Abstract
In wild-type FVB mice, leukocyte recruitment to lipopolysaccharide was sexually dimorphic, with a greater number of leukocytes recruited in females. In male β2-adrenergic receptor knock out mice (bred on a congenic FVB background) the number leukocytes recruited was increased ~4-fold, while in females there was no change, eliminating sexual dimorphism in leukocyte migration. While there were significantly fewer recruited CD62L+ and CD11a+ leukocytes in wild-type males, only in male β2-adrenergic receptor knock out mice was there an increase in the number of recruited CD11a+ leukocytes, again eliminating sexual dimorphism. Thus, leukocyte migration and CD11a+ adhesion molecule expression in male, but not in female, leukocytes is β2-adrenergic receptor-dependent. Our findings provide support for a role of β2-adrenergic receptor mechanisms in the inflammatory response, and suggest that β2-adrenergic receptor on male leukocytes contributes to sexual dimorphism in the effect of stress on inflammatory diseases.
Keywords: β2–adrenergic receptor, leukocyte recruitment, sexual dimorphism, knock out mice, adhesion molecules
1. Introduction
Leukocyte infiltration is an important pathological feature of many inflammatory diseases (Ajuebor et al., 2002; Edwards and Hallett, 1997; Goulding et al., 1998; Kasama et al., 2005), they are recovered from bronchoalveolar lavage fluid in asthmatic patients, indicating that they move through the endothelium and extracellular matrix and migrate across epithelium into airways (Liu et al., 1999), and are also found in arthritic joints at the pannus–cartilage junction, the site of joint-destroying erosions (Mohr and Menninger, 1980); neutrophils within the joint are now recognized to participate directly in chronic inflammation (Chen et al., 2006).
Stress can affect inflammatory diseases (see Black, 2002(Black, 2002a) for review), in part mediated by the effect of stress hormones on leukocyte function (Bierhaus et al., 2006; Bilbo et al., 2002; Dhabhar, 2002; Landmann et al., 1984; O’Leary et al., 1996; Shephard, 2003), and catecholamines mediate interactions between the sympathetic and the immune systems, to alter immune cell activity (Benschop et al., 1997; Downing and Miyan, 2000; Elenkov et al., 2000; Oberbeck, 2006; Straub et al., 1998). Norepinephrine, released from sympathetic nerve terminals, and epinephrine, released from the adrenal medulla (Elenkov et al., 2000), act primarily on α2 and β2 adrenergic receptors expressed on most resting and activated immune cells (Barnes, 1995; Barnes, 1999; Kin and Sanders, 2006; Maestroni, 2006; Wahle et al., 2005). Stimulation of the β2-adrenergic receptor has been shown to play a role in regulating leukocyte homing, immune cell surface phenotype and mature immune cell function. While the contribution of the β2-adrenergic receptor in inflammation is well established clinically, in view of their potent anti-inflammatory properties (Barnes, 1999; Cobelens et al., 2002; Sekut et al., 1995), its role in modulating leukocyte recruitment has received remarkably little attention (de Coupade et al., 2004; Straub et al., 2000b). The few studies investigating the role of β2-adrenergic receptor agonists on leukocyte function in vivo are mostly limited to examination of their effects on cytokine production and cell adhesion.
Many inflammatory diseases are sexually dimorphic, often more prevalent or severe in women (Ansar Ahmed et al., 1999; Castagnetta et al., 2002; Da Silva, 1995; Da Silva, 1999; Green, 1992; Wilder et al., 1982). Since β2-adrenergic mechanisms have been reported to be sexually dimorphic (Barker et al., 2005; de Coupade et al., 2004; Hinojosa-Laborde et al., 1999; Krieg et al., 1986; Mills et al., 1996; Tan et al., 1997; Wheeldon et al., 1994), the present study investigated the ability of β2-adrenergic receptors to modulate neutrophil recruitment, using wild-type (FVB) and β2-adrenergic receptor knockout mice (bred on a congenic FVB background). Of note, in a comparison of inflammation in the lungs of 9 mouse strains, after ovalbumin sensitization and aerosol challenge the FVB strain had fewer infiltrating leukocytes than 4 other strains, but similar to BALB/C, C57/BL and C3H/HeJ strains (Whitehead et al., 2003). Thus, strain differences in immune responses need to be taken into consideration when extrapolating data obtained from a single mouse strain, as reported in this current study.
Since most inflammatory responses and effector functions involve leukocyte adhesions, we also studied sex differences in two cell adhesion molecules that have been shown to be particularly affected by β-adrenergic receptor activation (Kurokawa et al., 1995; Miles et al., 1998; Mills et al., 2002a): i) CD62L (L-selectin), which mediates the early phase of leukocyte diapedesis, forming weak bonds to allow rolling adhesion on the endothelium, and ii) the integrin CD11a which forms stable bonds mediating leukocyte extravasation into sites of inflammation.
Even though β2-adrenergic receptor knockout mice express no alteration in their ability to mount adaptive immune responses (Sanders et al., 2003), in this study we show that in mice lacking β2-adrenergic receptors LPS-induced leukocyte recruitment is altered in males but not females, suggesting a mechanism for sexual dimorphism in inflammatory diseases.
2. Materials and Methods
Animals
Male and female FVB mice, 22-26 g (6 weeks old), were obtained from Charles River (Hollister, CA). Mice, with targeted deletions of the β2-adrenergic receptor, were a generous gift from Dr. Brian Kobilka (Stanford University, CA); β2-adrenergic receptor mice are bred on a congenic FVB background (Chruscinski et al., 1999).
Chemicals
Lipopolysaccharide (LPS) (Sigma Chemical Co., St. Louis, MO); anti-neutrophil fluorescein isothiocyanate (FITC)-labeled monoclonal antibody, a rat anti-mouse 7/4 antigen produced against neutrophil-rich cultured bone-marrow populations that defines a polymorphic neutrophil differentiation antigen (Hirsch and Gordon, 1983) (Cedarlane Laboratories Ltd. Hornby, Ontario, Canada); anti-mouse CD4 phycoerythrin (PE) (recognizing the L3T4 antigen, a marker for T helper lymphocytes; Caltag, South San Francisco, CA); PE anti-mouse CD11a and FITC anti-mouse CD62L (BD Biosciences, San Jose, CA).
Chemotaxis in vivo (murine air pouch)
Air pouches were produced on the backs of 6-week-old male and female β2-adrenergic receptor knockout and wild-type mice. Experiments were performed as described by Hachicha (Hachicha et al., 1999). Briefly, a dorsal air pouch was created by injecting 3 ml of sterile air subcutaneously in the back of the mouse on day zero, and again on day three. On day six, saline or LPS were directly injected in the pouch. Four hours after injection, mice were euthanized and their air pouches washed three times with 1 ml sterile phosphate buffered saline (PBS). This time point was chosen since we are interested in the neutrophil component of the early inflammatory response and neutrophil recruitment into the air pouch is maximal around four hours after LPS (Santos et al., 2003). The lavage exudates were centrifuged, cell pellets, containing ~1×106 cells, resuspended in 1 ml of PBS + 1% BSA + 1 μg/ml propidium iodide + 5 μg/ml Hoechst-33342 and viable cells counted by flow cytometry using a dual-laser FACStar cell sorter (Becton Dickinson, San Jose, CA). Data acquisition and analysis were accomplished with FlowJo version 8.3 software (Treestar, Ashland, OR).
Flow cytometry
Cells were incubated for 30 min at 4°C with saturating concentrations of the indicated mouse monoclonal antibodies labeled either with FITC or phycoerythrin (PE), specifically: FITC anti-PMN 0.25 pg/tube, PE anti-CD4 0.25 pg/tube, PE anti-CD11a 0.5 pg/tube, FITC anti CD62L 0.5 pg/ml. Cells (5×103) were then washed and resuspended in cold PBS-1% BSA and the cell-associated light scatter and fluorescence were determined by flow cytometry
Statistical analysis
Data are expressed as mean ± SEM of n distinct observations. Statistical analysis was performed using statistical software from SPSS, Inc. or with Origin 6.1. Statistical comparisons were made by a two-tailed Student’s t test (for one or two independent populations) and by one-way ANOVA for comparing multiple treatments.
3. Results
To establish β2-adrenergic receptor-dependence for leukocyte chemoattraction, a critical mechanism in the acute inflammatory response, we examined in vivo leukocyte recruitment induced by LPS in the murine dorsal air pouch of male and female β2-adrenergic receptor knockout compared to wild-type mice. Six-day old air pouches in the back of the mice were injected with saline or LPS and four hours later, mice were euthanized and leukocytes harvested for flow cytometric analysis.
Involvement of β2-adrenergic receptors in sexual dimorphism for neutrophil recruitment
In wild-type animals, LPS (100 ng/ml suspended in 1 ml sterile PBS) induced markedly higher recruitment of neutrophils into the air pouch in females than in males (~3-fold higher, wild-type males vs. wild-type females P<0.01, Figure 1). The absence of functional β2-adrenergic receptors had no significant effect on the number of leukocytes in the pouch following either vehicle (PBS) or LPS in females (wild-type females vs. knock out females P=0.311 and P=0.925, Figure 1). The number of peripheral blood leukocytes was also unchanged (wild-type females 7.10 ± 0.68×106 n=6 vs. knock out males 5.36 ± 0.71×106 N=6 P=0.107) In males, while there was no difference in number of leukocytes in the air pouch of wild-type and β2-adrenergic receptor knockouts after administration of vehicle (PBS), there was a dramatic, 3.6-fold, increase in numbers of leukocytes following administration of LPS (wild-type males vs. knock out males P<0.005, Figure 1). The number of peripheral blood leukocytes was significantly lower in knockouts (wild-type males 7.39 ± 0.83×106 n=6 vs. knock out males 4.79 ± 0.79×106 N=6 P=0.048). There was no sex difference in peripheral blood leukocyte counts in either the wild-type (P=0.793) or knockout mice (P=0.603). LPS-induced leukocyte recruitment in the β2-adrenergic receptor knock out male was not significantly different from that in the female (P=0.890, Figure 1).
Figure 1. In vivo effect of LPS on leukocyte recruitment in the air pouch.
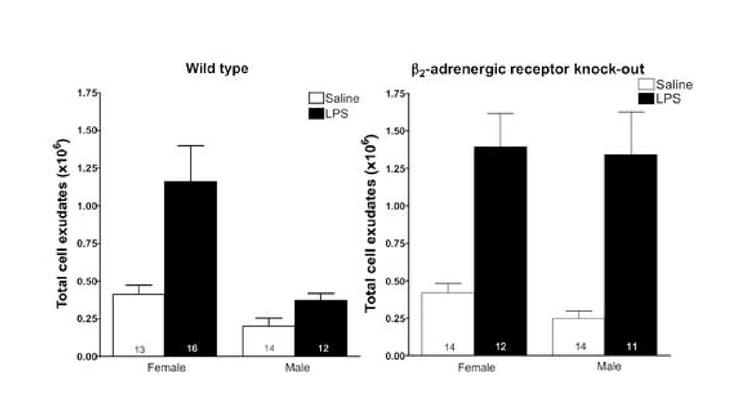
Total number of leukocytes in pouch lavage was determined by flow cytometry, as described in Materials and Methods. LPS elicits leukocyte recruitment in vivo. Sterile PBS (saline), alone, or containing 100 ng/ml LPS was injected into the 6-day murine air pouches of β2-adrenergic receptor knockout and control female and male animals. After 4 hours, exudates were collected and the total number of leukocytes was enumerated by flow cytometry as described in Materials and Methods. Number within bars indicates n.
Characterization of air pouch cellular infiltrate
To characterize the cells that infiltrated the air pouch in response to LPS, exudates were stained with a FITC–labeled anti-neutrophil antibody or with a phycoerythrin (PE) labeled anti-T helper lymphocyte CD4 antibody. Four hours following LPS administration leukocytes were recovered from the pouch. Most of these leukocytes were neutrophils, with a small proportion of lymphocytes; in table inset, Figure 1, the mean values are given for these populations. While the total number of cells recruited into the air pouch was greater in females (Figure 2), for LPS-recruited neutrophils there was no dimorphism in sex (female neutrophil wild-type vs. male neutrophil wild-type P=0.820; female neutrophil knockout vs. male neutrophil knockout P=0.738) or β2-adrenergic receptor (female neutrophil wild-type vs. female neutrophil knockout, P=0.912; male neutrophil wild-type vs. male neutrophil knockout, P=0.411), and for the percentage of LPS-recruited T cells, while there was a significant sex difference in wild types (female CD4 wild-type vs. male CD4 wild-type P=0.043), there was no sex dimorphism in knockouts (female CD4 knockout vs. male CD4 knockout P=0.554) or in β2-adrenergic receptor in wild-type or knockouts (male CD4 wild-type vs. male CD4 knockout P=0.072; female CD4 wild-type vs. female CD4 knockout, P=0.366).
Figure 2. Neutrophil accumulation in air pouch exudates in response to LPS.
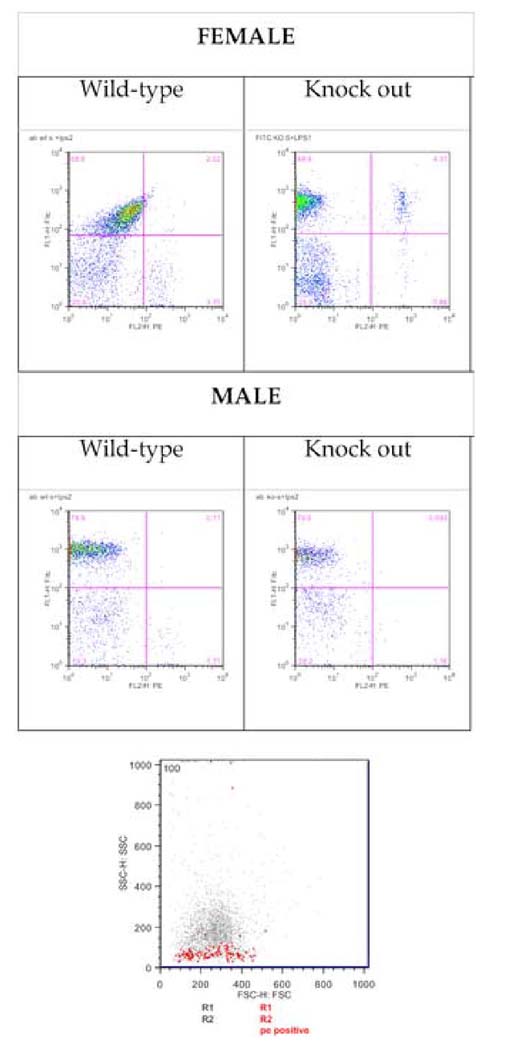
Cells were immunostained with a FITC anti-mouse neutrophil and with staining for T helper lymphocytes, phycoerythrin (PE)-CD4. The four panel part of figure shows typical flow cytometry dot plots; the abscissa shows fluorescence intensity for PE-labeled anti-CD4 and the ordinate shows the fluorescence intensity for FITC-labeled anti-neutrophil antibody. An FSC/SSC plot (bottom panel) shows the PE-labeled CD4 cell population; FSC and SSC were adjusted to include only live and nucleated cells (using propidium iodide and Hoeschst (R1 and R2), respectively). The plot shows PE positive (CD4) cells as red dots, with an SSC indicating a lymphocyte population separated from a population of larger and more dense monocytes and granulocytes.
CD62L and CD11a expressing leukocytes and density of adhesion molecule expression
In responses to LPS-stimulation, CD62L is down-regulated and shed from neutrophils prior to rolling adhesion on the vessel wall (Nakamura et al., 2001). Our data show that CD62L down-regulation did not differ significantly either by sex or between wild-type and β2-adrenergic receptor knock out groups of mice (data not shown). However, in contrast to CD62L, there were both sex– and β2-adrenergic receptor-dependent differences in CD11a cell populations. CD11a is expressed on all leukocytes and is involved in leukocyte adhesion to its ligands playing a role in most immune/inflammatory responses. In wild-type mice there was an almost 3-fold higher number of CD11a+ leukocytes recruited into the air pouch in female compared to male wild-type mice (P<0.05; Figure 3), but this difference was abolished in knock out mice. In knock out males, there was a significant increase (>2-fold increase over wild-type) in the numbers of CD11a+ cells migrating into the pouch (P<0.05; Figure 3); there was no significant difference in number of CD11a+ cells from the air pouch in wild-type and knock out females (P>0.05, Figure 3). Of note, there was a significant decrease in the fluorescent intensity for PE-CD11a in males (P<0.05), but no significant change in PE-CD11a fluorescence intensity in leukocytes from females, and no change for CD62L fluorescent intensity in either sex (Figure 4).
Figure 3. Number of leukocytes expressing adhesion molecules CD62L and CD11a recruited into the air pouch by LPS.
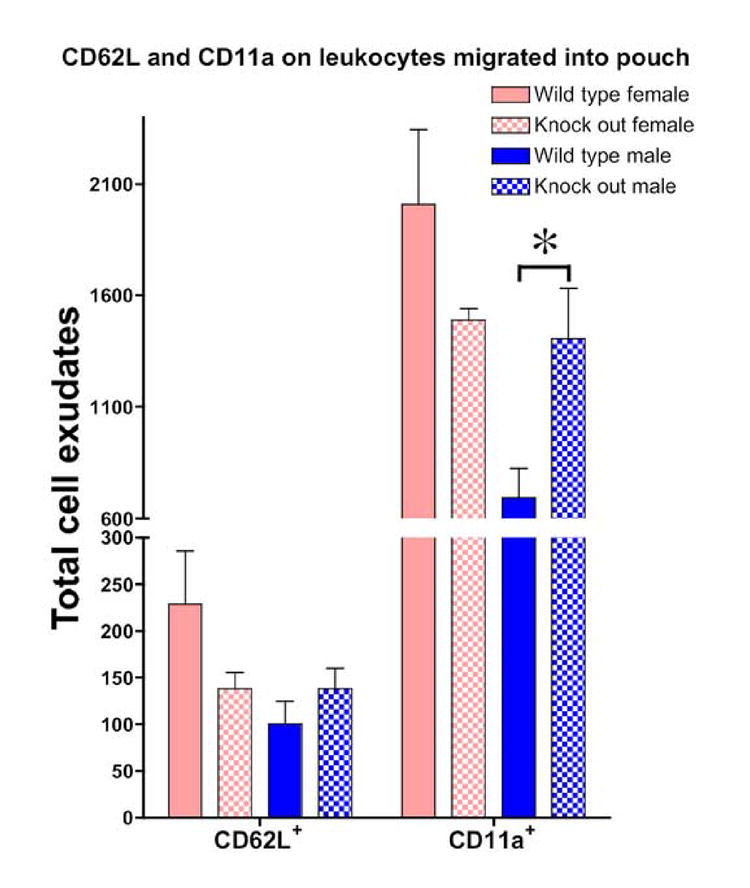
CD62L and CD11a expression on leukocytes found in the air pouch after LPS was injected into the pouch of male and female wild-type or knockout mice (n=6 for each group) ; * indicates P<0.05.
Figure 4. CD11a and CD62L adhesion molecule density as measured by fluorescent intensity on peripheral blood leukocyte.
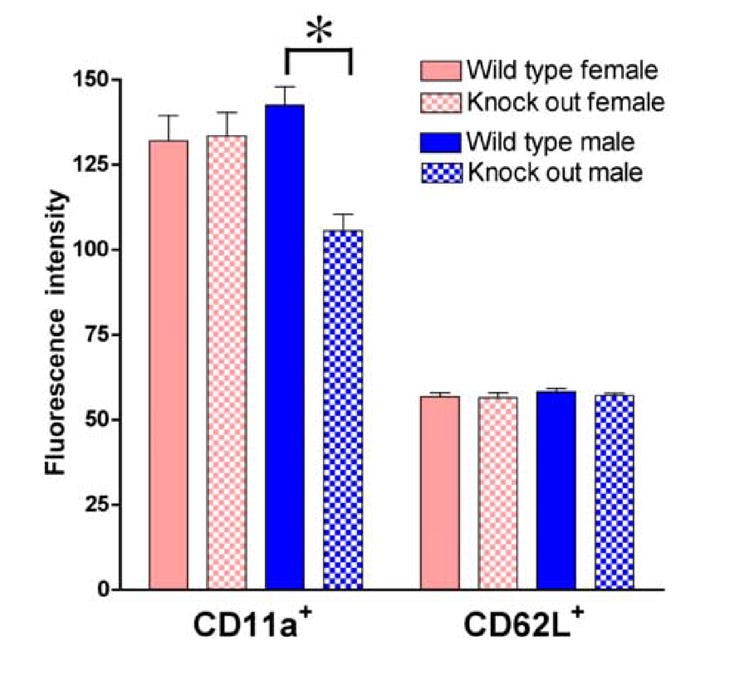
Mean fluorescence intensity significantly less in CD11a knock out males, while there was no change in females. Fluorescence intensity for CD62L was unchanged in knock out male and female mice (n=6 for each group); * indicates P<0.05.
4. Discussion
Using the murine air pouch model to characterize the impact of β2-adrenergic receptor mechanisms in leukocyte recruitment in vivo. In this model, an air pouch is established on the backs mice by injection of sterile air (3ml on day 0 and a further 3 ml on day 3), and into 6-day old pouch LPS (30 ng) is injected to recruit leukocytes that are harvested for flow cytometric analysis. We showed that the markedly greater leukocyte recruitment in females was abolished in β2-adrenergic receptor knockout mice; leukocyte recruitment was markedly enhanced in male but unchanged in female β2-adrenergic receptor knockout mice. There was also an enhancement in the number of CD11a+ leukocytes recruited into the air pouch in β2-adrenergic receptor knockout males, but not females.
In β2-adrenergic receptor knock out female mice there appeared to be an emergence of double-positive (neutrophil 7/4+/CD4+, Figure 1) cells. While the monoclonal 7/4 antibody defines a polymorphic neutrophil differentiation antigen (Hirsch and Gordon, 1983), more recently it has been shown to also weakly recognize immune activated macrophages (Kaposzta et al., 1998) and monocytes although the 7/4 antigen expression is reduced after exposure to LPS (Taylor et al., 2003). This emergence of this double positive 7/4+/CD4+ population in β2-adrenergic receptor knock out female mice may represent a further regulation of cell surface marker by β2-adrenergic receptor in a subpopulation of myeloid cells
Most actions of catecholamines on immune cells are believed to be mediated via β2- (Barnes, 1999), α1- (during chronic inflammation (Roupe van der Voort et al., 2000)), and α2- adrenergic receptors (Straub et al., 2000a). Our study demonstrates an increased LPS-induced neutrophil recruitment in β2-adrenergic receptor knockout male mice suggesting that the constitutive activity of the β2-adrenergic receptor suppresses cell migration. Importantly, β2-adrenergic receptors do not appear to have such a role in females. Our results are consistent with sex differences in host defense mechanisms reported by us, and others (Dina et al., 2001; Green et al., 2001; Majetschak et al., 2000; Spitzer, 1999; St Pierre Schneider et al., 1999). Since catecholamines augment the host response to LPS by inducing the synthesis of the acute phase reactant LPS binding protein, which neutralizes the toxicity of LPS (Black, 2002a), catecholamines are likely to play a key role in modulating the immune response, although it is unknown if this mechanism also occurs in females. In support of this hypothesis, sympathectomy increases recruitment of inflammatory cells into tissues during the innate immune response in male mice (although female mice were not evaluated in this study)(Rice et al., 2002).
While our current study concerned the role of β2-adrenergic receptors, it is possible that α-adrenergic receptors may also play a role in modulating immune cell function. The leukocyte population that we evaluated (i.e. that recruited into the pouch over the first 4 h post LPS) consisted predominantly (~70%) of neutrophil, as these cells are the first to be recruited by an inflammatory stimulus. While there do not appear to be reports describing the presence of α adrenergic receptors on neutrophils in rodents (Altenburg et al., 1997; Madden et al., 1995), α2-adrenergic receptors do appear to exist in low abundance on human neutrophils (Varani et al., 2002). Furthermore, in several species, including mice, other blood cells, notably platelets (Hamilton et al., 1986; Maes et al., 2002; Mishra et al., 1985), macrophages (Garcia et al., 2003; Ignatowski et al., 2000; Javierre et al., 1975; Miles et al., 1996) and lymphocytes (Amenta et al., 2002; Felsner et al., 1995; Veglio et al., 2001), appear to either express α adrenergic receptors or respond to direct action by α-adrenergic receptor agonists. While α-adrenergic receptor agonists may affect neutrophil mobilization, it has been hypothesized that this is due to an indirect effect of α-adrenergic receptor stimulation on lymphocytes to release pro-inflammatory factors such as TNFα which then act on neutrophils and other cells (Altenburg et al., 1997). With the greater involvement of other leukocytes, such as macrophages and monocytes in chronic inflammatory conditions it is possible that there could be a contribution of α-adrenergic receptors in modulating leukocyte function. Of note, estradiol decreases platelet α2-adrenergic receptors number and function (Brodde, 1983; Mishra et al., 1985), and the role of sex steroids in inflammation, which we have previously investigated in other models (Barker et al., 2005; Green et al., 2001; Green et al., 1999a; Green and Levine, 2005) is also an important question. However, to establish any sex steroid contribution to adrenergic mechanisms in leukocyte recruitment, at minimum we would need to perform male and female gonadectomy, sex steroid replacement with testosterone and dihydrotestosterone (to determine the contribution of estradiol) in males, and estradiol and progesterone in females. Future studies will address these key questions.
We also observed an increase in the number of CD11a+ cells in β2-adrenergic knock out males compared to wild-type males (no change was observed in females). This increase in CD11a+ may, at least in part, contribute to the enhanced recruitment of leukocytes in β2-adrenergic knock out males since CD11a is an important adhesion molecule facilitating migration of leukocytes into sites of inflammation (Parkos, 1997). While there is evidence that CD11a is regulated by adrenergic mechanisms (e.g. stress alters CD11a expression in leukocytes (Kuhlwein et al., 2001; Mills et al., 2002b)), to the best of our knowledge, sex differences in this relationship have not previously been reported.
Our data provide further evidence for a close relationship between the adrenergic system and the immune system, and adds a crucial finding of a marked sex dimorphism in this relationship. We have previously shown that chronic stress leads to a significant enhancement in neutrophil recruitment to LPS in male but not in female rats; this effect appeared to be β-adrenergic– and male sex steroid–dependent (Barker et al., 2005). This, as well as another study showing that high β2-adrenergic agonist levels produced during chronic stress in male mice increase neutrophil recruitment (Forsythe et al., 2004), appear to contrast with the current study in which there was an enhancement of leukocyte migration in β2-adrenergic knockout males. This difference may be related to chronic stress down regulating β2-adrenergic receptor density, as has been shown to occur on lymphocytes (Miller and Chen, 2006; Mills et al., 2004), or because the β2-adrenergic receptor gene deletion during embryogenesis may produce adaptive compensatory changes resulting in unexpected effects. This limitation on the use of knock out animals notwithstanding, the key finding of this study remains, namely the importance of β2-adrenergic–dependent mechanisms in leukocytes from male mice. In addition, age-related changes in β2-adrenergic receptor expression could contribute to differences in results from previous studies. Age-related changes in the expression of β2-adrenergic receptors have been reported, but are conflicting. β2- adrenergic receptor expression has exhibited age-related reduction (on human (Feldman et al., 1984) and murine (Kohno et al., 1986) lymphocytes), no change (on human (Landmann et al., 1981) or rat (De Blasi et al., 1987) monocytes and on human (Abrass and Scarpace, 1981) or rat (Pieri, 1991) lymphocytes), and increase (on human lymphocytes (Fitzgerald et al., 1984; Gietzen et al., 1989; O’Hara et al., 1985) and human (Pende et al., 1991) or murine (Vanscheeuwijck et al., 1990) monocytes).
This study has particular relevance in understanding sex differences in inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus, which have a markedly greater incidence in women, and in sub-acute stress such as trauma following burn injury in which women have a 50% increased risk of death when compared with men (Kerby et al., 2006). This sexual dimorphism is dependent not only on sex steroids but also on the hypothalamic–pituitary–adrenal and sympathoadrenal stress axes (Black, 2002b; Cutolo et al., 2003; Da Silva, 1999; Gaillard and Spinedi, 1998; Green et al., 1999b; Spinedi et al., 1997). Our data not only suggests novel relationships between sex, the β2-adrenergic receptor, leukocyte adhesion molecules and migration, but also suggests novel targets for therapeutic intervention in inflammatory diseases.
Table 1. Percent of neutrophils and CD4+ cells determined after counting 5,000 cells.
Four hours after challenge in the air pouch, neutrophils predominate among cells recovered from wild-type and β2-adrenergic receptor knockout mice. Data are presented as means ± SEM (n, number of mice)
| LPS-induced leukocyte population in air pouch exudate (%) | ||||||
|---|---|---|---|---|---|---|
| Wild-type | Knock out | |||||
| NEUTROPHILS
(FITC-neutrophil) |
T-HELPER
(PE-CD4) |
n | NEUTROPHILS
(FITC-neutrophil) |
T-HELPER
(PE-CD)4 |
n | |
| Female | 70.44±3.80 | 3.18±0.74 | 8 | 69.60±7.33 | 4.45±1.24 | 5 |
| Male | 71.66±3.26 | 1.11±0.34 | 6 | 66.55±5.21 | 3.39±1.17 | 5 |
Acknowledgments
This study was supported by a NIH R01AR05210.
Abbreviations
- LPS
lipopolysaccharide
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- PBS
phosphate buffered saline
- BSA
Bovine serum albumin
- ANOVA
analysis of variance
- N.S.
not significant
- SEM
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrass IB, Scarpace PJ. Human lymphocyte beta-adrenergic receptors are unaltered with age. J Gerontol. 1981;36:298–301. doi: 10.1093/geronj/36.3.298. [DOI] [PubMed] [Google Scholar]
- Ajuebor MN, Swain MG, Perretti M. Chemokines as novel therapeutic targets in inflammatory diseases. Biochem Pharmacol. 2002;63:1191–1196. doi: 10.1016/s0006-2952(02)00854-7. [DOI] [PubMed] [Google Scholar]
- Altenburg SP, Martins MA, Silva AR, Cordeiro RS, Castro-Faria-Neto HC. LPS-induced blood neutrophilia is inhibited by alpha 1-adrenoceptor antagonists: a role for catecholamines. J Leukoc Biol. 1997;61:689–694. doi: 10.1002/jlb.61.6.689. [DOI] [PubMed] [Google Scholar]
- Amenta F, El-Assouad D, Mignini F, Ricci A, Tayebati SK. Neurotransmitter receptor expression by peripheral mononuclear cells: possible marker of neuronal damage by exposure to radiations. Cell Mol Biol (Noisy-le-grand) 2002;48:415–421. [PubMed] [Google Scholar]
- Ansar Ahmed S, Hissong BD, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E. Gender and risk of autoimmune diseases: possible role of estrogenic compounds. Environ Health Perspect. 1999;107(Suppl 5):681–686. doi: 10.1289/ehp.99107s5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker LA, Dazin PF, Levine JD, Green PG. Sympathoadrenal-dependent sexually dimorphic effect of nonhabituating stress on in vivo neutrophil recruitment in the rat. Br J Pharmacol. 2005;145:872–879. doi: 10.1038/sj.bjp.0706257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Beta-adrenergic receptors and their regulation. Am J Respir Crit Care Med. 1995;152:838–860. doi: 10.1164/ajrccm.152.3.7663795. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Effect of beta-agonists on inflammatory cells. J Allergy Clin Immunol. 1999;104:S10–17. doi: 10.1016/s0091-6749(99)70269-1. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Schedlowski M, Wienecke H, Jacobs R, Schmidt RE. Adrenergic control of natural killer cell circulation and adhesion. Brain Behav Immun. 1997;11:321–332. doi: 10.1006/brbi.1997.0499. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Nawroth PP. Linking stress to inflammation. Anesthesiol Clin. 2006;24:325–340. doi: 10.1016/j.atc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc Natl Acad Sci U S A. 2002;99:4067–4072. doi: 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav Immun. 2002a;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behavior and Immunity. 2002b;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Brodde OE. Endogenous and exogenous regulation of human alpha- and beta-adrenergic receptors. J Recept Res. 1983;3:151–162. doi: 10.3109/10799898309041930. [DOI] [PubMed] [Google Scholar]
- Castagnetta L, Granata OM, Traina A, Cocciadiferro L, Saetta A, Stefano R, Cutolo M, Carruba G. A role for sex steroids in autoimmune diseases: a working hypothesis and supporting data. Ann N Y Acad Sci. 2002;966:193–203. doi: 10.1111/j.1749-6632.2002.tb04215.x. [DOI] [PubMed] [Google Scholar]
- Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–16700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- Cobelens PM, Kavelaars A, Vroon A, Ringeling M, van der Zee R, van Eden W, Heijnen CJ. The beta 2-adrenergic agonist salbutamol potentiates oral induction of tolerance, suppressing adjuvant arthritis and antigen-specific immunity. J Immunol. 2002;169:5028–5035. doi: 10.4049/jimmunol.169.9.5028. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Sulli A, Pizzorni C, Craviotto C, Straub RH. Hypothalamic-pituitary-adrenocortical and gonadal functions in rheumatoid arthritis. In: Sternberg EM, Haour FG, Smith CC, editors. Neuroendocrine and Neural Regulation of Autoimmune and Inflammatory Disease: Molecular, Systems, and Clinical Insights, Annals of the New York Academy of Sciences. Vol. 992. New York Academy of Sciences; New York: 2003. pp. 107–117. [DOI] [PubMed] [Google Scholar]
- Da Silva JA. Sex hormones, glucocorticoids and autoimmunity: facts and hypotheses. Ann Rheum Dis. 1995;54:6–16. doi: 10.1136/ard.54.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva JAP. Sex hormones and glucocorticoids: Interactions with the immune system. In: Cutolo M, editor. Neuroendocrine Immune Basis of the Rheumatic Diseases, Annals of the New York Academy of Sciences. Vol. 876. New York Academy of Sciences; New York: 1999. pp. 102–118. [DOI] [PubMed] [Google Scholar]
- De Blasi A, Fratelli M, Wielosz M, Lipartiti M. Regulation of beta adrenergic receptors on rat mononuclear leukocytes by stress: receptor redistribution and down-regulation are altered with aging. J Pharmacol Exp Ther. 1987;240:228–233. [PubMed] [Google Scholar]
- de Coupade C, Gear RW, Dazin PF, Sroussi HY, Green PG, Levine JD. beta2-Adrenergic receptor regulation of human neutrophil function is sexually dimorphic. Br J Pharmacol. 2004;143:1033–1041. doi: 10.1038/sj.bjp.0705972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. Stress-induced augmentation of immune function-The role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- Dina OA, Aley KO, Isenberg W, Messing RO, Levine JD. Sex hormones regulate the contribution of PKCepsilon and PKA signalling in inflammatory pain in the rat. Eur J Neurosci. 2001;13:2227–2233. doi: 10.1046/j.0953-816x.2001.01614.x. [DOI] [PubMed] [Google Scholar]
- Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–324. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Feldman RD, Limbird LE, Nadeau J, Robertson D, Wood AJ. Alterations in leukocyte beta-receptor affinity with aging. A potential explanation for altered beta-adrenergic sensitivity in the elderly. N Engl J Med. 1984;310:815–819. doi: 10.1056/NEJM198403293101303. [DOI] [PubMed] [Google Scholar]
- Felsner P, Hofer D, Rinner I, Porta S, Korsatko W, Schauenstein K. Adrenergic suppression of peripheral blood T cell reactivity in the rat is due to activation of peripheral alpha 2-receptors. J Neuroimmunol. 1995;57:27–34. doi: 10.1016/0165-5728(94)00158-k. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D, Doyle V, Kelly JG, O’Malley K. Cardiac sensitivity to isoprenaline, lymphocyte beta-adrenoceptors and age. Clin Sci (Lond) 1984;66:697–699. doi: 10.1042/cs0660697. [DOI] [PubMed] [Google Scholar]
- Forsythe P, Ebeling C, Gordon JR, Befus AD, Vliagoftis H. Opposing effects of short- and long-term stress on airway inflammation. Am J Respir Crit Care Med. 2004;169:220–226. doi: 10.1164/rccm.200307-979OC. [DOI] [PubMed] [Google Scholar]
- Gaillard RC, Spinedi E. Sex- and stress-steroids interactions and the immune system: Evidence for a neuroendocrine-immunological sexual dimorphism. Domestic Animal Endocrinology. 1998;15:345–352. doi: 10.1016/s0739-7240(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Garcia JJ, del Carmen Saez M, De la Fuente M, Ortega E. Regulation of phagocytic process of macrophages by noradrenaline and its end metabolite 4-hydroxy-3-metoxyphenyl-glycol. Role of alpha- and beta-adrenoreceptors. Mol Cell Biochem. 2003;254:299–304. doi: 10.1023/a:1027345820519. [DOI] [PubMed] [Google Scholar]
- Gietzen DW, Fregeau D, Goodman T, Weiler PG, Graf K, Magliozzi J. Lymphocyte beta-adrenoceptor/effector complex in aging and dementia of the Alzheimer type. Alzheimer Dis Assoc Disord. 1989;3:132–142. doi: 10.1097/00002093-198903030-00002. [DOI] [PubMed] [Google Scholar]
- Goulding NJ, Euzger HS, Butt SK, Perretti M. Novel pathways for glucocorticoid effects on neutrophils in chronic inflammation. Inflamm Res. 1998;47(Suppl3):S158–165. doi: 10.1007/s000110050310. [DOI] [PubMed] [Google Scholar]
- Green MS. The male predominance in the incidence of infectious diseases in children: a postulated explanation for disparities in the literature. Int J Epidemiol. 1992;21:381–386. doi: 10.1093/ije/21.2.381. [DOI] [PubMed] [Google Scholar]
- Green PG, Dahlqvist SR, Isenberg WM, Miao FJ, Levine JD. Role of adrenal medulla in development of sexual dimorphism in inflammation. Eur J Neurosci. 2001;14:1436–1444. doi: 10.1046/j.0953-816x.2001.01768.x. [DOI] [PubMed] [Google Scholar]
- Green PG, Dahlqvist SR, Isenberg WM, Strausbaugh HJ, Miao FJ, Levine JD. Sex steroid regulation of the inflammatory response: sympathoadrenal dependence in the female rat. J Neurosci. 1999a;19:4082–4089. doi: 10.1523/JNEUROSCI.19-10-04082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Dahlqvist SR, Isenberg WM, Strausbaugh HJ, Miao FJP, Levine JD. Sex steroid regulation of the inflammatory response: Sympathoadrenal dependence in the female rat. Journal of Neuroscience. 1999b;19:4082–4089. doi: 10.1523/JNEUROSCI.19-10-04082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PG, Levine JD. Sexual dimorphism in the effect of nonhabituating stress on neurogenic plasma extravasation. 2005 doi: 10.1111/j.1460-9568.2005.03872.x. [DOI] [PubMed] [Google Scholar]
- Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1alpha-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med. 1999;189:1923–1930. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CA, Deighton NM, Jones CR, Reid JL. Changes in rabbit platelet alpha and beta adrenoceptor number and platelet aggregation. Eur J Pharmacol. 1986;130:145–149. doi: 10.1016/0014-2999(86)90195-0. [DOI] [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clin Exp Pharmacol Physiol. 1999;26:122–126. doi: 10.1046/j.1440-1681.1999.02995.x. [DOI] [PubMed] [Google Scholar]
- Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- Ignatowski TA, Kunkel SL, Spengler RN. Interactions between the alpha(2)-adrenergic and the prostaglandin response in the regulation of macrophage-derived tumor necrosis factor. Clin Immunol. 2000;96:44–51. doi: 10.1006/clim.2000.4877. [DOI] [PubMed] [Google Scholar]
- Javierre MQ, Pinto LV, Lima AO, Sassine WA. Immunologic phagocytosis by macrophages: effect by stimulation of alpha adrenergic receptors. Rev Bras Pesqui Med Biol. 1975;8:271–274. [PubMed] [Google Scholar]
- Kaposzta R, Tree P, Marodi L, Gordon S. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4-deficient mice: role of macrophages in host defense against Candida albicans. Infect Immun. 1998;66:1708–1717. doi: 10.1128/iai.66.4.1708-1717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- Kerby JD, McGwin G, Jr, George RL, Cross JA, Chaudry IH, Rue LW., 3rd Sex differences in mortality after burn injury: results of analysis of the National Burn Repository of the American Burn Association. J Burn Care Res. 2006;27:452–456. doi: 10.1097/01.BCR.0000225957.01854.EE. [DOI] [PubMed] [Google Scholar]
- Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79:1093–1104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- Kohno A, Cinader B, Seeman P. Age-related changes in beta-adrenoceptors of lymphocytes. Immunol Lett. 1986;13:79–82. doi: 10.1016/0165-2478(86)90130-6. [DOI] [PubMed] [Google Scholar]
- Krieg RJ, Jr, Thorner MO, Evans WS. Sex differences in beta-adrenergic stimulation of growth hormone secretion in vitro. Endocrinology. 1986;119:1339–1342. doi: 10.1210/endo-119-3-1339. [DOI] [PubMed] [Google Scholar]
- Kuhlwein EC, Irwin MR, Ziegler MG, Woods VL, Kennedy B, Mills PJ. Propranolol affects stress-induced leukocytosis and cellular adhesion molecule expression. Eur J Appl Physiol. 2001;86:135–141. doi: 10.1007/s00421-001-0526-8. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y, Shinkai S, Torii J, Hino S, Shek PN. Exercise-induced changes in the expression of surface adhesion molecules on circulating granulocytes and lymphocytes subpopulations. Eur J Appl Physiol Occup Physiol. 1995;71:245–252. doi: 10.1007/BF00854986. [DOI] [PubMed] [Google Scholar]
- Landmann R, Bittiger H, Buhler FR. High affinity beta-2-adrenergic receptors in mononuclear leucocytes: similar density in young and old normal subjects. Life Sci. 1981;29:1761–1771. doi: 10.1016/0024-3205(81)90186-7. [DOI] [PubMed] [Google Scholar]
- Landmann RM, Muller FB, Perini C, Wesp M, Erne P, Buhler FR. Changes of immunoregulatory cells induced by psychological and physical stress: relationship to plasma catecholamines. Clin Exp Immunol. 1984;58:127–135. [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ridefelt P, Hakansson L, Venge P. Regulation of human eosinophil migration across lung epithelial monolayers by distinct calcium signaling mechanisms in the two cell types. J Immunol. 1999;163:5649–5655. [PubMed] [Google Scholar]
- Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- Maes M, Van Gastel A, Delmeire L, Kenis G, Bosmans E, Song C. Platelet alpha2-adrenoceptor density in humans: relationships to stress-induced anxiety, psychasthenic constitution, gender and stress-induced changes in the inflammatory response system. Psychol Med. 2002;32:919–928. doi: 10.1017/s0033291702005925. [DOI] [PubMed] [Google Scholar]
- Maestroni GJ. Sympathetic nervous system influence on the innate immune response. Ann N Y Acad Sci. 2006;1069:195–207. doi: 10.1196/annals.1351.017. [DOI] [PubMed] [Google Scholar]
- Majetschak M, Christensen B, Obertacke U, Waydhas C, Schindler AE, Nast-Kolb D, Schade FU. Sex differences in posttraumatic cytokine release of endotoxin-stimulated whole blood: relationship to the development of severe sepsis. J Trauma. 2000;48:832–839. doi: 10.1097/00005373-200005000-00006. discussion 839-840. [DOI] [PubMed] [Google Scholar]
- Miles BA, Lafuse WP, Zwilling BS. Binding of alpha-adrenergic receptors stimulates the anti-mycobacterial activity of murine peritoneal macrophages. J Neuroimmunol. 1996;71:19–24. doi: 10.1016/s0165-5728(96)00113-0. [DOI] [PubMed] [Google Scholar]
- Miles MP, Leach SK, Kraemer WJ, Dohi K, Bush JA, Mastro AM. Leukocyte adhesion molecule expression during intense resistance exercise. J Appl Physiol. 1998;84:1604–1609. doi: 10.1152/jappl.1998.84.5.1604. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta2-adrenergic receptor in children with asthma. Proc Natl Acad Sci U S A. 2006;103:5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills PJ, Adler KA, Dimsdale JE, Perez CJ, Ziegler MG, Ancoli-Israel S, Patterson TL, Grant I. Vulnerable caregivers of Alzheimer disease patients have a deficit in beta 2-adrenergic receptor sensitivity and density. Am J Geriatr Psychiatry. 2004;12:281–286. [PubMed] [Google Scholar]
- Mills PJ, Farag NH, Perez C, Dimsdale JE. Peripheral blood mononuclear cell CD62L and CD11a expression and soluble interstitial cell adhesion molecule-1 levels following infused isoproterenol in hypertension. J Hypertens. 2002a;20:311–316. doi: 10.1097/00004872-200202000-00022. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Perez CJ, Adler KA, Ziegler MG. The effects of spaceflight on adrenergic receptors and agonists and cell adhesion molecule expression. J Neuroimmunol. 2002b;132:173–179. doi: 10.1016/s0165-5728(02)00313-2. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Ziegler MG, Nelesen RA, Kennedy BP. The effects of the menstrual cycle, race, and gender on adrenergic receptors and agonists. Clin Pharmacol Ther. 1996;60:99–104. doi: 10.1016/S0009-9236(96)90172-1. [DOI] [PubMed] [Google Scholar]
- Mishra N, Hamilton CA, Jones CR, Leslie C, Reid JL. Alpha-adrenoceptor changes after oestrogen treatment in platelets and other tissues in female rabbits. Clin Sci (Lond) 1985;69:235–238. doi: 10.1042/cs0690235. [DOI] [PubMed] [Google Scholar]
- Mohr W, Menninger H. Polymorphonuclear granulocytes at the pannus-cartilage junction in rheumatoid arthritis. Arthritis Rheum. 1980;23:1413–1414. doi: 10.1002/art.1780231224. [DOI] [PubMed] [Google Scholar]
- Nakamura H, De Rosa SC, Yodoi J, Holmgren A, Ghezzi P, Herzenberg LA. Chronic elevation of plasma thioredoxin: Inhibition of chemotaxis and curtailment of life expectancy in AIDS. Proc Natl Acad Sci U S A. 2001;98:2688–2693. doi: 10.1073/pnas.041624998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara N, Daul AE, Fesel R, Siekmann U, Brodde OE. Different mechanisms underlying reduced beta 2-adrenoceptor responsiveness in lymphocytes from neonates and old subjects. Mech Ageing Dev. 1985;31:115–122. doi: 10.1016/s0047-6374(85)80022-1. [DOI] [PubMed] [Google Scholar]
- O’Leary EC, Marder P, Zuckerman SH. Glucocorticoid effects in an endotoxin-induced rat pulmonary inflammation model: differential effects on neutrophil influx, integrin expression, and inflammatory mediators. Am J Respir Cell Mol Biol. 1996;15:97–106. doi: 10.1165/ajrcmb.15.1.8679228. [DOI] [PubMed] [Google Scholar]
- Oberbeck R. Catecholamines: physiological immunomodulators during health and illness. Curr Med Chem. 2006;13:1979–1989. doi: 10.2174/092986706777584997. [DOI] [PubMed] [Google Scholar]
- Parkos CA. Cell adhesion and migration. I. Neutrophil adhesive interactions with intestinal epithelium. Am J Physiol. 1997;273:G763–768. doi: 10.1152/ajpgi.1997.273.4.G763. [DOI] [PubMed] [Google Scholar]
- Pende A, Musso NR, Vergassola C, Ioverno A, Galbariggi G, Lotti G. Absence of correlations between plasma catecholamine levels and mononuclear leukocyte beta 2-adrenergic receptors in the elderly. Biomed Pharmacother. 1991;45:383–386. doi: 10.1016/0753-3322(91)90002-b. [DOI] [PubMed] [Google Scholar]
- Pieri C. Food restriction slows down age-related changes in cell membrane parameters. Ann N Y Acad Sci. 1991;621:353–362. doi: 10.1111/j.1749-6632.1991.tb16991.x. [DOI] [PubMed] [Google Scholar]
- Rice PA, Boehm GW, Moynihan JA, Bellinger DL, Stevens SY. Chemical sympathectomy increases numbers of inflammatory cells in the peritoneum early in murine listeriosis. Brain Behav Immun. 2002;16:654–662. doi: 10.1016/s0889-1591(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Roupe van der Voort C, Heijnen CJ, Wulffraat N, Kuis W, Kavelaars A. Stress induces increases in IL-6 production by leucocytes of patients with the chronic inflammatory disease juvenile rheumatoid arthritis: a putative role for alpha(1)-adrenergic receptors. J Neuroimmunol. 2000;110:223–229. doi: 10.1016/s0165-5728(00)00328-3. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Kasprowicz DJ, Swanson-Mungerson MA, Podojil JR, Kohm AP. Adaptive immunity in mice lacking the beta(2)-adrenergic receptor. Brain Behav Immun. 2003;17:55–67. doi: 10.1016/s0889-1591(02)00056-9. [DOI] [PubMed] [Google Scholar]
- Santos DR, Calixto JB, Souza GE. Effect of a kinin B2 receptor antagonist on LPS- and cytokine-induced neutrophil migration in rats. Br J Pharmacol. 2003;139:271–278. doi: 10.1038/sj.bjp.0705236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekut L, Champion BR, Page K, Menius JA, Jr, Connolly KM. Anti-inflammatory activity of salmeterol: down-regulation of cytokine production. Clin Exp Immunol. 1995;99:461–466. doi: 10.1111/j.1365-2249.1995.tb05573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RJ. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. 2003;33:261–284. doi: 10.2165/00007256-200333040-00002. [DOI] [PubMed] [Google Scholar]
- Spinedi E, Chisari A, Pralong F, Gaillard RC. Sexual dimorphism in the mouse hypothalamic-pituitary-adrenal axis function after endotoxin and insulin stresses during development. Neuroimmunomodulation. 1997;4:77–83. doi: 10.1159/000097324. [DOI] [PubMed] [Google Scholar]
- Spitzer JA. Gender differences in some host defense mechanisms. Lupus. 1999;8:380–383. doi: 10.1177/096120339900800510. [DOI] [PubMed] [Google Scholar]
- St Pierre Schneider B, Correia LA, Cannon JG. Sex differences in leukocyte invasion in injured murine skeletal muscle. Res Nurs Health. 1999;22:243–250. doi: 10.1002/(sici)1098-240x(199906)22:3<243::aid-nur6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Straub RH, Linde HJ, Mannel DN, Scholmerich J, Falk W. A bacteria-induced switch of sympathetic effector mechanisms augments local inhibition of TNF-alpha and IL-6 secretion in the spleen. Faseb J. 2000a;14:1380–1388. doi: 10.1096/fj.14.10.1380. [DOI] [PubMed] [Google Scholar]
- Straub RH, Mayer M, Kreutz M, Leeb S, Scholmerich J, Falk W. Neurotransmitters of the sympathetic nerve terminal are powerful chemoattractants for monocytes. J Leukoc Biol. 2000b;67:553–558. doi: 10.1002/jlb.67.4.553. [DOI] [PubMed] [Google Scholar]
- Straub RH, Westermann J, Scholmerich J, Falk W. Dialogue between the CNS and the immune system in lymphoid organs. Immunol Today. 1998;19:409–413. doi: 10.1016/s0167-5699(98)01297-3. [DOI] [PubMed] [Google Scholar]
- Tan KS, McFarlane LC, Lipworth BJ. Effect of exogenous female sex-steroid hormones on beta 2-adrenoceptors in healthy males. Eur J Clin Pharmacol. 1997;52:281–283. doi: 10.1007/s002280050290. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 2003;33:2090–2097. doi: 10.1002/eji.200324003. [DOI] [PubMed] [Google Scholar]
- Vanscheeuwijck P, Van de Velde E, Fraeyman N. Characterization of the beta-adrenergic transduction system in spleen mononuclear leukocyte membranes of young and senescent rats. Biochem Pharmacol. 1990;39:2035–2040. doi: 10.1016/0006-2952(90)90626-v. [DOI] [PubMed] [Google Scholar]
- Varani K, Gessi S, Merighi S, Iannotta V, Cattabriga E, Spisani S, Cadossi R, Borea PA. Effect of low frequency electromagnetic fields on A2A adenosine receptors in human neutrophils. Br J Pharmacol. 2002;136:57–66. doi: 10.1038/sj.bjp.0704695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglio F, Tayebati SK, Schiavone D, Ricci A, Mulatero P, Bronzetti E, Rabbia F, Amenta F. Alpha1-adrenergic receptor subtypes in peripheral blood lymphocytes of essential hypertensives. J Hypertens. 2001;19:1847–1854. doi: 10.1097/00004872-200110000-00020. [DOI] [PubMed] [Google Scholar]
- Wahle M, Neumann RP, Moritz F, Krause A, Buttgereit F, Baerwald CG. Beta2-adrenergic receptors mediate the differential effects of catecholamines on cytokine production of PBMC. J Interferon Cytokine Res. 2005;25:384–394. doi: 10.1089/jir.2005.25.384. [DOI] [PubMed] [Google Scholar]
- Wheeldon NM, Newnham DM, Coutie WJ, Peters JA, McDevitt DG, Lipworth BJ. Influence of sex-steroid hormones on the regulation of lymphocyte beta 2-adrenoceptors during the menstrual cycle. Br J Clin Pharmacol. 1994;37:583–588. doi: 10.1111/j.1365-2125.1994.tb04308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol. 2003;285:L32–42. doi: 10.1152/ajplung.00390.2002. [DOI] [PubMed] [Google Scholar]
- Wilder RL, Calandra GB, Garvin AJ, Wright KD, Hansen CT. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982;25:1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]


