Abstract
There is much evidence suggesting early life events, such has handling or repeated separations from the nest, can have a long term effect on the biological and behavioral development of rats. The current study examined the effect of repeated maternal separation (MS) on the behavioral, cardiovascular, and neurobiological responses to stress in subjects vulnerable to environmental stressors as adults. Borderline hypertensive rats (BHR), which are the first generation offspring of spontaneously hyperternsive and Wistar-Kyoto rats, were separated from the dams for 3 hours per day from post-natal day 1 through 14. Non-separated controls remained in the home cage. When allowed to explore the open field chamber for 60 minutes as adults, MS subjects had significantly greater locomotor activity compared to controls. All subjects were exposed to 30 minutes of restraint stress during which time mean arterial pressure (MAP) and heart rate (HR) were measured. Although both groups had comparable increases in MAP, MS animals displayed significantly higher HR throughout the stress period. Finally, MS subjects had significantly more stress-induced Fos positive cells, an estimate of neuronal activation, in the central nucleus of the amygdala (CeA), paraventricular nucleus of the hypothalamus (PVN), and the bed nucleus of the stria terminalis (BNST), each of which plays an important role in organizing the biobehavioral response to stress. These results suggest that maternal separation can further enhance stress reactivity in this model and may represent a useful approach for studying the relationship between early life events and future vulnerability to stressful situations.
Keywords: maternal separation, stress, open field, cardiovascular, rats, Fos
Chronic exposure to stress is associated with a variety of physical and psychological disorders. Although multifactorial in etiology, prolonged exposure to adverse experience is widely thought to be contributory to the development of such divergent conditions as ulcer, cardiovascular disease, immunosuppression, anxiety disorders and depression, among many others (4,13,17). The biological pathways through which stressful events may lead to physiopathology include enhanced activation of the both the sympathoadrenal-medullary and hypothalamic-pituitary-adrenal (HPA) systems. This results in a constellation of responses that are normally adaptive during situations of acute challenge; increased adrenal release of catecholamines and glucocorticoids, elevation of blood pressure and heart rate, and enhanced cognitive processing of emotionally relevant stimuli. Although these events make good biological sense in the short term because they mobilize internal resources that increase the probability of successfully negotiating the stressful event, more long term stress-induced activation of these systems can shift from being adaptive to pathogenic.
However, stress is not a unitary phenomenon and considerable variation exists in individual responses to chronic exposure to stress; indeed, a negative health outcome is not a certainty. In order to more clearly understand what factors influence individual variation in the response to stress, and therefore the potential vulnerability to disease, much attention has focused on understanding the roles of mediating variables such as genetics, gender, experience, and coping skills. Of particular interest for the current study is the extent to which alterations in early life experience can shape future responses to stress. Maternal separation, which typically involves isolating each pup individually from the dam and littermates for at least 3 hours per day, is a widely used procedure that produces an adult biobehavioral profile of increased behavioral and endocrine responses to stress (21,22,26). On the other hand, separation for less time, usually 15 min per day (referred to as handling), produces a stress-immunization effect in adulthood characterized by substantial reductions in hypothalamic-pituitary adrenal axis and behavioral responses to stress (20,24). The precise mechanisms by which these early life events translate into long term changes in the offspring are unknown, but variations in the quality of maternal care, which may be exacerbated by the intervention, have received considerable attention (7,8). More recently, evidence has emerged suggesting that early life experience can produce a structural change of the DNA that alters gene expression in certain brain areas that may in turn affect stress responsivity (25,37,38).
Experiential factors typically do not exert their effects independently, however, but rather interact with other existing organismal variables, such as genetic family history. In recent years, we have become interested in using the borderline hypertensive rat (BHR) as a model with which to examine these relationships. The BHR is the first generation offspring of the spontaneously hypertensive rat (SHR) and the normotensive Wistar-Kyoto (WKY) rat. Although resting blood pressure in the BHR remains in the borderline range (c. 140/90 mmHg), this model shows considerable sensitivity to environmental stressors in that either chronic behavioral stress or increased dietary sodium elevates resting pressure to hypertensive levels (18,32). In addition, alterations in experience in the post-natal period are capable of affecting stress-induced cardiovascular responses in BHR. Specifically, cross-fostering BHR pups to WKY dams rather than leaving them with their natural SHR mothers, significantly reduces their blood pressure to acute stress (31). Thus, whereas adult BHR demonstrate a susceptibility to stress-induced hypertension, there is also evidence suggesting that early life events can modify future reactivity to stressful situations. To further understand how early experience may affect adult responses to stress in this model, the present study was designed to examine the extent to which maternal separation affects behavioral, neurobiological, and cardiovascular responses to stressful and novel situations in the BHR.
1. Methods
1.1 Subjects
Subjects used in this study were male borderline hypertensive rats (BHR). These animals were the first generation offspring of female spontaneously hypertensive rats (SHR) and male Wistar-Kyoto (WKY), both of which were purchased from Taconic Farms (Germantown, NY). Following harem breeding, pregnant SHR were housed individually in polyprophylene cages. Animals were housed in the vivarium with a 12:12h light dark cycle (lights on at 0600h). All animals had free access to food and water throughout the study. Day of birth was defined as pups being present by 1600 h. All litters were culled within 24 hours of birth to 8 pups, 4 males and 4 females where possible. No more than one male pup from each litter was used for any given measure. Subjects used in the study were housed individually following weaning at 3 weeks of age. All procedures were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
1.2 Maternal Separation
Maternal separation (MS) was conducted daily from postnatal day (PD) 1 until PD14. Dams were removed first from the home cage and kept in a separate cage during the 3 hour separation period. All pups were then removed and placed in individual plastic containers, maintained at 31°C, with sawdust. Following the 3-hour MS period, the pups were returned to the home cage followed by the dam. Non-maternal separated (N-MS) subjects were left undisturbed throughout the preweaning period. Cage changing was suspended during the two-week maternal separation period.
1.3. Open Field Testing
At 6−8 weeks of age, subjects were tested for 60 minutes in the open field arena, a behavioral test used to assess general locomotor behavior in a novel environment. This chamber is 22.1"W × 21.2"L × 15"H and is interfaced to a PC data collection system (Hamilton-Kinder, San Diego, CA). Animals were transported from the animal facility to a holding room adjacent to the test room at least 2 hours before the test. The arena was washed thoroughly between tests.
1.4. Cardiovascular Recording
Arterial catheters were made from a 3 cm Micro-Renathane (.025" OD) tip (Braintree Scientific, Braintree MA) fused to tygon tubing. Animals were anesthetized with ketamine hydrochloride+acepromazine (100 mg/kg, 2mg/kg, respectively, i.p.) and the catheter was introduced under aseptic conditions to the lower abdominal aorta via the right femoral artery. The catheter was tunneled up the back and exited dorsally at the neck. Each catheter was filled with heparinized saline (200U/ml) and capped with a stainless steel plug. Animals were tested after a 48−72 hour recovery period.
On the test day, each rat was transported in the home cage to the testing room and the arterial catheter was attached to a pressure transducer that was connected to PowerLab hardware and software (ADI, Grand Junction CO). Each subject was allowed to acclimate for at least 2 hours prior to recording a blood pressure and heart rate for 30 minutes. Following this baseline period, the animal was transferred to a cylindrical Plexiglas restraint tube for 30 minutes. At the end of the 30 minute restraint period, the subject was returned to the home cage for 90 minutes, at which time the brain was removed for immunohistochemistry.
1.5. Immunohistochemistry
Animals were anesthetized with sodium pentobarbital (65 mg/kg, i.p.) and perfused transcardially with 200 ml normal saline followed by 400 ml of 4% paraformaldehyde prepared in 0.1M phosphate buffer (PB), pH 7.4. Following removal, brains were post-fixed overnight in 4% paraformaldehyde and then stored in cryoprotectant (30% sucrose). Coronal sections (40μm) were taken using a freezing microtome/cryostat (Leica). Free-floating sections were washed several times in 0.01M PB before being soaked in 0.3% H2O2 to quench endogenous peroxidase activity. Following several washes in PB, sections were incubated in PB + 0.1% Triton x-100 and 2% normal goat serum to block non-specific binding. Following another series of washes, sections were incubated in the primary antibody (diluted 1:10,000, Santa Cruz Biotechnology, Santa Cruz CA) for 48 hours at 4° C.Sections were then incubated for 2 hours in the biotinylated secondary antibody (dilution 1:500, Vector), followed by several washes in PB before incubation for 2 hours in the avidinbiotin complex (dilution 1:1000, ABC Elite, Vector). The reaction product was developed using diaminobenzidine tetrahydrochloride (DAB, 0.5 mg/ml) in the presence of 0.02% H2O2. Stained sections were washed several times in PB and mounted on gelatin coated slides, dried, and coverslipped using Permount. Control procedures for Fos immunoreactivity included processing sections with sequential omission of the primary antibody, biotinylated secondary antibody, and the ABC reagent. Cell counts were obtained from a 1 in 4 series of immunostained 40 μm coronal sections through the areas of interest (areas PVN = 0.06mm2, BNST = 0.36mm2, CeA = 0.36mm2) using a Nikon Eclipse 300 microscope and Image J software (NIH).
1.6 Statistical Analyses
Total horizontal activity in the open field was collected for 60 minutes was analyzed using the student's t test. These data were also binned into six 10-minute blocks and analyzed using repeated measures analysis of variance (ANOVA) with time as the repeated measures factor and condition as the grouping factor. MAP and HR data collected during the 60 minute restraint stress period were analyzed using repeated measures ANOVA with time six 10-minute blocks) as the repeated measures factor and condition as the grouping factor. Significant interactions were followed up using Tukey's HSD test. Resting MAP and HR, total locomotor activity and Fos data were analyzed using the student's t test. A significance level of .05 was applied to all tests. Data are expressed as the mean ± SEM.
2. Results
2.1.Open field behavior
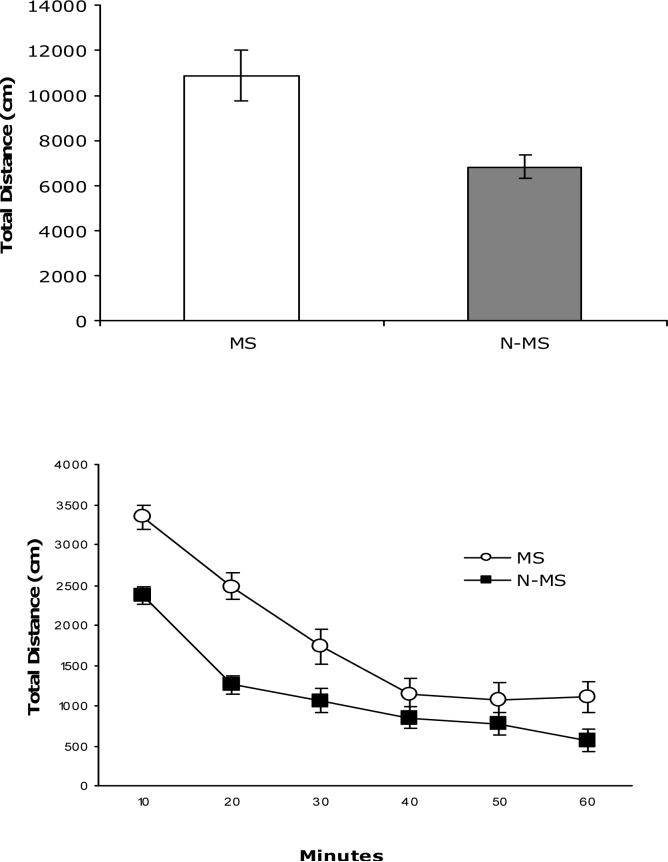
Figure 1 (top panel) shows the total spontaneous locomotor activity during the one hour test. MS BHR (n=9) displayed significantly more activity compared to N-MS (n=19) subjects, t(26)=3.78, p<.001. When analyzed as 10 minute bins, a mixed model ANOVA revealed a significant main effect of time F(5,130)=54.86, p<.0001, condition F(1,26)=22.71, p<.0001 and a significant time x condition interaction F(5,130)=3.31, p<.0008. As can be seen in Figure 1 (bottom panel), although both groups had comparable decreases in activity over the 60 minute test period, MS subjects were significantly more active at all but the 40 and 50 minute time points.
Figure 1.
Total exploratory activity in open field (top panel) during 60 min test and binned in 10 minute blocks (bottom panel) for MS (n= 9) and N-MS (n=19) subjects. Data are expressed as mean ± SEM.
2.2 Cardiovascular responses
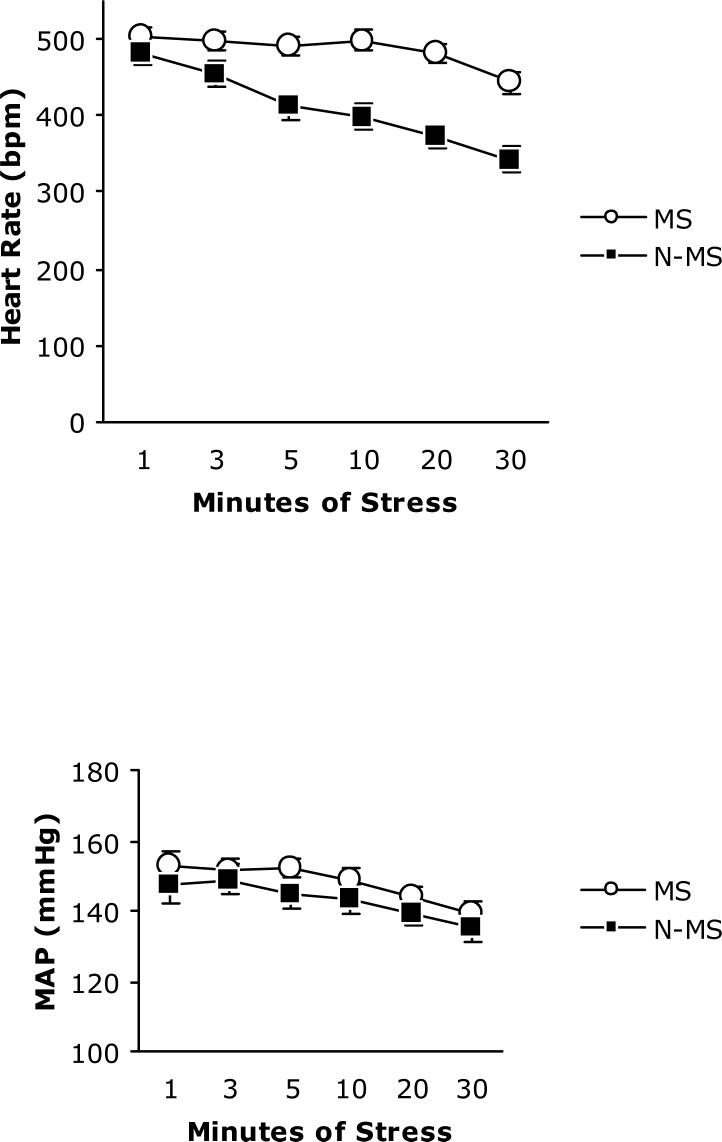
Analysis of MAP and HR data collected during a home cage rest session, shown in Table 1, (MS n=17; N-MN n=10) revealed no difference between groups t(25) = 1.07, p< .292 and t(25) =1.37, p< .81, respectively. Repeated measures ANOVA performed on HR during stress found a significant main effect of time F(5,100)=18.57, p< .001 and condition F(1,20)=26.60, p< .001 and a significant interaction of time x condition F(5,100)=4.67, p< .001; MS subjects had higher heart rate at all but the first time point (Figure 2, top panel). The same analysis of MAP (MS n=14; N-MS n=10) collected at 6 time points during the 30 min stress session (Figure 2, bottom panel) indicated that both groups tend to return to baseline over time F(5,100)=11.52,< p.001. There was not a significant main effect of condition F(1,20)= 1.21, p< .284 or of the time x condition interaction F(1,100)=.29, p< .917.
Table 1.
Resting MAP and HR (Mean ± SEM).
| MAP (mmHg) | HR (bpm) | |
|---|---|---|
| MS (n=17) | 126.5±2.0 | 327.3±6.1 |
| N-MS (n=10) | 123.1±2.2 | 313.9±7.6 |
Figure 2.
HR (top panel) and MAP (bottom panel) during 30 minutes of restraint stress in MS (n=14, open circle) and N-MS (n=10, closed squares) BHR. Data are expressed as mean ±SEM.
2.3 Immunohistochemistry
As seen in Figure 3, subjects exposed to the maternal separation procedure displayed significantly greater stress-induced Fos expression in each of the three brain areas examined (n=9−14 per group); BNST t(24)=3.01, p< .006, PVN t(17)=3.8 p<.001, and CeA t(18)=6.02 p <. 001.
Figure 3.
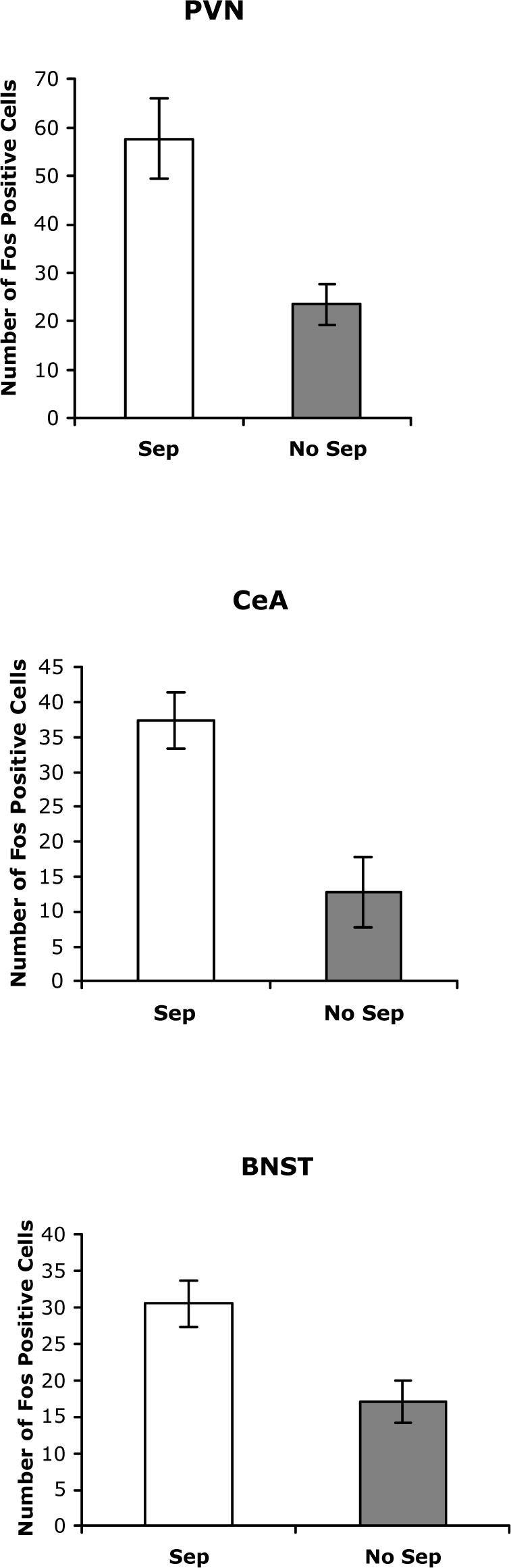
Fos immunoreactive cells in PVN (top panel), CeA (middle panel), and BST (bottom panel) in MS and N-MS BHR (n = 9−14 per group). Data are expressed as the mean±SEM.
3. Discussion
The purpose of the present study was to examine the effect of an early environmental challenge on the biobehavioral response to stress in rats that show enhanced stress reactivity as adults. As mentioned previously, this model is sensitive to several environmental stimuli as adults, leading us to questions regarding whether events early in life can modify this susceptibility. Since we were primarily interested in examining the extent to which daily maternal separation modulated adult responses in BHR, which is vulnerable to stress-induced cardiovascular disease as adults, rather than an analysis of varying genetic susceptibilities across strains, we did not examine WKY subjects in this study. Several lines of previous research suggest that WKY, as well as other normotensive strains of rats, are resistant to long-term perturbations in blood pressure caused by exposure to noxious environmental stimuli. For example, studies employing sensory stimulation/withdrawal (35), psychosocial stress (14) and classical (34) and operant (15) conditioning paradigms have all found little or no permanent effect of blood pressure and heart rate in normotensive strains of rodents. In addition, it has been reported that cross-fostering either WKY or Sprague-Dawley pups to SHR dams does permanently alter their adult blood pressure, suggesting resistance to the effects of a hypertensive maternal phenotype (9). Thus, given the range of studies failing to find any substantive cardiovascular effect of environmental intervention in normotensive strains, we used daily pup separation from the dam as a post-natal challenge to examine the effects of early adverse experience on BHR.
Animals placed in a novel environment typically display initially high levels of exploratory activity that drops off as the subject becomes more familiar with the environment. Differences in the level of total locomotion and the pattern of behavior across time have been used as indices of an organism's level of emotionality (11) as well as ability to process new information from a novel environment (19). Spontaneous motor activity was assessed in this experiment during a 60 minute trial in the open field chamber. These data were analyzed in two ways. First, BHR subjects exposed to maternal separation had significantly higher levels of total locomotor activity compared to non-separated BHR. In addition, when examined in 10 min bins across time, MS subjects start the trial at higher levels of activity that are maintained throughout most of the test. Although this study did not utilize a normotensive strain of rat, previous reports suggest that MS has little or no effect on these subjects. For example, Wistar rats separated for 6 h on post-natal days 1−21 showed no differences in behavior in the open field or elevated plus maze compared to controls (27). In addition, Wistar rats that are separated once for 24 h postnatal days 4, 9, or 18 show no evidence increased locomotor activity, although there is a tendency for females to be more active than males (19). Thus, our data suggest a level of hyperactivity produced by MS not seen in normotensive Wistar rats, although differences in MS procedures used in other studies (age, duration, etc) make precise comparisons difficult. Given that BHR are genetically predisposed toward heightened stress reactivity, the current data may suggest that MS further enhances this tendency, leading to increased escape behavior and hyperactivity in a novel environment.
Exposure to novel or threatening stimuli produces a complex pattern of endocrine, autonomic and behavioral activation designed to better enable the organism to negotiate the challenge. This study used the expression of the immediate early gene c-fos to estimate neuronal activation in brain regions important in mediating the response to stress. Although Fos expression is minimal under basal conditions, a variety of psychological and physiological challenges are capable of producing a significant elevation in Fos mRNA and the Fos protein product (5,29), thus making it a useful and frequently used method to map neural circuitry. We assessed stress-induced Fos expression in the BNST, CeA, and PVN because of the well-known role these structures play in orchestrating an organism's response to stress. The PVN has long been recognized as pivotal in producing stress-induced changes in the neuroendocrine profile (16,33). Stress-relevant information is received in the PVN by neurons that secrete corticotropin releasing hormone, setting in motion the HPA response. Successfully negotiating a stressful challenge, however, requires autonomic and behavioral responses in addition to neuroendocrine adjustments. Thus, limbic, cortical, brainstem, and hypothalamic regions become differentially involved depending on the characteristics of the stressor. For example, challenges that involve a psychological or emotional component, such as the open field or restraint, may be associated with more activation of hypothalamic and amygadoid nuclei compared with more physical stressors, such as ether inhalation or hemmorhage (1,12). In addition to stimulus-dependent activation of particular neural circuits, connections also exist that support specific biological adjustments, such as the CeA and BNST signaling the dorsal vagal complex to adjust cardiovascular functioning, thereby providing a way for higher brain centers to exert influence over blood pressure and heart rate (3).
The current study found that repeated maternal separation produced significant stress-induced increases in Fos immunoreactivity in the BNST, CeA, and PVN. This pattern of neural activation is consistent with several lines of relevant research. First, several studies have found that a single episode of acute stress produces substantial increases in Fos expression in the PVN, CeA, BNST as well as other areas (2,10). Also, the magnitude of neuronal activation has been shown to be sensitive to briefer periods of maternal separation. Specifically, handling, which involves 15 min of daily separation during the first two postnatal weeks, results in a reduction in Fos immunoreactivity in the PVN, BNST, CeA, as well as other areas (1). This is in line with an extensive body of research showing that handling generally produces a dampening of many endocrine and behavioral measures of stress (21,23,25). Finally, a recent study, employing a structural rather than functional approach, found that both brief (15 min) and long (3h) periods of maternal separation produced a significant reduction in the number of preautonomic limbic forebrain neurons (6). Using retrograde transport of the pseudorabies virus to map postnatal neural circuit development, these investigators found that daily separation led to significantly fewer neurons in the CeA and BNST on PD 8 and 10; an effect not observed in the PVN, probably because these circuits are formed earlier in development (28). Our results taken together with previous findings support the contention that early life events can substantially modify both structural and functional aspects of the neural circuits involved with processing emotionally relevant stimuli.
Since the BHR shows increased cardiovascular responses to both acute and chronic stress (30,32), we had hypothesized that repeated maternal separation would further enhance this tendency. In response to restraint stress we observed that while both groups had comparable increases in MAP, MS rats displayed a significant tachycardia during the stress period. Although this study did not examine how maternal separation may affect autonomic balance or cardiovascular regulation, several previous reports provide some context for understanding this observation. As previously described, maternal separation changes the brain circuitry in areas that have the potential to alter the central neural control of autonomic outflow (6), perhaps influencing parasympathetic and/or sympathetic tone. Of specific interest to the current study is a report that examined the ontogeny of autonomic control of heart rate in BHR, SHR, and WKY neonates (36). Using pharmacological blockade and surgery to compare the development of heart rate control in strains of rats with differing sensitivities to stress, it was found that at weaning BHR and SHR displayed enhanced adrenal medullary drive as well as greater parasympathetic restraint. Based on these observations, it is possible that superimposing maternal separation on the autonomic profile of the BHR, which resembles that of its hypertensive parent, further disrupts heart rate control making it more sensitive to environmental stress.
In summary, we observed that daily maternal separation for the first 14 days of life produces increases in behavioral, neurobiological, and cardiovascular responses to stressful or novel stimuli in BHR. These results contribute to a larger body of work suggesting that early life events are capable of producing enduring effects in the offspring. Moreover, given that the BHR is sensitive to environmental challenges, the current study provides a potentially useful approach studying the interaction between early life events and future stress reactivity.
ACKNOWLEDGEMENTS
This work was supported by NIH grant HL-073894.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
4. References
- 1.Abraham IM, Kovacs KJ. Postnatal handling alters the activation of stress-related neuronal circuitries. Eur J Neurosci. 2000;12:3003–3014. doi: 10.1046/j.1460-9568.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- 2.Arnold FJ, De Lucas Bueno M, Shiers H, Hancock DC, Evan GI, Herbert J. Expression of c-fos in regions of the basal limbic forebrain following intracerebroventricular corticotropin-releasing factor in unstressed or stressed male rats. Neuroscience. 1992;51:377–390. doi: 10.1016/0306-4522(92)90322-s. [DOI] [PubMed] [Google Scholar]
- 3.Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behav Brain Res. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 4.Bifulco A, Brown GW, Adler Z. Early sexual abuse and clinical depression in adult life. Br J Psychiatry. 1991;159:115–122. doi: 10.1192/bjp.159.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- 6.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- 8.Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 9.Cierpial MJ, McCarty R. Adult blood pressure reduction in spontaneously hypertensive rats reared by normotensive Sprague-Dawley mothers. Beh Neural Biol. 1991;56:262–270. doi: 10.1016/0163-1047(91)90409-j. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- 11.Elliott BM, Grunberg NE. Effects of social and physical enrichment on open field activity differ in male and female Sprague-Dawley rats. Behav Brain Res. 2005;165:187–196. doi: 10.1016/j.bbr.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 12.Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- 13.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 14.Harrap SB, Louis WJ, Doyle AE. Failure of psychosocial stress to induce hypertension in the rat. J Hyperten. 1984;2:653–662. doi: 10.1097/00004872-198412000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Herd AJ, Morse WH, Kelleher RT. Arterial hypertension in the squirrel monkey during behavioral experiments. Am J Physiol. 1969;217:424–429. doi: 10.1152/ajplegacy.1969.217.1.24. [DOI] [PubMed] [Google Scholar]
- 16.Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. J Neuroendocrinol. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 17.Holmes SJ, Robins LN. The role of parental disciplinary practices in the development of depression and alcoholism. Psychiatry. 1988;51:24–36. doi: 10.1080/00332747.1988.11024377. [DOI] [PubMed] [Google Scholar]
- 18.Lawler JE, Sanders BJ, Chen YF, Nagahama S, Oparil S. Hypertension produced by a high sodium diet in the borderline hypertensive rat (BHR) Clin Exp Hypertens A. 1987;9:1713–1731. doi: 10.3109/10641968709158968. [DOI] [PubMed] [Google Scholar]
- 19.Lehmann J, Pryce CR, Bettschen D, Feldon J. The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav. 1999;64:705–715. doi: 10.1016/s0091-3057(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 20.Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 21.Levine S. Psychosocial factors in growth and development. In: Levi L, editor. Society, Stress and Disease. Oxford University Press; London: 1975. pp. 43–50. [Google Scholar]
- 22.Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 24.Meaney MJ, Aitken DH, Sharma S, Viau V, Sarrieau A. Postnatal handling of rats alters the adrenocortical negative-feedback; a model for individual differences in the neuroendocrine response to stress. J Neuroendocrinol. 1990;50:597–604. [Google Scholar]
- 25.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paulus MP, Bakshi VP, Geyer MA. Isolation rearing affects sequential organization of motor behavior in post-pubertal but not pre-pubertal Lister and Sprague-Dawley rats. Behav Brain Res. 1998;94:271–280. doi: 10.1016/s0166-4328(97)00158-7. [DOI] [PubMed] [Google Scholar]
- 27.Roman E, Gustafsson L, Berg M, Nylander I. Behavioral profiles and stress-induced corticosteroid secretion in male Wistar rats subjected to short and prolonged periods of maternal separation. Horm Beh. 2006;50:736–747. doi: 10.1016/j.yhbeh.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Rinaman L, Levitt P, Card JP. Progressive postnatal assembly of limbic-autonomic circuits revealed by central transneuronal transport of pseudorabies virus. J Neurosci. 2000;20:2731–2741. doi: 10.1523/JNEUROSCI.20-07-02731.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 30.Sanders BJ, Cox RH, Lawler JE. Cardiovascular and renal responses to stress in borderline hypertensive rat. Am J Physiol. 1988;255:R431–438. doi: 10.1152/ajpregu.1988.255.3.R431. [DOI] [PubMed] [Google Scholar]
- 31.Sanders BJ, Gray MJ. Early environmental influences can attenuate the blood pressure response to acute stress in borderline hypertensive rats. Physiol Behav. 1997;61:749–754. doi: 10.1016/s0031-9384(96)00530-6. [DOI] [PubMed] [Google Scholar]
- 32.Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: a review and update. Neurosci Biobehav Rev. 1992;16:207–217. doi: 10.1016/s0149-7634(05)80181-2. [DOI] [PubMed] [Google Scholar]
- 33.Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro AP, Melhado J. Observations on blood pressure and other physiologic and biochemical mechanisms in rats with behavioral disturbances. J Psychosom Med. 1958;20:303–317. doi: 10.1097/00006842-195807000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Smookler HH, Buckley JP. Relationships between brain catecholamine synthesis, pituitary adrenal function and the production of hypertension during prolonged exposure to environmental stress. Int J Neuropharmacol. 1969;8:33–41. doi: 10.1016/0028-3908(69)90032-x. [DOI] [PubMed] [Google Scholar]
- 36.Tucker DC, Domino JV. Balance among autonomic controls of heart rate in neonatal spontaneously hypertensive and borderline hypertensive rats. J Auton Nerv Syst. 1988;22:11–21. doi: 10.1016/0165-1838(88)90149-x. [DOI] [PubMed] [Google Scholar]
- 37.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Fish E, Anisman H, Szyf M, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. Biol Psychol. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]