu Rev Pharmacol Toxicol. 2002;42:235–257. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg SM, Castellucci VF, Bayley H, Schwartz JH. A molecular mechanism for long-term sensitization in Aplysia. Nature. 1987;329:62–65. doi: 10.1038/329062a0. [DOI] [PubMed] [Google Scholar]
- 5.Sweatt JD, Kandel ER. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989;339:51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- 6.Muller U, Carew TJ. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron. 1998;21:1423–1434. doi: 10.1016/s0896-6273(00)80660-1. [DOI] [PubMed] [Google Scholar]
- 7.Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 8.Eppler CM, Bayley H, Greenberg SM, Schwartz JH. Structural studies on a family of cAMP-binding proteins in the nervous system of Aplysia. J Cell Biol. 1986;102:320–331. doi: 10.1083/jcb.102.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergold PJ, Beushausen SA, Sacktor TC, Cheley S, Bayley H, Schwartz JH. A regulatory subunit of the cAMP-dependent protein kinase down-regulated in aplysia sensory neurons during long-term sensitization. Neuron. 1992;8:387–397. doi: 10.1016/0896-6273(92)90304-v. [DOI] [PubMed] [Google Scholar]
- 10.Hegde AN, Goldberg AL, Schwartz JH. Regulatory subunits of cAMP-dependent protein kinases are degraded after conjugation to ubiquitin: a molecular mechanism underlying long-term synaptic plasticity. Proc Natl Acad Sci U S A. 1993;90:7436–7440. doi: 10.1073/pnas.90.16.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheley S, Panchal RG, Carr DW, Scott JD, Bayley H. Type II regulatory subunits of cAMP-dependent protein kinase and their binding proteins in the nervous system of Aplysia californica. J Biol Chem. 1994;269:2911–2920. [PubMed] [Google Scholar]
- 12.Liu J, Hu JY, Schacher S, Schwartz JH. The two regulatory subunits of aplysia cAMP-dependent protein kinase mediate distinct functions in producing synaptic plasticity. J Neurosci. 2004;24:2465–2474. doi: 10.1523/JNEUROSCI.4331-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chain DG, Hegde AN, Yamamoto N, Liu-Marsh B, Schwartz JH. Persistent activation of cAMP-dependent protein kinase by regulated proteolysis suggests a neuron-specific function of the ubiquitin system in Aplysia. J Neurosci. 1995;15:7592–7603. doi: 10.1523/JNEUROSCI.15-11-07592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chain DG, Schwartz JH, Hegde AN. Ubiquitin-mediated proteolysis in learning and memory. Mol Neurobiol. 1999;20:125–142. doi: 10.1007/BF02742438. [DOI] [PubMed] [Google Scholar]
- 15.Corbin JD, Sugden PH, West L, Flockhart DA, Lincoln TM, McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1978;253:3997–4003. [PubMed] [Google Scholar]
- 16.Potter RL, Taylor SS. Correlation of the cAMP binding domain with a site of autophosphorylation on the regulatory subunit of cAMP-dependent protein kinase II from porcine skeletal muscle. J Biol Chem. 1979;254:9000–9005. [PubMed] [Google Scholar]
- 17.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 18.Hegde AN, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain DG, Martin KC, Kandel ER, Schwartz JH. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 1997;89:115–126. doi: 10.1016/s0092-8674(00)80188-9. [DOI] [PubMed] [Google Scholar]
- 19.Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 20.Weber W, Hilz H. Stoichiometry of cAMP binding and limited proteolysis of protein kinase regulatory subunits R I and R II. Biochem Biophys Res Commun. 1979;90:1074–1081. doi: 10.1016/0006-291x(79)91935-1. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto E, Takio K, Krebs EG. Studies on the site in the regulatory subunit of type I cAMP-dependent protein kinase phosphorylated by cGMP-dependent protein kinase. J Biol Chem. 1981;256:5604–5607. [PubMed] [Google Scholar]
- 22.Bergold PJ, Sweatt JD, Winicov I, Weiss KR, Kandel ER, Schwartz JH. Protein synthesis during acquisition of long-term facilitation is needed for the persistent loss of regulatory subunits of the Aplysia cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1990;87:3788–3791. doi: 10.1073/pnas.87.10.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman UJ, Wang M, Nelson JB, Ekwunife FS, Liu L. Secretagogue-induced proteolysis of cAMP-dependent protein kinase in intact rat alveolar epithelial type II cells. Biochim Biophys Acta. 1996;1311:117–123. doi: 10.1016/0167-4889(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhuo M, Hu Y, Schultz C, Kandel ER, Hawkins RD. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994;368:635–639. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]
- 25.Santschi L, Reyes-Harde M, Stanton PK. Chemically induced, activity-independent LTD elicited by simultaneous activation of PKG and inhibition of PKA. J Neurophysiol. 1999;82:1577–1589. doi: 10.1152/jn.1999.82.3.1577. [DOI] [PubMed] [Google Scholar]
- 26.Zhuo M, Kandel ER, Hawkins RD. Nitric oxide and cGMP can produce either synaptic depression or potentiation depending on the frequency of presynaptic stimulation in the hippocampus. Neuroreport. 1994;5:1033–1036. doi: 10.1097/00001756-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Lewin MR, Walters ET. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci. 1999;2:18–23. doi: 10.1038/4520. [DOI] [PubMed] [Google Scholar]
- 28.Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem. 1998;5:246–256. [PMC free article] [PubMed] [Google Scholar]


