Abstract
Access to low-cost, effective diagnosis for leptospirosis is urgently needed in developing countries. The EIE-IgM-Leptospirose, a kit produced for public health laboratories in Brazil, was shown to have a sensitivity of 76% (77 of 102 patients) and 100% (102 of 102 patients) during acute and convalescent-phase leptospirosis respectively and a specificity of 93%–100% (total healthy and patient control subjects evaluated, 486). These findings indicate that the assay will be useful for diagnosis of this emerging infectious disease in Brazil and other developing countries.
Keywords: Leptospirosis, IgM ELISA, diagnosis, epidemics, urban, serovar, Leptospira, Copenhageni, Brazil
Leptospirosis is an emerging infectious disease due to large recent outbreaks in developed and developing countries (Bharti et al., 2003, Levett, 2001, McBride et al., 2005). Infection with Leptospira spirochetes produces a spectrum of disease presentations ranging from a mild subclinical infection to severe forms, such as Weil’s disease and severe pulmonary haemorrhage syndrome, associated with high mortality (5–50%) (Bharti et al., 2003, McBride et al., 2005). More than 500,000 cases of leptospirosis are estimated to occur worldwide each year (WHO, 1999). However these numbers significantly underestimate the true disease burden since (i) leptospirosis often goes unrecognized because of its non-specific presentation (Johnson et al., 2004, Murdoch et al., 2004, Russell et al., 2003, Segura et al., 2005), being misdiagnosed as dengue (Flannery et al., 2001, Karande et al., 2005, LaRocque et al., 2005), malaria (Ellis et al., 2006, Wongsrichanalai et al., 2003) or other causes of acute febrile illness, and (ii) standard diagnosis is based on antiquated methods, the microscopic agglutination test (MAT) and culture isolation, which are performed in few reference laboratories worldwide (Faine et al., 1999, WHO, 2003). Whole Leptospira-based serologic tests in ELISA and rapid formats are commercially-available and have a sensitivity of 28–72% and 75–94% to detect acute and convalescent-phase illness respectively (Bajani et al., 2003, Effler et al., 2000, Levett and Branch, 2002, Sehgal et al., 2003, Smits et al., 2001a, Smits et al., 2001b, Vijayachari et al., 2002). Limited access to effective diagnosis, especially in developing countries where the disease burden is greatest, is a major cause of under-reporting, which in turn has contributed to the perception of leptospirosis as a neglected disease.
In Brazil, epidemics of severe leptospirosis occur annually in favelas (slum communities) in all major urban centres during seasonal periods of heavy rainfall (Ko et al., 1999, Romero et al., 2003, Tassinari et al., 2004). More than 10,000 suspected cases are reported each year, yet less than 25% are laboratory-confirmed (SUS, Brazilian Health Ministry, http://www.saude.gov.br). In an initiative to reduce costs associated with diagnostic testing, decentralize diagnosis and improve case confirmation, Bio-Manguinhos, the vaccine and diagnostic test production facility of the Brazilian Ministry of Health, developed in 2002 a whole Leptospira-based IgM ELISA, EIE-IgM-Leptospirose which it now manufactures for use in public health laboratory and health care system. Herein we report the findings of a study performed to evaluate the performance of the kit during surveillance for urban leptospirosis.
During the study period (1996 to 1999) sera was collected from over 1,000 patients hospitalized during the urban epidemics of leptospirosis that occur regularly in the city of Salvador, Brazil. Of the 393 patients with paired serum samples, 102 were randomly selected for the evaluation. Case confirmation was defined as patients with a clinical suspicion of leptospirosis and (i) isolation of Leptospira spp. from a clinical sample or (ii) a fourfold or more rise in MAT titres between acute and convalescent serum samples or (iii) a single serum sample with a MAT titre of ≥800. Acute phase samples were collected from individuals between 2–19 days (on average 6 days) after onset of illness. Convalescent phase samples were collected between 18–194 days (on average 29 days) after onset of illness and after discharge of the patients from the hospital.
Sera were collected from 348 healthy individuals and 138 patients diagnosed with an illness other than leptospirosis. Of the healthy samples, 58 were randomly selected from a serum bank of 1,400 collected during a seroprevalence study of infectious disease in a leptospirosis endemic region of Salvador during 1998. A total of 184 samples were collected from individuals living in close proximity to neighbours diagnosed with severe leptospirosis during an epidemic in Salvador during 2001 (hyper-endemic group). Sera from a further 46 healthy blood bank donors resident in Salvador and 60 from healthy individuals resident in California, an area with a low incidence of leptospirosis, were included in the evaluation. The sera from the non-leptospirosis disease categories included patients from the endemic region for leptospirosis in Salvador, Brazil with viral hepatitis (HBsAg positive), dengue (IgM ELISA positive), Lyme disease (ELISA and immunoblot confirmed), syphilis (VDRL positive) and patients with an initial clinical suspicion of leptospirosis but who were subsequently diagnosed with another illness. To evaluate the duration of the IgM antibody response to severe leptospirosis, sera from 64 individuals were collected up to 5 years after the original illness.
The MAT was performed using a standard procedure (Cole et al., 1973) to determine the presence of antibodies to Leptospira antigens using a battery of 12 strains representing eight serogroups (Autumnalis, Ballum, Canicola, Cynopteri, Grippotyphosa, Hurstbridge, Icterohaemorrhagiae and Semaranga). A reduced battery of reference strains were used since previous studies at the study site (Ko et al., 1999) found that all agglutination reactions observed in patient samples from the study site were directed against strains represented by the eight serogroups.
The EIE-IgM-Leptospirose ELISA kit is based on a whole-cell antigen extract obtained from a clinical isolate of L. interrogans serovar Copenhageni. The strain was cultured in EMJH at 30°C (WHO, 2003) and the pellet recovered and washed 3× in PBS (6,000 g). Sonicated antigen preparations, containing phenylmethylsulphonylfluoride (1 mM), were adsorbed to polystyrene flat-bottomed 8-well microtitre strips (500 μg/well). A kit calibrator sample is provided for quality control purposes and calculation of a cut-off threshold that is used to determine positive reactions. The assay is performed with diluted patient serum samples according to the manufacturer’s instructions (http://www.bio.fiocruz.br). Detection of bound anti-human IgM antibody conjugated to horseradish peroxidase is determined by measuring OD450 after incubation with 3,3′,5,5′-tetramethylbenzidine substrate. An index ratio was determined by dividing the OD450 value for the test sample by 2.3 times the OD450 value of the kit’s calibrator sample. A positive reaction was defined as an index ratio >1.0. The evaluation was performed in a blinded manner by a research centre (Gonçalo Moniz Research Centre) of the Brazilian Ministry of Health, which is autonomous from the kit’s manufacturer. The study protocol was approved by the IRB committees of the Oswaldo Cruz Foundation and New York Presbyterian Hospital.
The sensitivity of the IgM-ELISA was defined as the proportion of samples from confirmed cases of leptospirosis that were positive by ELISA. Specificity was defined as the proportion of samples for each control group which were negative by IgM-ELISA. Statistical calculations were made using the Epitable program in Epi Info ver 6.04.
Evaluation of samples from 102 laboratory-confirmed cases identified during hospital-based surveillance in Salvador, Brazil found that the EIE-IgM Leptospirose had an overall sensitivity of 75.5% in identifying acute-phase leptospirosis (Table 1). Sensitivity was 62.1% during the first week of illness and increased significantly (P<0.001) to 91.7% by the second week. All paired convalescent-phase samples demonstrated positive reactivity in the assay. The EIE-IgM Leptospirose achieved significantly higher sensitivity (P<0.001) than the MAT screening criteria of titre ≥1:100 (Faine et al., 1999, WHO, 2003) throughout acute-phase illness (Table 1). Agreement between the assay and the MAT for acute and convalescent-phase leptospirosis ranged from good to excellent, with kappa scores of 0.78 and 0.96, respectively.
Table 1.
Reactivity of EIE-IgM-Leptospirose Kit with samples from leptospirosis patients and control subjects
| Subject group | ELISA positivea | MAT positiveb | % Positive reactions (95% CI) | |
|---|---|---|---|---|
| No. (total) | ELISA | MAT | ||
| Leptospirosis patients | ||||
| Acute-phase | 77 (102) | 41 (102) | 75.5 (65.8–83.2) | 40.2 (30.8–50.4) |
| 2 – 7 days | 36 (58) | 11 (58) | 62.1 (48.3–74.2) | 19.0 (10.3–31.8) |
| 8 – 13 days | 33 (36) | 23 (36) | 91.7 (76.4–97.8) | 63.9 (46.2–78.7) |
| 14 – 19 days | 8 (8) | 7 (8) | 100 (59.8–100) | 87.5 (46.7–99.3) |
| Convalescent-phase | 102 (102) | 102 (102) | 100 (95.5–100) | 100 (95.5–100) |
| Late convalescent-phase | ||||
| 6 months – 1 year | 17 (19) | ND | 89.5 (65.5–98.2) | ND |
| 1 – 2 years | 5 (6) | ND | 83.3 (36.5–99.1) | ND |
| 2 – 3 years | 7 (11) | ND | 63.6 (31.6–87.6) | ND |
| 3 – 4 years | 4 (7) | ND | 57.1 (20.2–88.2) | ND |
| 4 – 5 years | 10 (20) | ND | 50.0 (27.9–72.1) | ND |
| Healthy control groups | ||||
| Non-endemic community (US) | 0 (60) | ND | 0 (0–5.2) | ND |
| Blood bank donors (Brazil) | 3 (46) | ND | 6.5 (1.7–18.9) | ND |
| Endemic community (Brazil) | 0 (58) | ND | 0 (0–5.2) | ND |
| Hyper-endemic community (Brazil) | 4 (184) | ND | 2.2 (0.7–5.8) | ND |
| Patient control groups | ||||
| Initial clinical suspicion of leptospirosis | 2 (30) | ND | 6.7 (1.2–23.5) | ND |
| Hepatitis | 1 (19) | ND | 5.3 (0.3–28.1) | ND |
| Dengue | 0 (30) | ND | 0 (0–14.1) | ND |
| Syphilis | 1 (29) | ND | 3.4 (0.2–19.6) | ND |
| Lyme disease | 0 (30) | ND | 0 (0–14.1) | ND |
ND, not determined.
Defined as an index ratio of >1.0.
Defined as a MAT screening criteria of a titre of ≥ 1:100.
The specificity of the EIE-IgM Leptospirose was more than 93.3% during evaluation of healthy individual and patient control groups (Table 1). The specificity was 93.5 to 100% among healthy control subjects that included institutionalized individuals from California (non-endemic region), blood bank donors and participants in a citywide serosurvey from Salvador, Brazil (endemic community, Table 1). Furthermore, assay specificity was 97.8% among residents of a high-risk slum community (hyper-endemic) for annual leptospirosis epidemics. Reactivity was 5.3 and 0% in samples from patients with respectively, acute viral hepatitis and dengue, two diseases that have overlapping clinical presentations with leptospirosis, and was 6.7% among patients who were hospitalized with an initial suspicion for leptospirosis but later confirmed to have another disease. Samples from patients with other spirochetal infections, Lyme disease and syphilis, had limited reactivity (0 and 3.4%, respectively) in the IgM ELISA. Follow-up evaluations were performed six months to five years after hospitalization to obtain sera from leptospirosis cases (Table 1). Evaluation of late convalescent-phase samples found that IgM reactivity was 83.3% for samples collected one to two years after hospitalization, declining to 50.0% for samples collected four to five years post-infection (Fig. 1).
Fig. 1.
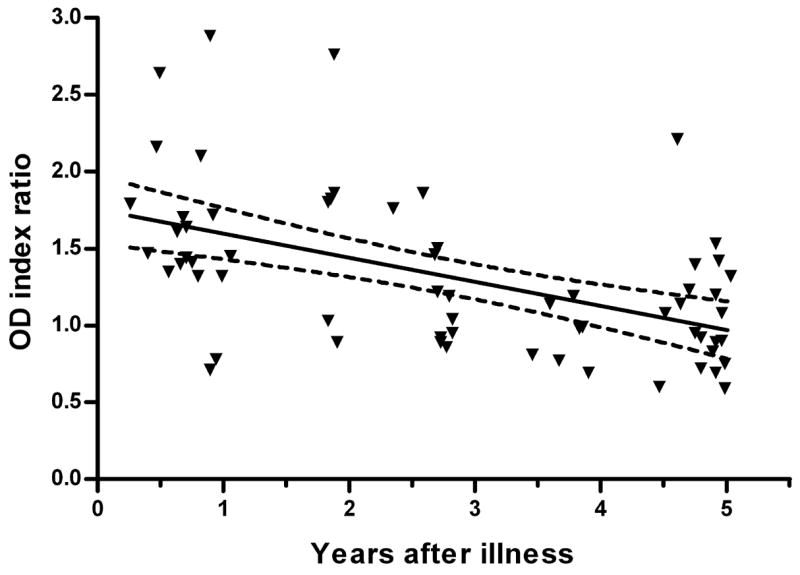
Persistence of anti-whole Leptospira IgM antibodies detected by the EIE-IgM Leptospirose kit in sera collected from individuals after recovery from leptospirosis. The solid and broken lines represent the slope and 95% confidence intervals, respectively, obtained by linear regression. Results are expressed as an index ratio of the absorbance (OD450) for the test sample and the kit’s calibrator sample. Index ratios >1.0 were defined as positive reactions.
The evaluation found that the EIE-IgM Leptospirose achieved similar performance characteristics as those reported for whole Leptospira-based serologic assays (Bajani et al., 2003, Effler et al., 2000, Levett and Branch, 2002, Sehgal et al., 2003, Smits et al., 2001a, Smits et al., 2001b, Vijayachari et al., 2002). This is not surprising for although the serovar source and preparation of antigen used in these assays may differ, they appear to detect an antibody response against the same immunodominant moiety, “broad reactive antigen” (Faine et al., 1999, Terpstra et al., 1985) which is expressed in the spectrum of pathogenic serovars. The EIE-IgM-Leptospirose demonstrated higher sensitivity (62.1%) during the first week of illness than reported (35–49%) for other whole Leptospira-based assays (Bajani et al., 2003, Effler et al., 2002, Vijayachari et al., 2002). However, this may relate more to differences among disease severity of patient populations evaluated in the trials. Direct comparison of the EIE-IgM-Leptospirose with other manufactured serologic kits will need to be performed in order to directly determine whether significant differences occur in the performance between these assays.
There are several caveats with respect to the evaluation’s findings. Firstly, the sensitivity of the EIE-IgM Leptospirose, as with other whole Leptospira-based assays, is low (62.1%) during the first week of illness. Of note, sensitivity increased to 91.7% during the second week of illness (Table 1). Therefore, testing of a late acute-phase sample (≥10th day of illness) is recommended when an initial sample is negative. Secondly, the findings of this evaluation may not be relevant to situations outside of urban leptospirosis or urban centers that have different epidemiological patterns such as that encountered in Salvador. The etiologic agent for urban outbreaks in Salvador, Brazil is L. interrogans serovar Copenhageni (Ko et al., 1999), which was the source of antigen for the assay. Therefore, the evaluation was conducted in the setting of “homologous” infections which may have contributed to the observed sensitivity of the assay. Performance may differ for other epidemiological settings, such as rural leptospirosis, where transmission is caused by other serovars. Urban epidemics are associated with severe disease presentations (Bharti et al., 2003, Levett, 2001, McBride et al., 2005), yet overall exposure rates may be lower than those in high endemic rural settings. In this evaluation the EIE-IgM-Leptospirose demonstrated a specificity of 93.5–100%. However, we found that 50.0% of patients had detectable IgM ELISA reactivity up to five years after hospitalization for leptospirosis (Table 1 and Figure 1). Although re-infection cannot be excluded, these findings provide additional evidence for prolonged persistence of anti-whole Leptospira antibodies after infection (Abdulkader et al., 2002, Cumberland et al., 2001, Everard and Bennett, 1990, Finsterer et al., 2005, Lupidi et al., 1991) which in turn, may adversely affect the applicability of the assay in regions of high endemic transmission. In fact, high false positive rates have been reported for whole Leptospira-based assays in settings of rural leptospirosis (Cumberland et al., 2001, da Silva et al., 1997).
Nevertheless the evaluation found that the EIE-IgM Leptospirose is a useful diagnostic tool for surveillance of urban leptospirosis. More than 96,000 individual tests are distributed nationally each year, thereby increasing access to leptospirosis diagnosis in Brazil. Furthermore, this kit demonstrated high specificity during evaluation of patient control groups that commonly confound the diagnosis of leptospirosis. Large outbreaks occur in marginalized slum communities in developing countries such as Brazil (Johnson et al., 2004, Karande et al., 2005, McBride et al., 2005). Diagnosis has become ever more critical in these settings with the emergence of dengue, which has hampered timely identification and treatment of leptospirosis (Karande et al., 2005, Ko et al., 1999, LaRocque et al., 2005, McBride et al., 2005). Yet, leptospirosis remains a neglected disease because of limited access to diagnosis, due to reliance on antiquated standard methods and the cost of commercially-available alternatives. Since conditions of urban poverty which favour rodent-borne transmission are present in much of the developing world, public sector initiatives to produce diagnostic assays, such as pursued by the Brazilian Ministry of Health, will be an important public health response to this emerging problem.
Acknowledgments
We thank the Central Laboratory for the State of Bahia (LACEN-BA), the HemoCentre for the State of Bahia (HEMOBA); Dr. Martin Schriefer from the Centre of Disease Control and Prevention at Fort Collins, Colorado, and the California State Health Department for providing serum samples. This work was supported by the Oswaldo Cruz Foundation, Brazilian Ministry of Health (09224-7, PDTIS), Brazilian National Research Council (300.861/96-6) and the National Institutes of Health, USA (AI052473 and TW00919).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulkader RC, Daher EF, Camargo ED, Spinosa C, da Silva MV. Leptospirosis severity may be associated with the intensity of humoral immune response. Rev Inst Med Trop Sao Paulo. 2002;44:79–83. doi: 10.1590/s0036-46652002000200005. [DOI] [PubMed] [Google Scholar]
- Bajani MD, Ashford DA, Bragg SL, Woods CW, Aye T, Spiegel RA, Plikaytis BD, Perkins BA, Phelan M, Levett PN, Weyant RS. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J Clin Microbiol. 2003;41:803–809. doi: 10.1128/JCM.41.2.803-809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- Cole JR, Jr, Sulzer CR, Pursell AR. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumberland P, Everard COR, Wheeler JG, Levett PN. Persistence of anti-leptospiral IgM, IgG and agglutinating antibodies in patients presenting with acute febrile illness in Barbados 1979–1989. Eur J Epidemiol. 2001;17:601–608. doi: 10.1023/a:1015509105668. [DOI] [PubMed] [Google Scholar]
- da Silva MV, Nakamura PM, Camargo ED, Batista L, Vaz AJ, Romero EC, Brandao AP. Immunodiagnosis of human leptospirosis by dot-ELISA for the detection of IgM, IgG, and IgA antibodies. Am J Trop Med Hyg. 1997;56:650–655. doi: 10.4269/ajtmh.1997.56.650. [DOI] [PubMed] [Google Scholar]
- Effler PV, Bogard AK, Domen HY, Katz AR, Higa HY, Sasaki DM. Evaluation of eight rapid screening tests for acute leptospirosis in Hawaii. J Clin Microbiol. 2002;40:1464–1469. doi: 10.1128/JCM.40.4.1464-1469.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effler PV, Domen HY, Bragg SL, Aye T, Sasaki DM. Evaluation of the indirect hemagglutination assay for diagnosis of acute leptospirosis in Hawaii. J Clin Microbiol. 2000;38:1081–1084. doi: 10.1128/jcm.38.3.1081-1084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RD, Fukuda MM, McDaniel P, Welch K, Nisalak A, Murray CK, Gray MR, Uthaimongkol N, Buathong N, Sriwichai S, Phasuk R, Yingyuen K, Mathavarat C, Miller RS. Causes of fever in adults on the Thai-Myanmar border. Am J Trop Med Hyg. 2006;74:108–113. [PubMed] [Google Scholar]
- Everard CO, Bennett S. Persistence of leptospiral agglutinins in Trinidadian survey subjects. Eur J Epidemiol. 1990;6:40–44. doi: 10.1007/BF00155547. [DOI] [PubMed] [Google Scholar]
- Faine SB, Adler B, Bolin C, Perolat P. MediSci. Melbourne, Australia: 1999. Leptospira and leptospirosis. [Google Scholar]
- Finsterer J, Stollberger C, Sehnal E, Stanek G. Mild leptospirosis with three-year persistence of IgG- and IgM-antibodies, initially manifesting as carpal tunnel syndrome. J Infect. 2005;51:E67–E70. doi: 10.1016/j.jinf.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Flannery B, Pereira MM, de Freitas Velloso L, de Castro Carvalho C, Goes de Codes L, de Saboia Orrico G, Dourado CMR, Riley LW, Reis MG, Ko AI. Referral pattern of leptospirosis cases during a large urban epidemic of dengue. Am J Trop Med Hyg. 2001;65:657–663. doi: 10.4269/ajtmh.2001.65.657. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Smith H, Joeph P, Gilman RH, Bautista CT, Campos KJ, Cespedes M, Klatsky P, Vidal C, Terry H, Calderon MM, Coral C, Cabrera L, Parmar PS, Vinetz JM. Environmental exposure and leptospirosis, Peru. Emerg Infect Dis. 2004;10:1016–1022. doi: 10.3201/eid1006.030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karande S, Gandhi D, Kulkarni M, Bharadwaj R, Pol S, Thakare J, De A. Concurrent Outbreak of Leptospirosis and Dengue in Mumbai, India, 2002. J Trop Pediatr. 2005;51:174–181. doi: 10.1093/tropej/fmh100. [DOI] [PubMed] [Google Scholar]
- Ko AI, Galvao Reis M, Ribeiro Dourado CM, Johnson WD, Jr, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- LaRocque RC, Breiman RF, Ari MD, Morey RE, Janan FA, Hayes JM, Hossain MA, Brooks WA, Levett PN. Leptospirosis during Dengue Outbreak, Bangladesh. Emerg Infect Dis. 2005;11:766–769. doi: 10.3201/eid1105.041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett PN, Branch SL. Evaluation of two enzyme-linked immunosorbent assay methods for detection of immunoglobulin M antibodies in acute leptospirosis. Am J Trop Med Hyg. 2002;66:745–748. doi: 10.4269/ajtmh.2002.66.745. [DOI] [PubMed] [Google Scholar]
- Lupidi R, Cinco M, Balanzin D, Delprete E, Varaldo PE. Serological follow-up of patients involved in a localized outbreak of leptospirosis. J Clin Microbiol. 1991;29:805–809. doi: 10.1128/jcm.29.4.805-809.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride AJ, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Curr Opin Infect Dis. 2005;18:376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- Murdoch DR, Woods CW, Zimmerman MD, Dull PM, Belbase RH, Keenan AJ, Scott RM, Basnyat B, Archibald LK, Reller LB. The etiology of febrile illness in adults presenting to Patan hospital in Kathmandu, Nepal. Am J Trop Med Hyg. 2004;70:670–675. [PubMed] [Google Scholar]
- Romero EC, Bernardo CC, Yasuda PH. Human leptospirosis: a twenty-nine-year serological study in Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2003;45:245–248. doi: 10.1590/s0036-46652003000500002. [DOI] [PubMed] [Google Scholar]
- Russell KL, Montiel GMA, Watts DM, Lagos-Figueroa RC, Chauca G, Ore M, Gonzalez JE, Moron C, Tesh RB, Vinetz JM. An outbreak of leptospirosis among Peruvian military recruits. Am J Trop Med Hyg. 2003;69:53–57. [PubMed] [Google Scholar]
- Segura ER, Ganoza CA, Campos K, Ricaldi JN, Torres S, Silva H, Cespedes MJ, Matthias MA, Swancutt MA, Lopez Linan R, Gotuzzo E, Guerra H, Gilman RH, Vinetz JM. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clin Infect Dis. 2005;40:343–351. doi: 10.1086/427110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal SC, Vijayachari P, Sugunan AP, Umapathi T. Field application of Lepto lateral flow for rapid diagnosis of leptospirosis. J Med Microbiol. 2003;52:897–901. doi: 10.1099/jmm.0.05064-0. [DOI] [PubMed] [Google Scholar]
- Smits HL, Chee HD, Eapen CK, Kuriakose M, Sugathan S, Gasem MH, Yersin C, Sakasi D, Lai-a-Fat RFM, Hartskeerl RA, Liesdek B, Abdoel TH, Goris MGA, Gussenhoven GC. Latex based, rapid and easy assay for human leptospirosis in a single test format. Trop Med Int Health. 2001a;6:114–118. doi: 10.1046/j.1365-3156.2001.00675.x. [DOI] [PubMed] [Google Scholar]
- Smits HL, Eapen CK, Sugathan S, Kuriakose M, Gasem MH, Yersin C, Sasaki D, Pujianto B, Vestering M, Abdoel TH, Gussenhoven GC. Lateral-flow assay for rapid serodiagnosis of human leptospirosis. Clin Diagn Lab Immunol. 2001b;8:166–169. doi: 10.1128/CDLI.8.1.166-169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassinari WdS, Pellegrini DdC, Sabroza PC, Carvalho MS. Spatial distribution of leptospirosis in the city of Rio de Janeiro, Brazil, 1996–1999. Cad Saude Publica. 2004;20:1721–1729. doi: 10.1590/s0102-311x2004000600031. [DOI] [PubMed] [Google Scholar]
- Terpstra WJ, Ligthart GS, Schoone GJ. ELISA for the detection of specific IgM and IgG in human leptospirosis. J Gen Microbiol. 1985;131:377–385. doi: 10.1099/00221287-131-2-377. [DOI] [PubMed] [Google Scholar]
- Vijayachari P, Sugunan AP, Sehgal SC. Evaluation of Lepto Dri Dot as a rapid test for the diagnosis of leptospirosis. Epidemiol Infect. 2002;129:617–621. doi: 10.1017/s0950268802007537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Leptospirosis worldwide, 1999. Wkly Epidemiol Rec. 1999;74:237–242. [PubMed] [Google Scholar]
- WHO. Human leptospirosis: guidance for diagnosis, surveillance and control. World Health Organization; Malta: 2003. [Google Scholar]
- Wongsrichanalai C, Murray CK, Gray M, Miller RS, McDaniel P, Liao WJ, Pickard AL, Magill AJ. Co-infection with malaria and leptospirosis. Am J Trop Med Hyg. 2003;68:583–585. doi: 10.4269/ajtmh.2003.68.583. [DOI] [PubMed] [Google Scholar]


