Abstract
Pseudomonas aeruginosa is one of the most important bacterial pathogens encountered by immunocompromised hosts and patients with cystic fibrosis (CF), and the lipopolysaccharide (LPS) elaborated by this organism is a key factor in virulence and both innate and acquired host responses to infection. The molecule has a fair degree of heterogeneity in its lipid A and O-antigen structure, and elaborates 2 different outer-core glycoforms, of which only one binds O-antigen. A close relatedness between the chemical structures and genes encoding biosynthetic enzymes has been established, with 11 major O-antigen groups identified. The lipid A can be variably penta-, hexa- or hepta-acylated, and these isoforms have differing potencies when activating host innate immunity via binding to Toll-like receptor 4. The O-antigen is a major target for protective immunity as evidenced by numerous animal studies, but attempts, to date, to produce a human vaccine targeting these epitopes have not been successful Newer strategies employing live attenuated P. aeruginosa, or heterologous attenuated bacteria expressing P. aeruginosa O-antigens are potential means to solve some of the existing problems related to making a P. aeruginosa LPS-specific vaccine. Overall, there is now a large amount of information available about the genes and enzymes needed to produce the P. aeruginosa LPS, detailed chemical structures have been determined for the major O-antigens, and significant biologic and immunologic studies have been conducted to define the role of this molecule in virulence and immunity to P. aeruginosa infection.
Keywords: Pseudomonas aeruginosa, Lipopolysaccharide, Innate immunity, Vaccine, Virulence
Introduction
While Pseudomonas aeruginosa has been appreciated as a significant bacterial pathogen since the 19th century, as discussed by Doggett (1979), it is within the past 50 years that this organism has become one of the most common causes of nosocomial bacterial infection, primarily due to modern medical therapies that provide the settings in which P. aeruginosa can colonize, infect and intoxicate susceptible patients (Cao et al., 2004; Garau and Gomez, 2003; Tredget et al., 2004). These settings arise primarily due to advances in patient care and treatment that prevent mortality from an initial injury or illness, leading to prolonged survival in a compromised state, hospitalizations and use of a variety of drugs that inadvertently promote P. aeruginosa infection and pathogenesis. Under these circumstances, P. aeruginosa can take advantage of the compromised health of susceptible individuals and colonize tissues, spread to vital organs and directly intoxicate host cells using bacterial factors, as well as initiate an overwhelming host inflammatory response that also contributes significantly to morbidity and mortality of infected patients. Common settings of P. aeruginosa infection include thermally injured patients (Tredget et al., 2004), immunocompromised hosts, notably those with neutropenia (Obritsch et al., 2005), patients receiving mechanical ventilation (Cao et al., 2004; Garau and Gomez, 2003) and those born with the genetic disease cystic fibrosis (CF) (Govan and Deretic, 1996; Lyczak et al., 2002; Saiman and Siegel, 2004). In all of these cases, the P. aeruginosa lipopolysaccharide (LPS) is a prominent factor in mediating both bacterial virulence and host responses, and the contribution of LPS to pathogenesis and immunity varies depending on the underlying patient basis for increased susceptibility to infection, the isoform of the LPS, particularly the lipid A component, and structural variation in the O-antigen side chain that impacts host immunity.
P. aeruginosa LPS structure
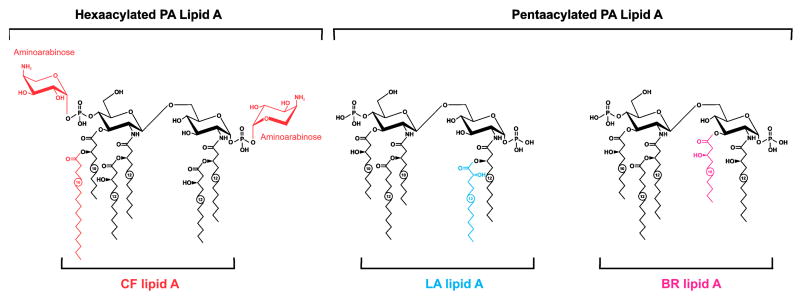
In order to understand how the P. aeruginosa LPS impacts virulence and immunity to this pathogen, a basic insight into the chemical structure and biologic activities of this molecule is essential. Fortunately, the concerted efforts of Y.A. Knirel and associates at the N.D. Zelinsky Institute of Organic Chemistry in Moscow (Russia) and numerous colleagues from around the world, have led to a very detailed knowledge of the fine chemical structure of the P. aeruginosa LPS. It is a typical gram-negative bacterial LPS, with a basic lipid A structure containing an N- and O-acylated diglucosamine bisphosphate backbone [4-P-β-D-GlcpNII-(1→6)-α-D-GlcpNI-(1→P] with chemical variation in the number of primary acyl groups and the types of fatty acids substituting the primary and secondary acyl groups (Fig. 1). Most of the laboratory-adapated strains of P. aeruginosa synthesize a penta-acylated (75% of the molecules) LPS, with some proportion made as a hexa-acylated LPS (25% of the molecules). The difference between the 2 isoforms is the lack of an O-linked 3-hydroxy decanoic acid (10:0(3-OH)) group at position 3 of the first glucosamine in the penta-acylated isoform. Growth conditions, notably magnesium levels, can affect the acylation pattern of P. aeruginosa lipid A. Among isolates from chronically infected CF patients, which are known to be mutants generally unable to synthesize O-antigen side chains, a hexa-acylated LPS form predominates (Fig. 1), although a hepta-acylated lipid A has been isolated, containing an additional palmitoyl (16:0) group linked to the primary 3-hydroxy decanoic acid group at position 3′ of glucosamine 2 (Ernst et al., 1999, 2003). The hexa- and hepta-acylated lipid A moieties also contain cationic 4-amino-4-deoxy-L-arabinose sugars (Fig. 1).
Fig. 1.
Structures of variant P. aeruginosa lipid A found to be predominantly expressed in isolates from CF patients (CF lipid A), bronchiectasis patients (BR lipid A) or from a laboratory adapted (LA lipid A) strain, PAK. Reprinted with permission from Nature Publishing Group from (Hajjar et al., 2002).
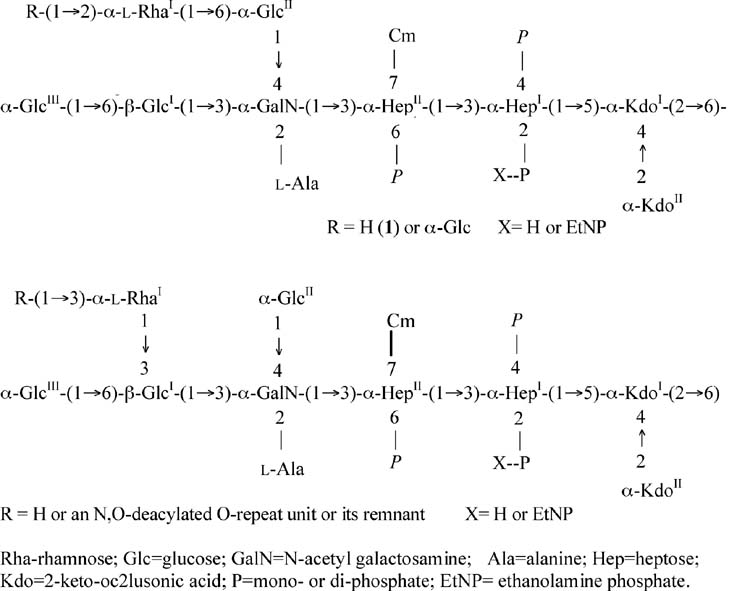
Bound to the lipid A is a relatively conserved inner-core structure which contains two D-manno-oct-2-ulosonic acid residues (KdoI and KdoII) and two L-glycero-D-manno-heptose residues (HepI and HepII) (Bystrova et al., 2003b, 2006) (Fig. 2). Bound to the second heptose residue, HepII, is a 7-O-carbamyl group (Beckmann et al., 1995), which is found in the LPS of other types of pseudomonads (Knirel et al., 1996). The 2 heptose residues are often phosphorylated at positions 2 and 4 of HepI and position 6 of HepII (Bystrova et al., 2002; Knirel et al., 2001; Sadovskaya et al., 2000). Phosphate substituents can be mono-, di- or even tri-phosphates, with most analyzed P. aeruginosa LPS having some triphosphate, which, to date, has only been detected in the LPS of this bacterial species. Also present in some P. aeruginosa LPS inner cores, but not in stoichiometric amounts, is ethanolamine mono- or di-phosphate. Of note, phosphorylation of the P. aeruginosa LPS inner core is essential for bacterial viability; a mutation in the waaP gene that phosphorylates position 4 of the HepI groups is lethal for the organism (Walsh et al., 2000).
Fig. 2.
General structure of the two glycoforms of the P. aeruginosa LPS core. The inner core comprises the 2 Kdo and 2 heptose (Hep) residues; the outer core the remainder of the molecule. For more detail see (Bystrova et al., 2006) where a similar version of the figure originally appeared. Rha-rhamnose; Glc=glucose; GalN=N-acetyl galactosamine; Ala=alanine; Hep=heptose; Kdo=2-keto-oc2lusonic acid; P=mono- or di-phosphate; EtNP= ethanolamine phosphate.
The outer core of the P. aeruginosa LPS is usually synthesized as 2 different isoforms or glycoforms by an individual strain (Bystrova et al., 2002, 2006; Sadovskaya et al., 2000). Both outer-core glycoforms contain an N-alanylated galactosamine residue, 3 D-glucose residues, and one L-rhamnose residue the position of which differs in the 2 glycoforms (Fig. 2). There is also some structural variation among the different P. aeruginosa strains in glycoform 1, with a less common variant having 4 glucose residues in the outer core (Bystrova et al., 2006). There is extensive O-acetylation of the hydroxyl groups in the outer-core sugars, but the acetates are not present in high amounts at any one position, making a clear structural determination of these substituents difficult. Also, O-acetyl groups are fairly labile under mild acid or base conditions and may be lost during LPS purification. The terminal rhamnose residue is often acetylated when it is not itself substituted with another monosaccharide. The role of O-acetylation of the outer core is not fully appreciated for P. aeruginosa, but could affect a number of important biologic properties.
Glycoform 2 of the P. aeruginosa outer core is the structure to which the O-antigen is attached (Bystrova et al., 2002). For most LPS-smooth strains studied, except for O-antigen serogroups O14 and O15, glycoform 2 is fully substituted with an O-antigen. LPS-rough strains, usually isolated from CF patients, have multiple mutations within the biosynthetic genes for the O-antigen (Evans et al., 1994) and do not have any O-antigen on glycoform 2. The relative amounts of glycoforms 1 and 2, and hence the degree of substitution of glycoform 2 with O-antigen in LPS-smooth strains, varies depending upon growth conditions, and levels ranging from 10–50% of the total LPS having O-antigen have been estimated.
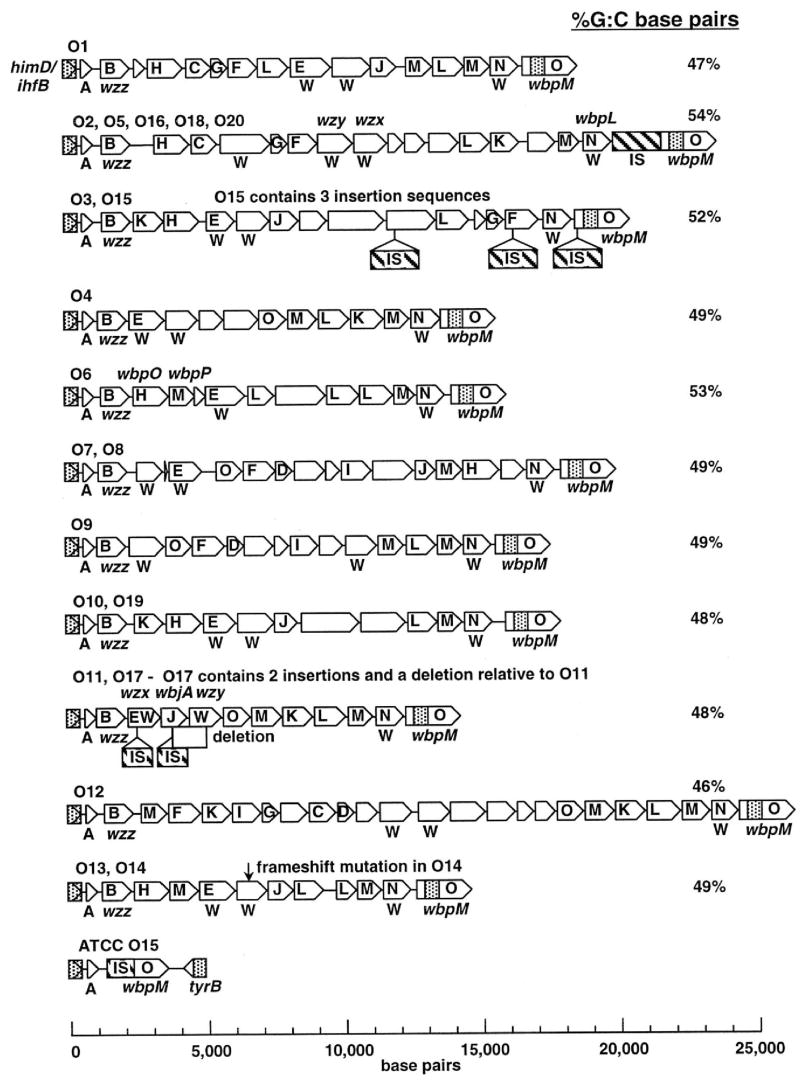
The O-antigen portion of the P. aeruginosa LPS is responsible for conferring serogroup specificity, which is defined by antibodies specific to the different variants of this antigen. Over the years a variety of serogrouping systems have been proposed by investigators from different countries and most of the commonly used ones can be reconciled when strains and antisera are cross-referenced (see (Bystrova et al., 2006)). In general, most investigators now refer to serogroups using the International Antigenic Typing System (IATS) schema. Chemically the O-antigens diverge into at least 11 structural variants (Bystrova et al., 2006), although within these variants there are minor differences among related structures in properties such as side-group substituents, linkages between sugars or conformations of different monosaccharides (Bystrova et al., 2006; Knirel, 1990). Of note, the serogroup O14 and O15 strains lack detectable O-antigens or LPS containing even a single O-antigen repeat linked to the glycoform 2 outer core (referred to as the SR-type LPS), suggesting they are likely LPS-rough strains in spite of the availability of serogroup-specific antisera. Likely the antisera to the serogroup O14 and O15 antigens are reacting with LPS core epitopes or other components on the serogroup O14 and O15 strains. These chemical findings are highly compatible with recent genetic results wherein the O-antigen biosynthetic loci have all been sequenced (Raymond et al., 2002). These investigators found 11 groups of O-antigen biosynthetic gene clusters among the 20 IATS-prototype strains analyzed (strains were obtained from the American Type Culture Collection (ATCC), Rockville, MD, USA). The 11 genetic groups corresponded precisely with the chemical structures reported for these different serogroups. Notably, the serogroup O15 strain from the ATCC was deleted for the O-antigen locus, and a second strain that was sequenced had a serogroup O3 O-antigen biosynthetic cluster interrupted by 3 insertion sequences. The O14 strain from the ATCC has an O13 O-antigen biosynthetic gene cluster with a frame shift mutation in the locus, likely accounting for lack of an O-antigen in these serogroups. In addition, serogroup O17 had 2 insertions and a deletion relative to the serogroup O11 locus which was in the proper site for O-antigen biosynthetic genes, and the actual biosynthetic locus for the O17 antigen has not yet been fully defined (Dean and Goldberg, 2000).
Typical sugars within the P. aeruginosa LPS O side chains include N-acyl derivatives of different amino sugars along with rhamnose (see (Bystrova et al., 2006) for details). The structures of some of these monosaccharides were first described in P. aeruginosa LPS. Rhamnose is also commonly found in some of the O-antigens. The monosaccharides are arranged in repeat units containing 3 to 4 individual monosaccharides, except for serogroup O7, which is a disaccharide repeat unit. The linkage of the monosaccharide in the first repeat unit to the rhamnose residue in glycoformII of the LPS outer core is usually a 2-N-acetyl derivative of a 6-deoxy-D-hexosamine, usually D-quinivosamine or D-fucosamine, although for serogroup O3 it is the 4-amino, 4-deoxy derivative of D-quinivosamine. This conservation in linkage of the O-antigen to the LPS core appears to be due to the conserved gene encoding the O-antigen ligase designated waaL. The WaaL protein catalyzes the binding of a 6-deoxyhexosamine to the terminal rhamnose residue of glycoform II of the LPS outer core, and forms a β-linkage, whereas in the repeat units that occur in the rest of the O-antigen chain the linkage between the 6-deoxy hexosamine and the next monosaccharide can be in either the α or the β configuration (Abeyrathne et al., 2005).
Genetics and biosynthesis of P. aeruginosa LPS
The genes encoding biosynthetic enzymes for the lipid A, core and O-antigen of P. aeruginosa LPS have not been as well studied as have those for other pathogenic Gram-negative organisms such as Escherichia coli (Reeves and Wang, 2002; Samuel and Reeves, 2003). Nonetheless, efforts initiated by J.B. Goldberg, first in Boston, MA then in Charlottesville, VA (Coyne and Goldberg, 1995; Dean et al., 1999, 2002; Dean and Goldberg, 2002; Evans et al., 1994; Goldberg et al., 1992, 1993) and by J. Lam in Guelph, Ontario (Dasgupta and Lam, 1995; de Kievit et al., 1997; Lightfoot and Lam, 1991, 1993; Rocchetta et al., 1999) provided some of the earliest insights into genome organization related to LPS biosynthesis. More recent, notably from Lam’s group, studies have further expanded our understanding of the genetics of P. aeruginosa LPS biosynthesis (Abeyrathne et al., 2005; Ishiyama et al., 2004; Miller et al., 2004; Mulrooney et al., 2005; Wenzel et al., 2005). Studies of the galU gene, which encodes UTP-glucose-1-phosphate uridylyltransferase, and the algC gene, which encodes a bifunctional phosphomannomutase/phosphoglucomutase, and the LPSs produced by strains with mutations in these loci (Dean and Goldberg, 2002; Goldberg et al., 1993) identified the necessity for these enzymes in LPS core biosynthesis. The deletion of algC led to a mutant producing the inner core that also lacked the conserved N-alanyl-galactosamine residue (see Fig. 2) while deletion of galU resulted in an LPS with the complete inner core but no outer-core sugars (see Fig. 2). In 1983 Rowe and Meadow (1983) characterized the structures of a set of LPSs produced by some chemical mutants of P. aeruginosa, which synthesized a variety of truncated LPS cores. The genes mutated in these strains were never identified. Nonetheless, it did appear feasible to make deeper-rough mutants in at least some P. aeruginosa strains. Walsh et al. (1999) identified 3 genes in a locus unlinked to the O-antigen biosynthetic locus which encoded synthesis of the inner core Kdo by P. aeruginosa. In another study it was reported that phosphorylation of the P. aeruginosa LPS inner core by a waaP homologue and two other genes unique to P. aeruginosa, wapP and wapQ, was essential for viability and intrinsic drug resistance (Walsh et al., 2000). No viable mutants deleted for these genes could be produced (Walsh et al., 2000). De Kievit and Lam (1997) identified the waaC and waaF genes in P. aeruginosa by homology to the same genes in Salmonella, where they function as heptosyl transferases, adding heptoseI and heptoseII to the LPS inner core. Overall, many of the genes involved in biosynthesis of LPS core sugars or proteins that are needed to produce the core oligosaccharide have been identified.
Lam’s group also proposed that a second oligosaccharide, which they termed “A-band” (and used the term “B-band” to refer to the O-antigen) was present on the P. aeruginosa LPS molecule, but Pier and colleagues found that these 2 polysaccharides were not on the same molecule (Hatano et al., 1993) and could be separated using immunoprecipitation with antibodies specific to different A-band and B-band antigens. The A-band oligosaccharide is a polymer of D-rhamnose, and several studies have analyzed the genes encoding proteins needed for synthesis of components of the A-band LPS (Rocchetta et al., 1998a,b; Rocchetta and Lam, 1997). In addition, A-band polysaccharide was produced by a galU mutant of PAO1 that could only synthesize the inner-core oligosaccharide (Choudhury et al., 2005), and structural analyses of the core oligosaccharides from a number of P. aeruginosa strains have failed to detect A-band polysaccharide linked to the inner core (Knirel et al., 2001; Bystrova et al., 2002, 2003a, b, 2004, 2006; Choudhury et al., 2005). Moreover, in the study of Choudhury et al. (2005) purified A-band material lacked typical lipid A or LPS-core sugars, as determined by glycosyl composition, linkage analyses, and NMR spectroscopy, further indicating the A-band oligosaccharide is unlikely to be attached to the same lipid A and core oligosaccharide as is the B-band O-antigen. Also, a role for the A-band antigen in pathogenesis or immunity, either as a component of the usual LPS or as a separate molecule, has not yet been found.
A major advance came in 2002 when the studies of Raymond et al. (2002) provided a detailed analysis of the major genes encoding the biosynthesis of P. aeruginosa LPS O-antigens found in the 20 IATS serogroup prototype strains (Fig. 3). As noted earlier, they found 11 distinct biosynthetic loci, 3 strains (O14, O15 and O17) with one of these 11 loci but with mutations in the gene clusters preventing O-antigen production, and attributed the rest of the serologic variation associated with P. aeruginosa LPS O-antigens to minor chemical variations found among strains within closely related serogroups. A complete annotation of each of the proteins associated with the genes found in the 11 O-antigen biosynthetic clusters can be found at http://www.genome.washington.edu/uwgc/O-Antigen/. All of the O-antigen biosynthetic loci are located between the conserved himD/ihfB and wbpM genes present in the chromosomes of all 20 IATS serotypes. Of note, the WbpM protein is needed for synthesis of LPS by all P. aeruginosa isolates and encodes an inner-membrane UDP-N-acetyl-glucosamine C6 dehydratase (Creuzenet and Lam, 2001). As UDP-N-acetyl glucosamine is likely the precursor to all of the important monosaccharides synthesized by bacteria, and all of the LPS O-antigens contain a C6-deoxy amino or neutral sugar, the essentiality of the WbpM protein for O-antigen biosynthesis can be explained.
Fig. 3.
Schematic depiction of the different O-antigen biosynthetic gene clusters of P. aeruginosa. Genes are identified by arrows drawn to scale and protein families shown as single-letter designations. Open reading frames with possible membrane-spanning domains are designated with a W. When known, specific genes are shown above the clusters. The C-terminal coding region of wbpM, shown as an open arrow, is presumed to extend rightward of the cloned region, as shown. Shown along the right-hand edge is the G+C content for each of the biosynthetic loci. Reprinted with permission of the American Society for Microbiology from (Raymond et al., 2002).
In regard to demonstrating an actual enzymatic role of a particular protein in biosynthesis, this area has made some headway with definitive studies of cloned P. aeruginosa LPS biosynthetic enzymes, but most of the determinations of function are now carried out by genetic studies, wherein P. aeruginosa genes are cloned into heterologous organisms such as E. coli to complement LPS synthetic defects caused by loss of production of proteins with known enzymatic functions. Additionally, bioinformatic in silico analysis is used more frequently now to define function by homology, comparing P. aeruginosa proteins to other bacterial LPS biosynthetic proteins that have a high degree of similarity in amino acid sequence or in predicted 3-dimensional structure. The genes encoding these related proteins are usually contained within homologous LPS biosynthetic genetic loci. Most investigators are aware of the pitfalls of these homology assumptions, but the difficulty of actually demonstrating an enzymatic function for an isolated, recombinant protein is well appreciated among those who have attempted this type of experiment. Virtually no precursors are readily available and must often be made using multiple cloned and purified proteins that participate in the biosynthetic pathway combined with chemical characterization by chromatography and mass spectrometry of products produced which cannot be compared to a defined, synthetic standard. Functional predictions are often made by elegant chemical studies that do not directly demonstrate the actual production of a product from a precursor, but show functional homology to related proteins by a variety of measures such as Far-UV circular dichroism spectroscopy, SDS-PAGE for determination of multimeric products, matrix-assisted laser desorption/ionization-time of flight mass spectrometry, and gel filtration analyses, such as was carried out with the product of the wbpD gene of P. aeruginosa PAO1 (Wenzel et al., 2005). These analysis indicated that the WbpD protein is a 3-N-acetyltransferase, that could be important for synthesizing the 2,3, diacetamido, 2,3 dideoxy uronic acids found in the O-antigen of some serogroups of P. aeruginosa. The enzymatic function of the AlgC protein as both a phosphoglucomutase (Coyne et al., 1994) and phosphomannomutase has been demonstrated directly (Goldberg et al., 1993).
Interaction of P. aeruginosa LPS with pattern recognition molecules of the innate immune system
Ever since the discovery in 1997 that the mammalian innate immune system possesses pattern-recognition receptors (PRR) such as Toll-like receptors (TLR) (Medzhitov et al., 1997) found in invertebrates, there has been an explosion of interest in how PRR interact with bacterial factors, referred to as pathogen-associated molecular patterns (PAMPs), and how these interactions impact pathogenesis and immunity to infection. One of the first, and best studied, TLR is TLR-4, which is essential for the recognition of the lipid A portion of bacterial LPS and mediates both effective host resistance to infection as well as some of the pathology associated with LPS-induced shock (Fitzgerald and Chen, 2006; Schnare et al., 2006). These findings are particularly pronounced when studying Enterobacteriaceae, where C3H/HeJ and C57BL/10ScCr mice, which are unable to produce functional TLR4 (Poltorak et al., 1998) are highly susceptible to Salmonella infection compared with C3H/HeN mice with intact responses to LPS (O’Brien et al., 1982). However, such mice are often highly resistant to the effects of injection with purified LPS (Rosenstreich et al., 1977). Thus, TLR4-dependent early recognition of the presence of infection confers resistance to infection whereas a hyperinflammatory response induced by injection of LPS can be reduced by lack of functional TLR4.
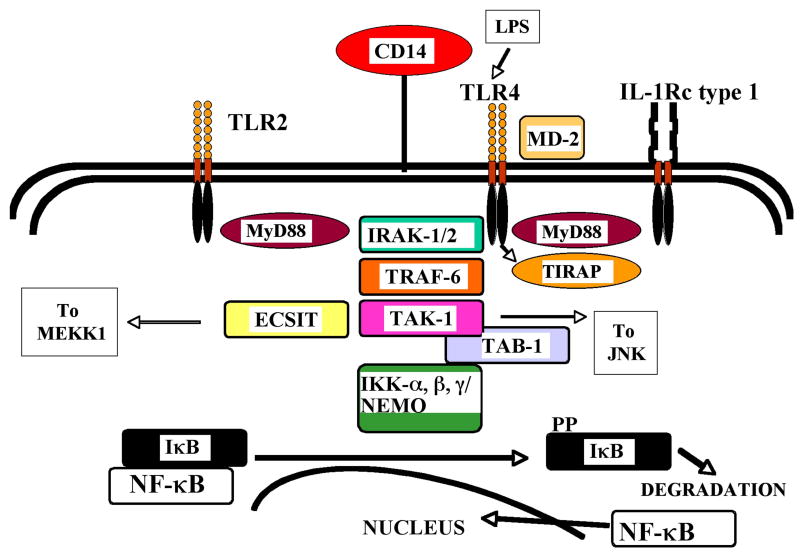
Recognition of lipid A by TLR4 is somewhat complex and involves both extracellular recognition factors and intracellular signal-transducing molecules (Fig. 4). LPS can be bound by LPS-binding protein (LBP), a 58–60-kDa glycosylated polypeptide synthesized constitutively by the liver with levels increased about 10-fold in response to inflammatory stimuli (Zweigner et al., 2006). LBP binds to lipid A with high affinity and transfers LPS to membrane-bound CD14, which is part of the cellular complex that recognizes LPS. CD14 can also be found as a soluble molecule, and LBP transfer of LPS to soluble CD14 enhances the responses of CD14-negative cells. It has also been reported that LBP can bind whole bacteria as well as molecular LPS (Wright et al., 1989). However, recently Blander and Medzhitov (2006) showed that binding of LPS to TLR4 following bacterial uptake by dendritic cells occurred within the acidified endosome, not on the cell surface, which was important for sorting foreign and self antigens into appropriate compartments for initiating immune responses or maintaining tolerance of self antigens.
Fig. 4.
Activation of innate immune signaling pathways by binding of LPS to TLR4-MD-2, facilitated by CD14. TLR4, along with other TLRs such as TLR2, and cytokine receptors such as the interleukin-1 receptor type 1 (IL-1Rc type 1) utilize a variety of adaptor molecules to transmit the information of ligand binding to the nucleus in order to induce cytokine and other responses. MyD88 (myeloid differentiation factor 88); TIRAP: (TIR domain-containing adaptor protein); TRAF-6 (tumor necrosis factor receptor–associated factor 6); TAK-1 (transforming growth factor β-activating kinase 1); TAB1 TAB2, and TAB3 (TAK1-binding protein 1, 2 or 3); IKK-α, -β, and -γ. IKK-γ (Inducible kinase, IKK-γ is also called NEMO [nuclear factor κB (NF-κB) essential modulator]); IκB (inhibitor of NF-κB); PP-IκB (phosphorylated IκB); JNK (c-Jun N-terminal kinase). Modified from (Pier, 2004) and reprinted with permission from McGraw-Hill publishers.
LPS bound to CD14 can interact with the extracellular or intraluminal domains of TLR4 in the presence of a co-factor, MD2. Whether this occurs on the cell surface as thought by many, or within the lumen of endosomes following phagocytosis or endocytosis of whole bacteria or free LPS, as shown by Blander and Medzhitov (2006) for dendritic cells, is not extensively known. Binding of LPS to TLR4 brings adaptor molecules containing Toll/IL-1 receptor (TIR) domains to interact with the cytoplasmic portion of TLR4. These adaptors include TIRAP/Mal and TRAM, which function to sort out the signals to be transduced by TLR4-MD2-bound LPS (Fitzgerald and Chen, 2006). The next set of molecules essential for activating cellular responses to LPS are MyD88 and TRIF, which themselves interact with additional adaptors to lead to activation of transcription factors, notably NF-κB, which enters the nucleus and promotes production of inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor α and a host of other factors. The balance of production of these factors, which undergo complex transcriptional and translational regulation, determines the outcome of the host response to LPS.
In regard to P. aeruginosa LPS and interaction with TLR4, it was generally regarded for a long time that this organism’s LPS stimulated less inflammation and lower overall host responses compared with that of enterobacterial LPS, such as that from E. coli (Pier et al., 1981a). However, more recent studies showing a high degree of variability in the P. aeruginosa lipid A structure that can be synthesized depending on the strain and the growth conditions have shown that TLR4-mediated responses are highly dependent on the level of acylation of the lipid A. In general, production of a fully hexa-acylated lipid A is associated with a more vigorous inflammatory response induced by P. aeruginosa (Ernst et al., 2003) whereas production of lipid A with lower levels of acylation results in reduced cellular responses and production of inflammatory cytokines. Growth of P. aeruginosa strain PAK in a low-magnesium medium (8 μM) promoted the production of hexa-acylated LPS (Ernst et al., 1999) and increased the level of stimulation of cells.
Ernst et al. (1999) additionally found that more of the hexa-acylated form of the P. aeruginosa LPS was produced by strains isolated from chronically-infected lungs of CF patients, and this LPS isoform stimulated greater amounts of inflammation when tested on cells in vitro when compared with the penta-acylated form primarily produced during in vitro growth of non-CF strains. Hajjar et al. (2002) found that the human TLR4-MD-2, but not the mouse homologue, transduced more vigorous responses to the hexa-acylated form of P. aeruginosa LPS. Another interesting finding showed that the penta-acylated form of P. aeruginosa LPS could antagonize TLR4-dependent responses of the human T24 bladder epithelial carcinoma cell line to hexa-acylated LPS from E. coli (Backhed et al., 2003). Thus, it appears that when testing less than fully hexa-acylated LPS, or organisms that fail to produce such an isoform of LPS when grown in vitro, the full spectrum of TLR4-dependent signaling to P. aeruginosa LPS may not be observed.
As one of the major sites of P. aeruginosa infection is the lung, there has been a great deal of interest in determining the level of expression of TLR molecules in the epithelial cells of this tissue, which are felt to be among the cells most likely to initially encounter P. aeruginosa bacteria and LPS and mediate initial host responses to respiratory tract infection. By RT-PCR, mRNA for TLRs 1–10, MD2, and MyD88 were detected in both normal and CF cultured airway epithelial cells (Muir et al., 2004), although in humans TLR10 is not made as a functional protein. Immunohistochemistry revealed TLR2 was in the apical membrane of the cells whereas TLR4 had a more basolateral distribution, and there was no cytokine response to LPS by these cells (Muir et al., 2004). Similarly, mRNA for TLRs 1, 2, 4, 6 and 9 were detected in a CF tracheal epithelial cell line, CFTE29o(−) and in a bronchial epithelial cell line, 16HBE14o(−), with wild-type (WT) CF transmembrane conductance regulator (CFTR) (Greene et al., 2005). However, this latter study reported LPS, along with other TLR stimuli, could induce IL-8 and IL-6 protein secretion, and there was no major difference between the WT and mutant CFTR cell lines. Of note, Hauber et al. (2005) analyzed TLR2 and TLR4 expression in the bronchial epithelium of CF and normal subjects, and found there was actually reduced expression of TLR4 and TLR2 in the bronchial epithelium of patients with CF, but higher expression in the submucosal neutrophils, likely a result of chronic P. aeruginosa infection. Overall, it is difficult to know how these differing measures of TLR expression and responses of cultured cells versus in vivo tissue samples can be reconciled. One aspect in need of more study is to determine if humans with chronic P. aeruginosa infections become hyporesponsive to LPS, which occurs after injection of a low dose of LPS followed within the next few days by a more potent dose which now has reduced activity (Greer and Rietschel, 1978; Greisman et al., 1964). If so, then perhaps TLR4 expression in the lung may be more relevant to susceptibility and resistance to acute P. aeruginosa infection as opposed to chronic infection typical of CF, where chronic exposure to LPS might put the host into an LPS-hyporesponsive state in order to minimize damage from LPS-induced inflammation.
In regard to acute P. aeruginosa lung infection, most studies in mice seem to indicate that a lack of TLR4 on its own has little effect on P. aeruginosa pathogenesis when measuring bacterial clearance or lethality, but TLR4 may impact the overall cytokine response. George et al. (1993) first showed no major difference in the LD50 of several P. aeruginosa strains in C3H/HeN and C3H/HeJ mice, which we now know to be TLR4-producing and TLR4-deficient, respectively. In contrast, Faure et al. (2004) found C3H/HeJ mice to be more susceptible to lung infection and pathology from a highly cytotoxic strain of P. aeruginosa, PA103 that produces high levels of the ExoU phospholipase cytotoxin, when compared to TLR4-sufficient mice, but there was no difference in susceptibility of these mice to a non-cytotoxic mutant of strain PA103. However, this is a highly pathogenic strain of P. aeruginosa with other known genetic defects affecting production of virulence factors, and thus may not be very representative of typical P. aeruginosa strains. Several other studies have shown that TLR4-deficent mice do not appear to be more susceptible to P. aeruginosa pulmonary infection (Feuillet et al., 2006; Ramphal et al., 2005; Skerrett et al., 2007), but when a TLR4 deficiency is bred into a mouse with another TLR deficiency, such as TLR5, then there is increased susceptibility to P. aeruginosa lung infection, at least when using strain PAK (Feuillet et al., 2006). Also, TLR4 knock-out mice respond to P. aeruginosa lung infection with reduced production of inflammatory cytokines, but normal bacterial clearance (Ramphal et al., 2005; Skerrett et al., 2007). Thus, the redundancy of effector functions of host PRR likely abrogates a significant deficiency from lack of a single TLR such as TLR4.
Another major tissue site of P. aeruginosa infection is the eye, specifically the cornea, wherein prolonged wear of contact lenses increases the likelihood of a severe P. aeruginosa keratitis (Verhelst et al., 2005, 2006). In the eye, corneal inflammation is the primary cause of pathology, leading to inflammation-based opacification and loss of visual acuity. Thus, activation of inflammation in this tissue can be pathologic as opposed to protective. Studies on the expression of TLRs, including TLR4, in the corneal epithelium has been limited, with contrasting findings. Song et al. (2001) reported CD14 and TLR4 were expressed in the corneal epithelial cell line 10.014 pRSV-T and these cells responded to LPS by secretion of cytokines and chemokines. Johnson et al. (2005) found by RT-PCR that TLRs 2, 4 and 9 were expressed in the corneal epithelium of C57Bl/6 mice but did not demonstrate the presence or location of these proteins within the corneal epithelium. In contrast, Blais et al. (2005) found human wing and basal corneal epithelial cells, but not the superficial epithelial cells overlying the top of the cornea, expressed MD-2 and TLR4. Additionally, in cultured SV-40-immortalized human corneal epithelial cells, MD-2 and TLR4 proteins were readily detected. Ueta et al. (2004) found that TLR2 and TLR4 were only expressed intracellularly by human primary corneal epithelial cells and a human corneal epithelial cell line (HCE-T), and suggested this lack of ability to respond to LPS could be important in maintaining the non-inflammatory status of the cornea. Overall, it appears that intact human corneal tissue has low or no surface expression of TLR4 and MD-2 whereas expression in cultured cells is seen and expression may be different in murine corneal cells on an intact eye.
When purified P. aeruginosa LPS was applied to the abraded corneal epithelium of BALB/c, C3H/HeJ, and C3H/HeN mice, the endotoxin induced an increase in stromal thickness and corneal haze, indicative of pathology due to inflammation. This response was not seen in C3H/HeJ mice lacking functional TLR4, and was attributed to a lack of production of 2 inflammatory cytokines, platelet endothelial cell adhesion molecule-1 and macrophage inflammatory protein (MIP)-2 (Khatri et al., 2002). Similarly, mice lacking the MyD88 adaptor protein that mediates some of the signal transduction from TLR4 did not respond to LPS with an increase in corneal thickness or haze compared with WT controls (Johnson et al., 2005), and there was no production of the chemokines MIP-2 or KC, which mediates neutrophil entry into injured mouse tissues. Thus, in these studies a role for TLR4 and the adaptor protein MyD88 in corneal pathology in response to LPS was seen. Of note, many aspects of the host responses in the lung and in the cornea to P. aeruginosa infection can result in opposing pathologies and outcomes. Lack of inflammation in the lung, such as is manifest by neutropenia (Oishi et al., 1993; Zweerink et al., 1990) or deficiency of the MyD88 adaptor protein (Power et al., 2004; Skerrett et al., 2004) results in severe susceptibility to P. aeruginosa infection and subsequent death, whereas limiting neutrophil inflammation (Fromer and Klintworth, 1975; Steuhl et al., 1987) or the lack of MyD88-mediated responses (Johnson et al., 2005) protects the cornea from LPS-induced inflammation.
Interaction of P. aeruginosa LPS and the cystic fibrosis transmembrane conductance regulator (CFTR)
The well known hypersusceptibility of CF patients to chronic P. aeruginosa lung infection has provoked numerous studies into the molecular and cellular basis for the inability of these otherwise immunocompetent patients to resist this infection. A number of non-mutually exclusive hypotheses have been proposed, including: 1) increased susceptibility of CF patients to infection due to altered antimicrobial factors in the airway surface epithelium (Travis et al., 2001); 2) increased acidification of the trans-Golgi network (Poschet et al., 2002) leading to altered surface glycoproteins and changes in the airway mucosa promoting infection and inflammation; 3) decreased acidification of the macrophage phagolysosome following phagocytosis of P. aeruginosa resulting in decreased bacterial killing (Di et al., 2006); 4) reduced production of the airway surface liquid overlying the epithelium leading to decreased transport of the upper mucus layer (Boucher, 2004), which inhibits bacterial clearance, and 5) an inability of CF patients to respond to P. aeruginosa with the proper innate immune effectors due to lack of functional CFTR that directly binds to the outer-core oligosaccharide of the P. aeruginosa LPS (Pier et al., 1996, 1997). While the first 4 hypotheses could be contributing factors, none of them adequately explains why it is that over 80% of CF patients become infected with one predominant pathogen, P. aeruginosa, and eventually succumb to chronic infection with this pathogen (Accurso, 2006). To date, only the hypothesis that CFTR-dependent binding of P. aeruginosa LPS and activation of innate immunity provides an explanation for the specificity of the CFTR defect that leads to the overwhelming propensity of CF patients to get P. aeruginosa infections. In the presence of some of the other aspects of CF, such as dehydrated airway surface liquid, thickened mucus, and reduced mucus transport, a complete picture of all of the factors contributing to chronic P. aeruginosa infection in this patient population is beginning to emerge.
In 1996, the conserved outer-core oligosaccharide of the P. aeruginosa LPS was found to be the bacterial ligand mediating entry of P. aeruginosa into lung and corneal epithelial cells (Pier et al., 1996; Zaidi et al., 1996), a process that was defective in cells lacking WT CFTR. The next year it was found that CFTR itself was the host receptor mediating P. aeruginosa epithelial cell internalization (Pier et al., 1997). Importantly, inhibition of P. aeruginosa uptake by airway epithelial cells in the lungs of infected mice by co-administration of either purified LPS core oligosaccharide (Pier et al., 1996) or a peptide composed of CFTR amino acids 108–117, identified as the portion of CFTR that binds P. aeruginosa LPS (Pier et al., 1997), led to reduced bacterial clearance and increased bacterial levels. This was confirmed by studies in transgenic CF mice (Schroeder et al., 2001). It appears that CFTR can actually extract the LPS from the P. aeruginosa outer membrane (Schroeder et al., 2002), promoting the internalization of both the bacterial cells and the LPS molecule. The result of this interaction between P. aeruginosa LPS core and CFTR is activation of a rapid (within 15 min) NF-κB nuclear translocation response in airway epithelial cells of both infected mice and transformed human cells that is absent when either mutant ΔF508 CFTR is present or no CFTR is made (Schroeder et al., 2002). Downstream effects of NF-κB nuclear translocation, including production of IL-6, IL-8, CXC1 and intercellular adhesion molecule-1 are also reduced in the setting of CF (Reiniger et al., 2005), suggesting a lag in the innate immune response that allows P. aeruginosa to establish a niche within the lung from which it cannot be eliminated. Likely this niche is within the dehydrated mucus, as this is where P. aeruginosa is always seen to reside in pathology sections taken from the lung of deceased or transplanted CF patients (Baltimore et al., 1989; Jeffery and Brain, 1988; Worlitzsch et al., 2002).
Further evidence for the importance of the CFTR-P. aeruginosa LPS oligosaccharide interaction in mediating resistance to infection via epithelial cell binding has also been published. Schroeder et al. (2001) showed that mice with WT CFTR readily bound P. aeruginosa to the tracheal epithelium, resulting in clearly visualized cellular internalization and desquamation of epithelial cells loaded with P. aeruginosa bacteria. This was also seen in epithelial cells of infected monkey tracheas (Schroeder et al., 2001). In contrast, tracheal epithelial cells in CFTR knock-out mice, and mice producing ΔF508 CFTR, failed to bind P. aeruginosa and, in fact, by scanning electron microscopy, their tracheas looked comparable to uninfected mice. However, the CF mice had reduced clearance of P. aeruginosa and higher bacterial burdens, indicative of the importance of the CFTR-P. aeruginosa interaction in mediating innate immunity. Confirmation that levels of expression of CFTR affected the host’s ability to clear P. aeruginosa came in a study showing transgenic CF mice were hypersusceptible to chronic P. aeruginosa infection following oral infection (Coleman et al., 2003). In this study, CF mice were mostly unable to clear P. aeruginosa from the lungs whereas WT mice generally did so rapidly. Of note, a line of CFTR knock-out mice that were additionally engineered to over-express human CFTR in the lung under the control of the surfactant protein C promoter had a significantly enhanced ability to clear P. aeruginosa from the lung (Coleman et al., 2003). Thus, producing more CFTR increased clearance of this pathogen from the lung, supporting a role for the bacterial-CFTR interaction in activating host immunity in the setting of WT CFTR.
Both supporting and contravening data have been published regarding the role of CFTR in binding P. aeruginosa LPS and mediating epithelial cell internalization. Esen et al. (2001) found that invasion of human epithelial cells by P. aeruginosa was inhibited by CFTR peptide 108–117, and Kong et al. (2006) found that P. aeruginosa pyocyanin decreased CFTR expression and localization in the cell plasma membrane, and the pyocyanin thus decreased epithelial cell uptake of P. aeruginosa. In addition to P. aeruginosa, Salmonella enterica serovar Typhi has also been shown to use CFTR to cross the intestinal mucosa and initiate infection, a process that leads to typhoid fever (Pier et al., 1998). Epithelial translocation was reduced in mice heterozygous for non-functional CFTR and almost eliminated in transgenic CF mice. The resistance of heterozygous carriers of mutant CFTR to typhoid fever has been proposed as one of the possible selective pressures that might have maintained mutant CFTR alleles in a heterozygous state among European populations at a level of 4–5%. Confirmation of CFTR-dependent intestinal epithelial cell uptake of Salmonella enterica serovar Typhi has been published (Hoare et al., 2006; Tsui et al., 2003).
In contrast, a number of studies have not found CFTR-dependent binding or internalization of P. aeruginosa (Darling et al., 2004; Ko et al., 1997; Zulianello et al., 2006) by cultured epithelial cells, nor an increased susceptibility of transgenic CF mice to P. aeruginosa infection (Chroneos et al., 2000). However, some of these studies used mucoid, LPS-rough strains of P. aeruginosa, which not only do not express the bacterial ligand for CFTR (Pier et al., 1996) but also, the over-expression of alginate inhibits P. aeruginosa uptake by epithelial cells (Massengale et al., 2000; Pier et al., 1996). Indeed, it was shown that a clinical P. aeruginosa LPS-rough, mucoid isolate, FRD-1, lacked the ability to bind CFTR (Schroeder et al., 2001), thus directly explaining the negative findings of Chroneos et al. (2000), who used strain FRD-1 in their studies of susceptibility to infection in transgenic CF mice.
Other studies not finding a P. aeruginosa-CFTR interaction also had experimental design problems that would have interfered with the detection of this interaction. It was reported polarized airway epithelial cells do not bind P. aeruginosa readily (Hybiske et al., 2004; Lee et al., 1999; Plotkowski et al., 1999), or that P. aeruginosa uses a transcellular pathway to penetrate polarized monolayers (Zulianello et al., 2006). However, the trans-epithelial resistance (TER) initially established with these monolayers was very high, usually >1000 Ohms, which is 5 to 20 times that measured in the intact human airway (Barker et al., 1995; Coyne et al., 2003; Knowles et al., 1984). Thus, these over-polarized cell cultures are not physiologic. Of note, when the TER is lowered by EDTA treatment to more closely approximate that of the intact human airway epithelium (60–100 Ohms) (Barker et al., 1995; Coyne et al., 2003; Knowles et al., 1984), then P. aeruginosa interactions and internalization by the polarized cells were observed.
Another problem encountered in the study of the P. aeruginosa-CFTR interaction is the use of transiently transfected cells to express either WT or mutant CFTR (Darling et al., 2004). Transient transfection does not mimic normal CFTR production, glycosylation level, trafficking or biologic effector function (Varga et al., 2004), and without a demonstration that nearly 100% of the cells transiently transfected with WT CFTR have WT properties, it cannot be concluded that a population of cells with WT CFTR function has been produced. Indeed, in a stably transfected dog kidney cell line using green fluorescent protein-labeled CFTR (Moyer et al., 1996), it was shown that only about 20% of the cells expressing intermediate levels of CFTR actually interacted with P. aeruginosa (Gerceker et al., 2000). Cells expressing too much or too little CFTR could not internalize P. aeruginosa. Thus, in the study of Darling et al. (2004) wherein the cell lines only expressed 30% or 50% of the WT levels of CFTR, as determined by immunostaining, there was likely a large under-expression of the WT level of CFTR, preventing normal CFTR-dependent cellular responses. In addition, they did not show if the CFTR produced was in the fully glycosylated form or not, an essential feature for WT CFTR trafficking (Varga et al., 2004). Moreover, their use of sodium butyrate to stimulate CFTR production could have resulted in improper expression of CFTR, along with increased expression of other molecules the transcription of which is increased in the presence of butyrate. Furthermore, as noted above, 2 of the 3 P. aeruginosa strains they studied were LPS-rough, mucoid strains lacking the ability to interact with CFTR. Overall, while there have been a number of studies not finding a role for CFTR in epithelial cell uptake or responses to P. aeruginosa, all have had significant experimental flaws that render the final conclusions suspect.
Role of P. aeruginosa LPS in virulence and immunity to infection
For quite a while we have known that expression of the smooth form of the P. aeruginosa LPS has been essential for full-fledged virulence in acute infection, as evidenced by experimental results from multiple investigators (Cryz Jr. et al., 1984; Goldberg et al., 1995; Ohno et al., 1995; Pier and Thomas, 1982). However, we have also known for over 20 years that LPS rough isolates unable to produce O-antigens predominate in the lungs of chronically infected CF patients (Hancock et al., 1983; Penketh et al., 1983). Other studies have suggested that LPS-rough P. aeruginosa isolates can also be obtained from acutely-infected patients with pneumonia (Hirakata et al., 2000) who rarely get systemic infection, which does require an LPS-smooth strain. Animal studies using a galU mutant of strain PAO1, which only produces a rough LPS, showed that this strain was unable to spread systemically in mice following intranasal inoculation, but at a high enough dose was lethal due to fulminant pneumonia without septicemia (Priebe et al., 2004). Overall, it appears that LPS O-antigens are needed for systemic spread of P. aeruginosa strains but not for infection confined to the lung, likely due to the ability of serum complement to readily kill LPS-rough strains (Hancock et al., 1983; Pier and Ames, 1984).
As P. aeruginosa emerged as a significant nosocomial pathogen in the 1960s attempts to develop vaccines were initiated by a number of pharmaceutical companies. A heptavalent vaccine based on extracts from 7 different strains defined as immunotypes due to their protective activity in mice (Fisher et al., 1969) underwent several clinical trials (Alexander and Fisher, 1974; Alexander et al., 1969) as did a vaccine based on growth in a defined medium and extraction of the culture (Jones et al., 1978, 1979, 1980; Miler et al., 1977). In both of these cases the protective factor was found to be LPS (Crowder et al., 1972; MacIntyre et al., 1986a,b). However, due to the toxicity of these vaccines their final development into a useful clinical product was never realized. Nonetheless, these studies established the principle that P. aeruginosa LPS O-antigens were major targets of highly effective immunity, particularly as noted in animal studies.
A less toxic version of the LPS O-antigens was pursued as a vaccine candidate in the 1970s and 1980s, and consisted of a fraction of the O-antigens that were of a large enough molecular size to be immunogenic on their own and were referred to as high-molecular-weight polysaccharides (Pier, 1982; Pier et al., 1978, 1981b; Pier and Thomas, 1982, 1983). Animal studies clearly showed high-level protection from infection in a number of settings with these antigens, and human studies showed the polysaccharides were immunogenic (Pier, 1982, 1985, 1988; Pier and Thomas, 1983). While antibody-mediated protection clearly predominated as a mediator of immunity, it was also shown that immune T cells played a role (Powderly et al., 1988), which was eventually found to be due to a cytotoxic T cell armed with antibody to the O-antigen mediating an antibody-dependent cellular cytotoxicity killing of the bacterial cells (Markham et al., 1991). However, when some of the serologically related O-antigens from serogroups O2 and O5 were combined into a multivalent vaccine, it was found that antagonistic immune interactions were produced in mice and rabbits (Hatano and Pier, 1998). Attempts to overcome the inherent difficulties in using the LPS O-antigens as a multivalent vaccine by developing monoclonal antibodies to the major LPS O serogroups shows promise as a means for passive therapy (Hemachandra et al., 2001; Lai et al., 2005) of acute P. aeruginosa infection.
A related approach was taken by Cryz and colleagues, who developed an octavalent conjugate vaccine consisting of 8 P. aeruginosa O-antigens conjugated to P. aeruginosa exotoxin A (Cryz Jr. et al., 1988, 1989). The conjugation process detoxified the exotoxin A. This vaccine was evaluated in a non-blinded, retrospectively controlled trial in CF patients (Cryz et al., 1994, 1997; Lang et al., 2004; Langford and Hiller, 1984; Schaad et al., 1990) with encouraging reports of efficacy. However, it was recently reported on the Web site of Crucell N.V. (http://cws.huginonline.com/C/132631/PR/200607/1064252_5_5.html) that a phase III trial involving 476 patients from 46 centers in four European countries did not show efficacy in preventing P. aeruginosa infection in CF patients. A hyperimmune intravenous IgG product derived from the plasma of donors given the octavalent O-antigen conjugate vaccine was evaluated in intensive care units of United States Department of Veterans Affairs and Department of Defense hospitals but again showed no efficacy (Donta et al., 1996). Give the strong protective efficacy of LPS-O-antigen-based vaccines in animals it is difficult to discern what exactly went wrong in these clinical trials, with no clear explanation currently available for their failure.
One potential reason is that the immunogenicity of the vaccines as formulated for human use is poor. Thus newer attempts to use modern molecular genetic tools to produce vaccines with enhanced immunogenicity are being evaluated. A highly attenuated strain of P. aeruginosa has been made by deleting the aroA gene needed for synthesis of aromatic amino acids which are not available in host tissues for P. aeruginosa to incorporate and use (Priebe et al., 2002). This organism has shown good efficacy at inducing primarily serotype-specific immunity for lung infection (Priebe et al., 2003), but interestingly induced non-serotype-specific immunity against eye infections (Zaidi et al., 2006). Goldberg and colleagues have cloned the biosynthetic genes for the O11 LPS O-antigen into an attenuated Salmonella enterica serovar Typhimurium vector (Evans et al., 1994; Goldberg et al., 1992; Pier et al., 1995) and shown that oral and systemic immunization provides some protection against infection (DiGiandomenico et al., 2004). Further development of these vaccine candidates could result in a more effective means to exploit the sensitivity of P. aeruginosa to elimination in the presence of high titers of O-antigen-specific antibody.
A few investigators have tried to use epitopes in the more conserved core of the P. aeruginosa LPS as targets for protective antibodies (Harrison et al., 1997; Noguchi et al., 1991; Terashima et al., 1991; Yokota et al., 1990), but these have not yet shown any efficacy in clinical trials. Other investigators failed to find protective efficacy associated with LPS core epitopes (Hatano et al., 1995). Most likely this is due to the ability of the long O side chains to protect the organism from phagocytosis even when opsonized by antibody and complement fragments deposited close to the outer membrane. These opsonins must be bound to the surface where they are accessible for binding to Fc and complement receptors on phagocytes (Brown et al., 1983; Moffitt and Frank, 1994).
As the problem with P. aeruginosa infections in the intensive care units, among CF patients and in mechanically ventilated patients has not abated in 40 years, while increasing antibiotic resistance and loss of effective antimicrobial treatments has increased (Collin et al., 2001; Gales et al., 2001; Ramphal, 2004), a need for effective immunotherapies remains. While the O-antigen is a high-value target for these immunologic reagents, the diversity of serogroups, the current high cost of multivalent conjugate vaccines or multivalent human monoclonal antibodies, pose major challenges for realizing useful reagents in the near future. Success with attenuated or recombinant bacterial vaccines could advance the field if they can be shown to be safe and immunogenic, although ultimately there will always be a large question of who to immunize to demonstrate clinical efficacy and what will be the outcome parameters documenting efficacy? But the P. aeruginosa LPS is a fascinating and complex molecule with many more properties and facets to be revealed before we get a complete picture of how this important bacterial structure contributes to the manifestation of virulence and immunity to P. aeruginosa infection.
Acknowledgments
Support for the actual research from my laboratory related to the P. aeruginosa LPS has come from the National Institutes of Health, primarily grant AI 22535-20. I want to thank all of my collaborators, colleagues and associates whose names appear on many of our publications for helping to produce the body of data that has advanced our understanding of P. aeruginosa LPS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeyrathne PD, Daniels C, Poon KK, Matewish MJ, Lam JS. Functional characterization of WaaL, a ligase associated with linking O-antigen polysaccharide to the core of Pseudomonas aeruginosa lipopolysaccharide. J Bacteriol. 2005;187:3002–3012. doi: 10.1128/JB.187.9.3002-3012.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accurso FJ. Update in cystic fibrosis 2005. Am J Respir Crit Care Med. 2006;173:944–947. doi: 10.1164/rccm.2601006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JW, Fisher MW, MacMillan BG, Altemeier WA. Prevention of invasive Pseudomonas infection in burns with a new vaccine. Arch Surg. 1969;99:249–256. doi: 10.1001/archsurg.1969.01340140121018. [DOI] [PubMed] [Google Scholar]
- Alexander JW, Fisher MW. Immunization against Pseudomonas infection after thermal injury. J Infect Dis. 1974;130:S152–S158. doi: 10.1093/infdis/130.supplement.s152. [DOI] [PubMed] [Google Scholar]
- Backhed F, Normark S, Schweda EKH, Oscarson S, Richter-Dahlfors A. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 2003;5:1057–1063. doi: 10.1016/s1286-4579(03)00207-7. [DOI] [PubMed] [Google Scholar]
- Baltimore RS, Christie CDC, Smith GJW. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis – Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989;140:1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Barker PM, Boucher RC, Yankaskas JR. Bioelectric properties of cultured monolayers from epithelium of distal human fetal lung. Am J Physiol. 1995;268:L270–277. doi: 10.1152/ajplung.1995.268.2.L270. [DOI] [PubMed] [Google Scholar]
- Beckmann F, Moll H, Jager KE, Zahringer U. 7-O-carbamoyl-L-glycero-D-manno-heptose: a new core constituent in the lipopolysaccharide of Pseudomonas aeruginosa. Carbohydr Res. 1995;267:C3–7. doi: 10.1016/0008-6215(94)00371-l. [DOI] [PubMed] [Google Scholar]
- Blais DR, Vascotto SG, Griffith M, Altosaar I. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:4235–4244. doi: 10.1167/iovs.05-0543. [DOI] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Joiner KA, Frank MM. The role of complement in host resistance to bacteria. Springer Semin Immunopathol. 1983;6:349–360. doi: 10.1007/BF02116279. [DOI] [PubMed] [Google Scholar]
- Bystrova OV, Shashkov AS, Kocharova NA, Knirel YA, Lindner B, Zahringer U, Pier GB. Structural studies on the core and the O-polysaccharide repeating unit of Pseudomonas aeruginosa immunotype 1 lipopolysaccharide. Eur J Biochem. 2002;269:2194–2203. doi: 10.1046/j.1432-1033.2002.02875.x. [DOI] [PubMed] [Google Scholar]
- Bystrova OV, Lindner B, Moll H, Kocharova NA, Knirel YA, Zahringer U, Pier GB. Structure of the lipopolysaccharide of Pseudomonas aeruginosa O-12 with a randomly O-acetylated core region. Carbohydr Res. 2003a;338:1895–1905. doi: 10.1016/s0008-6215(03)00290-8. [DOI] [PubMed] [Google Scholar]
- Bystrova OV, Shashkov AS, Kocharova NA, Knirel YA, Zahringer U, Pier GB. Elucidation of the structure of the lipopolysaccharide core and the linkage between the core and the O-antigen in Pseudomonas aeruginosa immunotype 5 using strong alkaline degradation of the lipopolysaccharide. Biochemistry (Mosc) 2003b;68:918–925. doi: 10.1023/a:1025759217501. [DOI] [PubMed] [Google Scholar]
- Bystrova OV, Lindner B, Moll H, Kocharova NA, Knirel YA, Zahringer U, Pier GB. Full structure of the lipopolysaccharide of Pseudomonas aeruginosa immunotype 5. Biochemistry (Mosc) 2004;69:170–175. doi: 10.1023/b:biry.0000018947.60328.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrova OV, Knirel YA, Lindner B, Kocharova NA, Kondakova AN, Zahringer U, Pier GB. Structures of the core oligosaccharide and O-units in the R- and SR-type lipopolysaccharides of reference strains of Pseudomonas aeruginosa O-serogroups. FEMS Immunol Med Microbiol. 2006;46:85–99. doi: 10.1111/j.1574-695X.2005.00004.x. [DOI] [PubMed] [Google Scholar]
- Cao B, Wang H, Sun H, Zhu Y, Chen M. Risk factors and clinical outcomes of nosocomial multi-drug resistant Pseudomonas aeruginosa infections. J Hosp Infect. 2004;57:112–118. doi: 10.1016/j.jhin.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Choudhury B, Carlson RW, Goldberg JB. The structure of the lipopolysaccharide from a galU mutant of Pseudomonas aeruginosa serogroup-O11. Carbohydr Res. 2005;340:2761–2772. doi: 10.1016/j.carres.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Chroneos ZC, Wert SE, Livingston JL, Hassett DJ, Whitsett JA. Role of cystic fibrosis transmembrane conductance regulator in pulmonary clearance of Pseudomonas aeruginosa in vivo. J Immunol. 2000;165:3941–3950. doi: 10.4049/jimmunol.165.7.3941. [DOI] [PubMed] [Google Scholar]
- Coleman FT, Mueschenborn S, Meluleni G, Ray C, Carey VJ, Vargas SO, Cannon CL, Ausubel FM, Pier GB. Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection. Proc Natl Acad Sci USA. 2003;100:1949–1954. doi: 10.1073/pnas.0437901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin Infect Dis. 2001;33:947–953. doi: 10.1086/322604. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- Coyne MJ, Goldberg JB. Cloning and characterization of the gene (rfc) encoding O-antigen polymerase of Pseudomonas aeruginosa PAO1. Gene. 1995;167:81–86. doi: 10.1016/0378-1119(95)00595-1. [DOI] [PubMed] [Google Scholar]
- Coyne MJ, Russell KS, Coyle CL, Goldberg JB. The Pseudomonas aeruginosa algC gene encodes phosphoglucomutase, required for the synthesis of a complete lipopolysaccharide core. J Bacteriol. 1994;176:3500–3507. doi: 10.1128/jb.176.12.3500-3507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creuzenet C, Lam JS. Topological and functional characterization of WbpM, an inner membrane UDP-GlcNAc C6 dehydratase essential for lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Mol Microbiol. 2001;41:1295–1310. doi: 10.1046/j.1365-2958.2001.02589.x. [DOI] [PubMed] [Google Scholar]
- Crowder JG, Fisher MW, White A. Type-specific immunity in Pseudomonas disease. J Lab Clin Med. 1972;79:47–54. [PubMed] [Google Scholar]
- Cryz S, Jr, Pitt T, Furer E, Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984;44:508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz SJ, Jr, Sadoff JC, Ohman D, Furer E. Characterization of the human immune response to a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. J Lab Clin Med. 1988;111:701–707. [PubMed] [Google Scholar]
- Cryz SJ, Jr, Sadoff JC, Fürer E. Octavalent Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Microb Pathog. 1989;6:75–80. doi: 10.1016/0882-4010(89)90010-7. [DOI] [PubMed] [Google Scholar]
- Cryz SJ, Jr, Wedgwood J, Lang AB, Ruedeberg A, Que JU, Furer E, Schaad UB. Immunization of noncolonized cystic fibrosis patients against Pseudomonas aeruginosa. J Infect Dis. 1994;169:1159–1162. doi: 10.1093/infdis/169.5.1159. [DOI] [PubMed] [Google Scholar]
- Cryz SJ, Jr, Lang A, Rudeberg A, Wedgwood J, Que JU, Furer E, Schaad U. Immunization of cystic fibrosis patients with a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Behring Inst Mitt. 1997:345–349. [PubMed] [Google Scholar]
- Darling KE, Dewar A, Evans TJ. Role of the cystic fibrosis transmembrane conductance regulator in internalization of Pseudomonas aeruginosa by polarized respiratory epithelial cells. Cell Microbiol. 2004;6:521–533. doi: 10.1111/j.1462-5822.2004.00380.x. [DOI] [PubMed] [Google Scholar]
- Dasgupta T, Lam JS. Identification of rfbA, involved in B-band lipopolysaccharide biosynthesis in Pseudomonas aeruginosa serotype O5. Infect Immun. 1995;63:1674–1680. doi: 10.1128/iai.63.5.1674-1680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kievit TR, Lam JS. Isolation and characterization of two genes, waaC (rfaC) and waaF (rfaF), involved in Pseudomonas aeruginosa serotype O5 inner-core biosynthesis. J Bacteriol. 1997;179:3451–3457. doi: 10.1128/jb.179.11.3451-3457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kievit TR, Staples T, Lam JS. Pseudomonas aeruginosa rfc genes of serotypes O2 and O5 could complement O-polymerase-deficient semi-rough mutants of either serotype. FEMS Microbiol Lett. 1997;147:251–257. doi: 10.1111/j.1574-6968.1997.tb10250.x. [DOI] [PubMed] [Google Scholar]
- Dean CR, Goldberg JB. The wbpM gene in Pseudomonas aeruginosa serogroup O17 resides on a cryptic copy of the serogroup O11 O antigen gene locus. FEMS Microbiol Lett. 2000;187:59–63. doi: 10.1111/j.1574-6968.2000.tb09137.x. [DOI] [PubMed] [Google Scholar]
- Dean CR, Goldberg JB. Pseudomonas aeruginosa galU is required for a complete lipopolysaccharide core and repairs a secondary mutation in a PA103 (serogroup O11) wbpM mutant. FEMS Microbiol Lett. 2002;210:277–283. doi: 10.1111/j.1574-6968.2002.tb11193.x. [DOI] [PubMed] [Google Scholar]
- Dean CR, Franklund CV, Retief JD, Coyne MJ, Hatano K, Evans DJ, Pier GB, Goldberg JB. Characterization of the serogroup O11 O antigen locus of Pseudomonas aeruginosa PA103. J Bacteriol. 1999;181:4275–4284. doi: 10.1128/jb.181.14.4275-4284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean CR, Datta A, Carlson RW, Goldberg JB. WbjA adds glucose to complete the O-antigen trisaccharide repeating unit of the lipopolysaccharide of Pseudomonas aeruginosa serogroup O11. J Bacteriol. 2002;184:323–326. doi: 10.1128/JB.184.1.323-326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, Palfrey HC, Nelson DJ. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- DiGiandomenico A, Rao J, Goldberg JB. Oral vaccination of BALB/c mice with Salmonella enterica serovar Typhimurium expressing Pseudomonas aeruginosa O antigen promotes increased survival in an acute fatal pneumonia model. Infect Immun. 2004;72:7012–7021. doi: 10.1128/IAI.72.12.7012-7021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett RG. Microbiology of Pseudomonas aeruginosa. In: Doggett RG, editor. Clinical Manifestations of Infection and Current Therapy. Academic Press; New York: 1979. pp. 1–8. [Google Scholar]
- Donta ST, Peduzzi P, Cross AS, Sadoff J, Haakenson C, Cryz SJ, Kauffman C, Bradley S, Gafford G, Elliston D, Beam TR, John JF, Ribner B, Cantey R, Welsh CH, Ellison RT, Young EJ, Hamill RJ, Leaf H, Schein RMH, Mulligan M, Johnson C, Abrutyn E, Griffiss JM, Hamadeh R, Eliasson AH, Mcclain JB, Melcher GP, Kelly JW, Byrne WR, Wallace M, Amundson D, Gumpert B, Slagle D. Immunoprophylaxis against Klebsiella and Pseudomonas aeruginosa infections. J Infect Dis. 1996;174:537–543. doi: 10.1093/infdis/174.3.537. [DOI] [PubMed] [Google Scholar]
- Ernst RK, Yi EC, Guo L, Lim KB, Burns JL, Hackett M, Miller SI. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science. 1999;286:1561–1565. doi: 10.1126/science.286.5444.1561. [DOI] [PubMed] [Google Scholar]
- Ernst RK, Hajjar AM, Tsai JH, Moskowitz SM, Wilson CB, Miller SI. Pseudomonas aeruginosa lipid A diversity and its recognition by Toll-like receptor 4. J Endotoxin Res. 2003;9:395–400. doi: 10.1179/096805103225002764. [DOI] [PubMed] [Google Scholar]
- Esen M, Grassme H, Riethmuller J, Riehle A, Fassbender K, Gulbins E. Invasion of human epithelial cells by Pseudomonas aeruginosa involves src-like tyrosine kinases p60Src and p59Fyn. Infect Immun. 2001;69:281–287. doi: 10.1128/IAI.69.1.281-287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DJ, Pier GB, Coyne MJ, Goldberg JB. The rfb locus from Pseudomonas aeruginosa strain PA103 promotes the expression of O antigen by both LPS-rough and LPS-smooth isolates from cystic fibrosis patients. Mol Microbiol. 1994;13:427–434. doi: 10.1111/j.1365-2958.1994.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Faure K, Sawa T, Ajayi T, Fujimoto J, Moriyama K, Shime N, Wiener-Kronish JP. TLR4 signaling is essential for survival in acute lung injury induced by virulent Pseudomonas aeruginosa secreting type III secretory toxins. Respir Res. 2004;5:1. doi: 10.1186/1465-9921-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci USA. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MW, Devlin HB, Gnabasik F. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969;98:835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KA, Chen ZJ. Sorting out Toll signals. Cell. 2006;125:834–836. doi: 10.1016/j.cell.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Fromer CH, Klintworth GK. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. II. Studies on the effect of leukocytic elimination on corneal vascularization. Am J Pathol. 1975;81:531–544. [PMC free article] [PubMed] [Google Scholar]
- Gales AC, Jones RN, Turnidge J, Rennie R, Ramphal R. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin Infect Dis. 2001;32(Suppl 2):S146–155. doi: 10.1086/320186. [DOI] [PubMed] [Google Scholar]
- Garau J, Gomez L. Pseudomonas aeruginosa pneumonia. Curr Opin Infect Dis. 2003;16:135–143. doi: 10.1097/00001432-200304000-00010. [DOI] [PubMed] [Google Scholar]
- George SE, Kohan MJ, Gilmour MI, Taylor MS, Brooks HG, Creason JP, Claxton LD. Pulmonary clearance and inflammatory response in C3H/HeJ mice after intranasal exposure to Pseudomonas spp. Appl Environ Microbiol. 1993;59:3585–3591. doi: 10.1128/aem.59.11.3585-3591.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerceker AA, Zaidi T, Marks P, Golan DE, Pier GB. Impact of heterogeneity within cultured cells on bacterial invasion: analysis of Pseudomonas aeruginosa and Salmonella enterica serovar Typhi entry into MDCK cells by using a green fluorescent protein-labelled cystic fibrosis transmembrane conductance regulator receptor. Infect Immun. 2000;68:861–870. doi: 10.1128/iai.68.2.861-870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JB, Hatano K, Meluleni GS, Pier GB. Cloning and surface expression of Pseudomonas aeruginosa O antigen in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:10716–10720. doi: 10.1073/pnas.89.22.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JB, Hatano K, Pier GB. Synthesis of lipopolysaccharide O side chains by Pseudomonas aeruginosa strain PAO1 requires the enzyme phosphomannomutase. J Bacteriol. 1993;175:1605–1611. doi: 10.1128/jb.175.6.1605-1611.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JB, Coyne MJ, Neely AN, Holder IA. Avirulence of a Pseudomonas aeruginosa algC mutant in a burned-mouse model of infection. Infect Immun. 1995;63:4166–4169. doi: 10.1128/iai.63.10.4166-4169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan JRW, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene CM, Carroll TP, Smith SG, Taggart CC, Devaney J, Griffin S, O’Neill SJ, McElvaney NG. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J Immunol. 2005;174:1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- Greer GG, Rietschel ET. Inverse relationship between the susceptibility of lipopolysaccharide (lipid A)-pretreated mice to the hypothermic and lethal effect of lipopolysaccharide. Infect Immun. 1978;20:366–374. doi: 10.1128/iai.20.2.366-374.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisman SE, Wagner HN, Jr, Iio M, Hornick RB. Mechanisms of endotoxin tolerance: ii. relationship between endotoxin tolerance and reticuloendothelial system phagocytic activity in man. J Exp Med. 1964;119:241–264. doi: 10.1084/jem.119.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- Hancock REW, Mutharia LM, Chan L, Darveau RP, Speert DP, Pier GB. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: A class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983;42:170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FJ, Rohm D, Kohzuki T, Noguchi H. Pharmacokinetics, tolerability, and preliminary efficacy of human anti-Pseudomonas aeruginosa monoclonal antibodies in pneumonia and burn infection patients. Hybridoma. 1997;16:413–420. doi: 10.1089/hyb.1997.16.413. [DOI] [PubMed] [Google Scholar]
- Hatano K, Pier GB. Complex serology and immune response of mice to variant high-molecular-weight O polysaccharides isolated from Pseudomonas aeruginosa serogroup O2 strains. Infect Immun. 1998;66:3719–3726. doi: 10.1128/iai.66.8.3719-3726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano K, Goldberg JB, Pier GB. Pseudomonas aeruginosa lipopolysaccharide – evidence that the O-side chains and common antigens are on the same molecule. J Bacteriol. 1993;175:5117–5128. doi: 10.1128/jb.175.16.5117-5128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano K, Goldberg JB, Pier GB. Biologic activities of antibodies to the neutral-polysaccharide component of the Pseudomonas aeruginosa lipopolysaccharide are blocked by O side chains and mucoid exopolysaccharide (alginate) Infect Immun. 1995;63:21–26. doi: 10.1128/iai.63.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber HP, Tulic MK, Tsicopoulos A, Wallaert B, Olivenstein R, Daigneault P, Hamid Q. Toll-like receptors 4 and 2 expression in the bronchial mucosa of patients with cystic fibrosis. Can Respir J. 2005;12:13–18. doi: 10.1155/2005/648984. [DOI] [PubMed] [Google Scholar]
- Hemachandra S, Kamboj K, Copfer J, Pier G, Green LL, Schreiber JR. Human monoclonal antibodies against Pseudomonas aeruginosa lipopolysaccharide derived from transgenic mice containing megabase human immunoglobulin loci are opsonic and protective against fatal Pseudomonas sepsis. Infect Immun. 2001;69:2223–2229. doi: 10.1128/IAI.69.4.2223-2229.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakata Y, Finlay BB, Simpson DA, Kohno S, Kamihira S, Speert DP. Penetration of clinical isolates of Pseudomonas aeruginosa through MDCK epithelial cell monolayers. J Infect Dis. 2000;181:765–769. doi: 10.1086/315276. [DOI] [PubMed] [Google Scholar]
- Hoare A, Bittner M, Carter J, Alvarez S, Zaldivar M, Bravo D, Valvano MA, Contreras I. The outer core lipopolysaccharide of Salmonella enterica serovar Typhi is required for bacterial entry into epithelial cells. Infect Immun. 2006;74:1555–1564. doi: 10.1128/IAI.74.3.1555-1564.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hybiske K, Ichikawa JK, Huang V, Lory SJ, Machen TE. Cystic fibrosis airway epithelial cell polarity and bacterial flagellin determine host response to Pseudomonas aeruginosa. Cell Microbiol. 2004;6:49–63. doi: 10.1046/j.1462-5822.2003.00342.x. [DOI] [PubMed] [Google Scholar]
- Ishiyama N, Creuzenet C, Lam JS, Berghuis AM. Crystal structure of WbpP, a genuine UDP-N-acetylglucosamine 4-epimerase from Pseudomonas aeruginosa: substrate specificity in udp-hexose 4-epimerases. J Biol Chem. 2004;279:22635–22642. doi: 10.1074/jbc.M401642200. [DOI] [PubMed] [Google Scholar]
- Jeffery PK, Brain PR. Surface morphology of human airway mucosa: normal, carcinoma or cystic fibrosis. Scan Microsc. 1988;2:553–560. [PubMed] [Google Scholar]
- Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, Lass JH, Pearlman E. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Roe EA, Gupta JL. Low mortality in burned patients in a Pseudomonas vaccine trial. Lancet. 1978;2:401–403. doi: 10.1016/s0140-6736(78)91868-8. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Roe EA, Gupta JL. Controlled trials of a polyvalent Pseudomonas vaccine in burns. Lancet. 1979;2:977–982. doi: 10.1016/s0140-6736(79)92559-5. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Roe EA, Gupta JL. Controlled trial of Pseudomonas immunoglobulin and vaccine in burn patients. Lancet. 1980;2:1263–1265. doi: 10.1016/s0140-6736(80)92334-x. [DOI] [PubMed] [Google Scholar]
- Khatri S, Lass JH, Heinzel FP, Petroll WM, Gomez J, Diaconu E, Kalsow CM, Pearlman E. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and Toll-like receptor 4. Invest Ophthalmol Vis Sci. 2002;43:2278–2284. [PubMed] [Google Scholar]
- Knirel YA. Polysaccharide antigens of Pseudomonas aeruginosa. CRC Crit Rev Microbiol. 1990;17:273–304. doi: 10.3109/10408419009105729. [DOI] [PubMed] [Google Scholar]
- Knirel YA, Helbig JH, Zahringer U. Structure of a decasaccharide isolated by mild acid degradation and dephosphorylation of the lipopolysaccharide of Pseudomonas fluorescens strain ATCC 49271. Carbohydr Res. 1996;283:129–139. doi: 10.1016/0008-6215(95)00401-7. [DOI] [PubMed] [Google Scholar]
- Knirel YA, Bystrova OV, Shashkov AS, Lindner B, Kocharova NA, Senchenkova SN, Moll H, Zahringer U, Hatano K, Pier GB. Structural analysis of the lipopolysaccharide core of a rough, cystic fibrosis isolate of Pseudomonas aeruginosa. Eur J Biochem. 2001;268:4708–4719. doi: 10.1046/j.1432-1327.2001.02396.x. [DOI] [PubMed] [Google Scholar]
- Knowles M, Murray G, Shallal J, Askin F, Ranga V, Gatzy J, Boucher R. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol. 1984;56:868–877. doi: 10.1152/jappl.1984.56.4.868. [DOI] [PubMed] [Google Scholar]
- Ko YH, Delannoy M, Pedersen PL. Cystic fibrosis, lung infections, and a human tracheal antimicrobial peptide (HTAP) FEBS Lett. 1997;405:200–208. doi: 10.1016/s0014-5793(97)00189-0. [DOI] [PubMed] [Google Scholar]
- Kong F, Young L, Chen Y, Ran H, Meyers M, Joseph P, Cho YH, Hassett DJ, Lau GW. Pseudomonas aeruginosa pyocyanin inactivates lung epithelial vaculoar ATPase-dependent cystic fibrosis transmembrane conductance regulator expression and localization. Cell Microbiol. 2006;8:1121–1133. doi: 10.1111/j.1462-5822.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- Lai Z, Kimmel R, Petersen S, Thomas S, Pier G, Bezabeh B, Luo R, Schreiber JR. Multi-valent human monoclonal antibody preparation against Pseudomonas aeruginosa derived from transgenic mice containing human immunoglobulin loci is protective against fatal Pseudomonas sepsis caused by multiple serotypes. Vaccine. 2005;23:3264–3271. doi: 10.1016/j.vaccine.2005.01.088. [DOI] [PubMed] [Google Scholar]
- Lang AB, Rudeberg A, Schoni MH, Que JU, Furer E, Schaad UB. Vaccination of cystic fibrosis patients against Pseudomonas aeruginosa reduces the proportion of patients infected and delays time to infection. Pediatr Infect Dis J. 2004;23:504–510. doi: 10.1097/01.inf.0000129688.50588.ac. [DOI] [PubMed] [Google Scholar]
- Langford DT, Hiller J. Prospective, controlled study of a polyvalent Pseudomonas vaccine in cystic fibrosis – Three year results. Arch Dis Child. 1984;59:1131–1134. doi: 10.1136/adc.59.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Chow D, Haus B, Tseng W, Evans D, Fleiszig S, Chandy G, Machen T. Airway epithelial tight junctions and binding and cytotoxicity of Pseudomonas aeruginosa. Am J Physiol. 1999;277:L204–217. doi: 10.1152/ajplung.1999.277.1.L204. [DOI] [PubMed] [Google Scholar]
- Lightfoot J, Lam JS. Molecular cloning of genes involved with expression of A-band lipopolysaccharide, an antigenically conserved form, in Pseudomonas aeruginosa. J Bacteriol. 1991;173:5624–5630. doi: 10.1128/jb.173.18.5624-5630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot J, Lam JS. Chromosomal mapping, expression and synthesis of lipopolysaccharide in Pseudomonas aeruginosa – a role for guanosine diphospho (GDP)-D-mannose. Mol Microbiol. 1993;8:771–782. doi: 10.1111/j.1365-2958.1993.tb01620.x. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre S, Lucken R, Owen P. Smooth lipopolysaccharide is the major protective antigen for mice in the surface extract from IATS serotype 6 contributing to the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986a;52:76–84. doi: 10.1128/iai.52.1.76-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre S, McVeigh T, Owen P. Immunochemical and biochemical analysis of the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986b;51:675–686. doi: 10.1128/iai.51.2.675-686.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham RB, Pier GB, Schreiber JR. The role of cytophilic IgG3 antibody in T-cell-mediated resistance to infection with the extracellular bacterium Pseudomonas aeruginosa. J Immunol. 1991;146:316–320. [PubMed] [Google Scholar]
- Massengale AR, Quinn FJ, Williams A, Gallagher S, Aronoff SC. The effect of alginate on the invasion of cystic fibrosis respiratory epithelial cells by clinical isolates of Pseudomonas aeruginosa. Exp Lung Res. 2000;26:163–178. doi: 10.1080/019021400269853. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Miler JM, Spilsbury JF, Jones RJ, Roe EA, Lowbury EJL. A new polyvalent Pseudomonas vaccine. J Med Microbiol. 1977;10:19–27. doi: 10.1099/00222615-10-1-19. [DOI] [PubMed] [Google Scholar]
- Miller WL, Wenzel CQ, Daniels C, Larocque S, Brisson JR, Lam JS. Biochemical characterization of WbpA, a UDP-N-acetyl-D-glucosamine 6-dehydrogenase involved in O-antigen biosynthesis in Pseudomonas aeruginosa PAO1. J Biol Chem. 2004;279:37551–37558. doi: 10.1074/jbc.M404749200. [DOI] [PubMed] [Google Scholar]
- Moffitt MC, Frank MM. Complement resistance in microbes. Springer Semin Immunopathol. 1994;15:327–344. doi: 10.1007/BF01837364. [DOI] [PubMed] [Google Scholar]
- Moyer B, Tabb J, Schweibert E, Guggino W, Langford G, Stanton B. Intracellular trafficking of CFTR in living human airway cells using green fluorescent protein (GFP) Pediatr Pulmonol. 1996;(Suppl 13):209. Abstract no 2. [Google Scholar]
- Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:777–783. doi: 10.1165/rcmb.2003-0329OC. [DOI] [PubMed] [Google Scholar]
- Mulrooney EF, Poon KK, McNally DJ, Brisson JR, Lam JS. Biosynthesis of UDP-N-acetyl-L-fucosamine, a precursor to the biosynthesis of lipopolysaccharide in Pseudomonas aeruginosa serotype O11. J Biol Chem. 2005;280:19535–19542. doi: 10.1074/jbc.M500612200. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Yokota S, Ohtsuka H, Kohzuki T, Terashima M, Irie K. Broad-spectra human monoclonal antibodies that protect mice infected with Pseudomonas aeruginosa in Human Diseases. In: Homma JY, Tanimoto H, Holder IA, Hoiby N, Doring G, editors. Antibiotics and Chemotherapy. Vol. 44. S. Karger AG; Basel: 1991. pp. 172–184. [DOI] [PubMed] [Google Scholar]
- O’Brien AD, Metcalf ES, Rosenstreich DL. Defect in macrophage effector function confers Salmonella typhimurium susceptibility on C3H/HeJ mice. Cell Immunol. 1982;67:325–333. doi: 10.1016/0008-8749(82)90224-6. [DOI] [PubMed] [Google Scholar]
- Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25:1353–1364. doi: 10.1592/phco.2005.25.10.1353. [DOI] [PubMed] [Google Scholar]
- Ohno A, Isii Y, Tateda K, Matumoto T, Miyazaki S, Yokota S, Yamaguchi K. Role of LPS length in clearance rate of bacteria from the bloodstream in mice. Microbiol UK. 1995;141:2749–2756. doi: 10.1099/13500872-141-10-2749. [DOI] [PubMed] [Google Scholar]
- Oishi K, Sonoda F, Iwagaki A, Ponglertnapagorn P, Watanabe K, Nagatake T, Siadak A, Pollack M, Matsumoto K. Therapeutic effects of a human antiflagella monoclonal antibody in a neutropenic murine model of Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother. 1993;37:164–170. doi: 10.1128/aac.37.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penketh A, Pitt T, Roberts D, Hodson ME, Batten JC. The relationship of phenotype changes in Pseudomonas aeruginosa to the clinical condition of patients with cystic fibrosis. Am Rev Respir Dis. 1983;127:605–608. doi: 10.1164/arrd.1983.127.5.605. [DOI] [PubMed] [Google Scholar]
- Pier GB. Safety and immunogenicity of a high molecular weight polysaccharide vaccine to immunotype 1 Pseudomonas aeruginosa. J Clin Invest. 1982;69:303–308. doi: 10.1172/JCI110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB. Pseudomonas aeruginosa surface polysaccharide vaccines. New therapeutic approaches from basic research. In: Speert DP, Hancock REW, editors. Pseudomonas aeruginosa: New Therapeutic Approaches from Basic Research. Vol. 36. S. Karger; Basel: 1985. pp. 157–167. [PubMed] [Google Scholar]
- Pier GB. Polysaccharide antigens of Pseudomonas aeruginosa. Rev Infect Dis. 1988;10:S337–S340. doi: 10.1093/cid/10.supplement_2.s337. [DOI] [PubMed] [Google Scholar]
- Pier GB. Molecular Mechanisms of Microbial Pathogenesis. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AF, Kasper DL, editors. Harrison’s Principles of Internal Medicine. 16. McGraw-Hill; New York: 2004. pp. 700–706. [Google Scholar]
- Pier GB, Ames P. Mediation of the killing of rough, mucoid isolates of Pseudomonas aeruginosa from patients with cystic fibrosis by the alternative pathway of complement. J Infect Dis. 1984;150:223–228. doi: 10.1093/infdis/150.2.223. [DOI] [PubMed] [Google Scholar]
- Pier GB, Thomas DM. Lipopolysaccharide and high molecular weight polysaccharide serotypes of Pseudomonas aeruginosa. J Infect Dis. 1982;145:217–223. doi: 10.1093/infdis/145.2.217. [DOI] [PubMed] [Google Scholar]
- Pier GB, Thomas DM. Characterization of the human immune response to a polysaccharide vaccine from Pseudomonas aeruginosa. J Infect Dis. 1983;148:206–213. doi: 10.1093/infdis/148.2.206. [DOI] [PubMed] [Google Scholar]
- Pier GB, Sidberry HF, Sadoff JC. Protective immunity induced in mice by immunization with a high molecular weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1978;22:919–925. doi: 10.1128/iai.22.3.919-925.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Markham RB, Eardley DD. Correlation of the biological response of C3H/HeJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981a;127:184–191. [PubMed] [Google Scholar]
- Pier GB, Sidberry HF, Sadoff JC. High-molecular-weight polysaccharide antigen from Pseudomonas aeruginosa immunotype 2. Infect Immun. 1981b;34:461–468. doi: 10.1128/iai.34.2.461-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Meluleni G, Goldberg JB. Clearance of Pseudomonas aeruginosa from the murine gastrointestinal tract is effectively mediated by O-antigen-specific circulating antibodies. Infect Immun. 1995;63:2818–2825. doi: 10.1128/iai.63.8.2818-2825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Grout M, Zaidi TS, Olsen JC, Johnson LG, Yankaskas JR, Goldberg JB. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Grout M, Zaidi T, Meluleni G, Mueschenborn SS, Banting G, Ratcliff R, Evans MJ, Colledge WH. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature. 1998;392:79–82. doi: 10.1038/30006. [DOI] [PubMed] [Google Scholar]
- Plotkowski MC, de Bentzmann S, Pereira SH, Zahm JM, Bajolet-Laudinat O, Roger P, Puchelle E. Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am J Respir Cell Mol Biol. 1999;20:880–890. doi: 10.1165/ajrcmb.20.5.3408. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Poschet J, Perkett E, Deretic V. Hyperacidification in cystic fibrosis: links with lung disease and new prospects for treatment. Trends Mol Med. 2002;8:512–519. doi: 10.1016/s1471-4914(02)02414-0. [DOI] [PubMed] [Google Scholar]
- Powderly W, Schreiber JR, Pier GB, Markham RB. T cells recognizing polysaccharide-specific B cells function as contrasuppressor cells in the generation of T cell immunity to Pseudomonas aeruginosa. J Immunol. 1988;140:2746–2752. [PubMed] [Google Scholar]
- Power MR, Peng Y, Maydanski E, Marshall JS, Lin TJ. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J Biol Chem. 2004;279:49315–49322. doi: 10.1074/jbc.M402111200. [DOI] [PubMed] [Google Scholar]
- Priebe GP, Brinig MM, Hatano K, Grout M, Coleman FT, Pier GB, Goldberg JB. Construction and characterization of a live, attenuated aroA deletion mutant of Pseudomonas aeruginosa as a candidate intranasal vaccine. Infect Immun. 2002;70:1507–1517. doi: 10.1128/IAI.70.3.1507-1517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe GP, Meluleni GJ, Coleman FT, Goldberg JB, Pier GB. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect Immun. 2003;71:1453–1461. doi: 10.1128/IAI.71.3.1453-1461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe GP, Dean CR, Zaidi TS, Meluleni GJ, Coleman FT, Coutinho YS, Noto MJ, Urban TA, Pier GB, Goldberg JB. The galU gene of Pseudomonas aeruginosa is required for corneal infection and efficient systemic spread following pneumonia but not for infection confined to the lung. Infect Immun. 2004;72:4224–4232. doi: 10.1128/IAI.72.7.4224-4232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R. Changes in the etiology of bacteremia in febrile neutropenic patients and the susceptibilities of the currently isolated pathogens. Clin Infect Dis. 2004;39(Suppl 1):S25–31. doi: 10.1086/383048. [DOI] [PubMed] [Google Scholar]
- Ramphal R, Balloy V, Huerre M, Si-Tahar M, Chignard M. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J Immunol. 2005;175:3927–3934. doi: 10.4049/jimmunol.175.6.3927. [DOI] [PubMed] [Google Scholar]
- Raymond CK, Sims EH, Kas A, Spencer DH, Kutyavin TV, Ivey RG, Zhou Y, Kaul R, Clendenning JB, Olson MV. Genetic variation at the O-antigen biosynthetic locus in Pseudomonas aeruginosa. J Bacteriol. 2002;184:3614–3622. doi: 10.1128/JB.184.13.3614-3622.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PP, Wang L. Genomic organization of LPS-specific loci. Curr Top Microbiol Immunol. 2002;264:109–135. [PubMed] [Google Scholar]
- Reiniger N, Ichikawa JK, Pier GB. Influence of cystic fibrosis transmembrane conductance regulator on gene expression in response to Pseudomonas aeruginosa infection of human bronchial epithelial cells. Infect Immun. 2005;73:6822–6830. doi: 10.1128/IAI.73.10.6822-6830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchetta HL, Lam JS. Identification and functional characterization of an ABC transport system involved in polysaccharide export of A-band lipopolysaccharide in Pseudomonas aeruginosa. J Bacteriol. 1997;179:4713–4724. doi: 10.1128/jb.179.15.4713-4724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchetta HL, Burrows LL, Pacan JC, Lam JS. Three rhamnosyltransferases responsible for assembly of the A-band D-rhamnan polysaccharide in Pseudomonas aeruginosa: a fourth transferase, WbpL, is required for the initiation of both A-band and B-band lipopolysaccharide synthesis. Mol Microbiol. 1998a;28:1103–1119. doi: 10.1046/j.1365-2958.1998.00871.x. [DOI] [PubMed] [Google Scholar]
- Rocchetta HL, Pacan JC, Lam JS. Synthesis of the A-band polysaccharide sugar D-rhamnose requires Rmd and WbpW: identification of multiple AlgA homologues, WbpW and ORF488, in Pseudomonas aeruginosa. Mol Microbiol. 1998b;29:1419–1434. doi: 10.1046/j.1365-2958.1998.01024.x. [DOI] [PubMed] [Google Scholar]
- Rocchetta HL, Burrows LL, Lam JS. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 1999;63:523–553. doi: 10.1128/mmbr.63.3.523-553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich DL, Glode LM, Mergenhagen SE. Action of endotoxin on lymphoid cells. J Infect Dis. 1977;136(Suppl):S239–245. doi: 10.1093/infdis/136.supplement.s239. [DOI] [PubMed] [Google Scholar]
- Rowe PS, Meadow PM. Structure of the core oligosaccharide from the lipopolysaccharide of Pseudomonas aeruginosa PAC1R and its defective mutants. Eur J Biochem. 1983;132:329–337. doi: 10.1111/j.1432-1033.1983.tb07366.x. [DOI] [PubMed] [Google Scholar]
- Sadovskaya I, Brisson JR, Thibault P, Richards JC, Lam JS, Altman E. Structural characterization of the outer core and the O-chain linkage region of lipopolysaccharide from Pseudomonas aeruginosa serotype O5. Eur J Biochem. 2000;267:1640–1650. doi: 10.1046/j.1432-1327.2000.01156.x. [DOI] [PubMed] [Google Scholar]
- Saiman L, Siegel J. Infection control in cystic fibrosis. Clin Microbiol Rev. 2004;17:57–71. doi: 10.1128/CMR.17.1.57-71.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel G, Reeves P. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr Res. 2003;338:2503–2519. doi: 10.1016/j.carres.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Schaad UB, Lang AB, Wedgwood J, Ruedberg A, Furer E, Que JU, Cryz SJ., Jr Immunization of non-colonized cystic fibrosis patients with a Pseudomonas aeruginosa O-polysaccharide-toxin A conjugate vaccine. Pediatr Pulmonol Suppl. 1990;5:91–92. [Google Scholar]
- Schnare M, Rollinghoff M, Qureshi S. Toll-like receptors: sentinels of host defence against bacterial infection. Int Arch Allergy Immunol. 2006;139:75–85. doi: 10.1159/000090001. [DOI] [PubMed] [Google Scholar]
- Schroeder TH, Reiniger N, Meluleni G, Grout M, Coleman FT, Pier GB. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J Immunol. 2001;166:7410–7418. doi: 10.4049/jimmunol.166.12.7410. [DOI] [PubMed] [Google Scholar]
- Schroeder TH, Lee MM, Yacono PW, Cannon CL, Gerceker AA, Golan DE, Pier GB. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-kappa B translocation. Proc Natl Acad Sci USA. 2002;99:6907–6912. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol. 2004;172:3377–3381. doi: 10.4049/jimmunol.172.6.3377. [DOI] [PubMed] [Google Scholar]
- Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2007;292:L312–322. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- Song PI, Abraham TA, Park Y, Zivony AS, Harten B, Edelhauser HF, Ward SL, Armstrong CA, Ansel JC. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877. [PubMed] [Google Scholar]
- Steuhl KP, Doring G, Henni A, Thiel HJ, Botzenhart K. Relevance of host-derived and bacterial factors in Pseudomonas aeruginosa corneal infections. Invest Ophthalmol Vis Sci. 1987;28:1559–1568. [PubMed] [Google Scholar]
- Terashima M, Uezumi I, Tomio T, Kato M, Irie K, Okuda T, Yokota S, Noguchi H. A protective human monoclonal antibody directed to the outer core region of Pseudomonas aeruginosa lipopolysaccharide. Infect Immun. 1991;59:1–6. doi: 10.1128/iai.59.1.1-6.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis SM, Singh PK, Welsh MJ. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr Opin Immunol. 2001;13:89–95. doi: 10.1016/s0952-7915(00)00187-4. [DOI] [PubMed] [Google Scholar]
- Tredget EE, Shankowsky HA, Rennie R, Burrell RE, Logsetty S. Pseudomonas infections in the thermally injured patient. Burns. 2004;30:3–26. doi: 10.1016/j.burns.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Tsui IS, Yip CM, Hackett J, Morris C. The type IVB pili of Salmonella enterica serovar Typhi bind to the cystic fibrosis transmembrane conductance regulator. Infect Immun. 2003;71:6049–6050. doi: 10.1128/IAI.71.10.6049-6050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta M, Nochi T, Jang MH, Park EJ, Igarashi O, Hino A, Kawasaki S, Shikina T, Hiroi T, Kinoshita S, Kiyono H. Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173:3337–3347. doi: 10.4049/jimmunol.173.5.3337. [DOI] [PubMed] [Google Scholar]
- Varga K, Jurkuvenaite A, Wakefield J, Hong JS, Guimbellot JS, Venglarik CJ, Niraj A, Mazur M, Sorscher EJ, Collawn JF, Bebok Z. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- Verhelst D, Koppen C, Van Looveren J, Meheus A, Tassignon MJ. Clinical, epidemiological and cost aspects of contact lens related infectious keratitis in Belgium: results of a seven-year retrospective study. Bull Soc Belge Ophtalmol. 2005:7–15. [PubMed] [Google Scholar]
- Verhelst D, Koppen C, Van Looveren J, Meheus A, Tassignon MJ. Contact lens-related corneal ulcers requiring hospitalization: a 7-year retrospective study in Belgium. Acta Ophthalmol Scand. 2006;84:522–526. doi: 10.1111/j.1600-0420.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- Walsh AG, Burrows LL, Lam JS. Genetic and biochemical characterization of an operon involved in the biosynthesis of 3-deoxy-D-manno-octulosonic acid in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1999;173:27–33. doi: 10.1111/j.1574-6968.1999.tb13480.x. [DOI] [PubMed] [Google Scholar]
- Walsh AG, Matewish MJ, Burrows LL, Monteiro MA, Perry MB, Lam JS. Lipopolysaccharide core phosphates are required for viability and intrinsic drug resistance in Pseudomonas aeruginosa. Mol Microbiol. 2000;35:718–727. doi: 10.1046/j.1365-2958.2000.01741.x. [DOI] [PubMed] [Google Scholar]
- Wenzel CQ, Daniels C, Keates RA, Brewer D, Lam JS. Evidence that WbpD is an N-acetyltransferase belonging to the hexapeptide acyltransferase superfamily and an important protein for O-antigen biosynthesis in Pseudomonas aeruginosa PAO1. Mol Microbiol. 2005;57:1288–1303. doi: 10.1111/j.1365-2958.2004.04767.x. [DOI] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Doring G. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Tobias PS, Ulevitch RJ, Ramos RA. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989;170:1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Kaya S, Noguchi H. Antigenic epitope in Pseudomonas aeruginosa lipopolysaccharide immunologically cross-reactive with Escherichia coli 026 lipopolysaccharide. FEMS Microbiol Lett. 1990;68:245–248. [PubMed] [Google Scholar]
- Zaidi TS, Fleiszig SMJ, Preston MJ, Goldberg JB, Pier GB. Lipopolysaccharide outer core is a ligand for corneal cell binding and ingestion of Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 1996;37:976–986. [PubMed] [Google Scholar]
- Zaidi TS, Priebe GP, Pier GB. A live-attenuated Pseudomonas aeruginosa vaccine elicits outer membrane protein-specific active and passive protection against corneal infection. Infect Immun. 2006;74:975–983. doi: 10.1128/IAI.74.2.975-983.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulianello L, Canard C, Kohler T, Caille D, Lacroix JS, Meda P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect Immun. 2006;74:3134–3147. doi: 10.1128/IAI.01772-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink HJ, Detolla LJ, Gammon MC, Hutchison CF, Puckett JM, Sigal NH. A human monoclonal antibody that protects mice against Pseudomonas-induced pneumonia. J Infect Dis. 1990;162:254–257. doi: 10.1093/infdis/162.1.254. [DOI] [PubMed] [Google Scholar]
- Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8:946–952. doi: 10.1016/j.micinf.2005.10.006. [DOI] [PubMed] [Google Scholar]