Abstract
Rickets, the state of vitamin D deficiency, has reemerged as a potential problem in the United States. At the dawn of the 20th century, rickets was pervasive among infants residing in the polluted cities of Europe and the northeastern United States.
Important milestones in the history of rickets were the understanding that photosynthesized vitamin D and dietary vitamin D were similar, the discernment of the antirachitic potency of artificial and natural ultraviolet rays, and the discovery that ultraviolet irradiation could render various foods antirachitic. Clinical guidelines were instituted to promote sensible exposure to sunlight and artificial ultraviolet radiation.
In addition, irradiated ergosterol from yeast became the major vitamin D source for food fortification and the treatment of rickets, leading to a public health campaign to eradicate rickets by the 1930s. We review the sequence and turn of events pertaining to the discovery of vitamin D and the strategies for the eradication of the reemerging rickets problem.
RICKETS, THE CLINICAL disorder of vitamin D deficiency during infancy and childhood, is deemed a relic of the past by modern-day clinicians in the United States. However, this apparently vanquished nutritional or sunlight-deprivation disorder is making a comeback. Recent case reports highlight the resurgence of rickets among infants who had been exclusively breastfed beyond 6 months of age without vitamin D supplementation.1–5 The majority of the affected infants were dark skinned (Black, Afro-Caribbean, or of Asian descent),1–6 residents of northern latitudes, or both.7,8
BREASTFEEDING AND THE REEMERGENCE OF RICKETS
Maternal vitamin D status is an important factor for the development of rickets in breastfed infants.9 Typically, breastmilk lacks adequate vitamin D10,11 unless the nursing mother is adequately supplemented with vitamin D or exposed to enough sunlight.12 Humans meet their vitamin D needs from sunlight exposure and diet; however, very few foods are naturally rich in vitamin D.9–11,13–15
The vitamin D precursor (7-dehydrocholesterol) in the skin is photolyzed into previtamin D3 upon exposure to a narrow band of solar ultraviolet-B (UV-B) photons (290–315 nm).9,14,15 Previtamin D3 is rapidly converted to vitamin D3, which is further hydroxylated in the liver to 25-hydroxyvitamin D3 [25(OH)D3] and then in the kidney to 1,25-dihydroxyvitamin D3, the active form of vitamin D3.10,14 25(OH)D is the major circulating vitamin D metabolite, and its measure reflects the vitamin D status of an individual.9
Vitamin D3 photoproduction is influenced by season, latitude of residence, and skin color.16–21 Wintertime vitamin D3 photoproduction is compromised in latitudes above 35°. During winter in the higher latitudes, sunlight has a longer tangential path to reach the earth’s surface, resulting in the absorption and loss of the UV-B photons in the ozone stratosphere. The role of skin color in vitamin D3 photoproduction hinges on the function of melanin. Epidermal melanin concentration determines an individual’s skin color, and dark-skinned individuals have higher levels of cutaneous melanin. The vitamin D3 precursor, 7-dehydrocholesterol, is predominantly concentrated in the viable deeper layers of the epidermis, namely the stratum spinosum and stratum basale.19 Melanin acts as a natural filter (sunscreen) and efficiently absorbs the UV-B photons, compromising vitamin D3 photoproduction in dark-skinned people.20 These individuals need upwards of 6-times greater exposure to UV-B radiation to raise their vitamin D3 levels to the same level as in White individuals.22 Dark-skinned people residing in the northeastern United States are vulnerable year-round—but especially in the winter—to hypovitaminosis D (i.e., vitamin D insufficiency) because of their skin color and inadequate sunlight exposure.9,10,14,15
It is apparent that the current reemergence of rickets has coincided with a resurgence of breast-feeding without adequate vitamin D supplementation.13 The prevalence of hypovitaminosis D is particularly marked among Black women, 42% of whom in the reproductive age group (age 15–49 years) have the condition.23 Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000 showed that non-Hispanic Blacks had consistently lower intakes of vitamin D from food and supplements than did Whites.24 It is therefore not surprising that we are seeing cases of rickets among breastfed dark-skinned infants. Currently, however, there are no guidelines for optimizing and monitoring the vitamin D status of dark-skinned nursing mothers.
Recognizing that prolonged breastfeeding without adequate vitamin D supplementation is an important risk factor for rickets, the American Academy of Pediatrics in April 2003 recommended that all breastfed infants, irrespective of skin color or latitude of residence, be given 200 IU of vitamin D per day.25 This recommendation, however, is still inadequate for addressing the problem of reemerging rickets.9 The Canadian Government has mandated that all breastfed infants receive 400 IU of vitamin D daily.9,26
The reemergence of rickets could also be ascribed to the avoidance of direct exposure to sunlight among infants less than 6 months of age,9 as a preventive measure for reducing the risk of skin cancer during adulthood associated with early exposure to ultraviolet radiation from sunlight.12,13 Even though overt vitamin D deficiency and rickets remain uncommon in the United States, hypovitaminosis D, characterized by low levels of 25(OH)D (< 20 ng/mL), is thought to be more pervasive. Epidemiological and clinical studies have highlighted the excessive prevalence of hypovitaminosis D among apparently healthy children, adolescents, and adults worldwide.9,23,27–35 In this context, we reviewed the history of rickets, focusing on the discovery of vitamin D, a seminal event in public health’s conquest of the rickets epidemic of the early 20th century.
HISTORY OF RICKETS
Rickets, being a sun-deprivation disorder, most likely affected early residents of the world’s temperate climates.36 Soranus of Ephesus, a famous physician of the Greco-Roman Era (1st–2nd century AD), observed bony deformities suggestive of rickets among infants residing in Rome.36–39 It was not until the mid-17th century, however, that rickets was readily recognized as a distinct disease.38 By then, rickets was endemic among residents of the southwest counties of Dorset and Somerset in England.36–39 The mid-17th-century “endemic” of rickets could be explained by this population’s urbanization.36 The concomitant atmospheric pollution resulting in smoke and smog would have hindered the sun-mediated vitamin D synthesis in the population and increased their vulnerability for rickets.
The first published account of rickets as a clinical disease is credited to Daniel Whistler in 1645.40 Whistler wrote his monograph Inaugural Medical Disputation on the Disease of English Children Which Is Popularly Termed the Rickets, a concise description of clinical features and symptoms of rickets, as a thesis for his doctor of medicine degree in October 1645 from Leyden, in the Netherlands.38–40 Whistler’s work, however, was soon eclipsed by Francis Glisson’s treatise on rickets, De Rachitide, or On Rickets, published in 1650.41 Unlike Whistler, Glisson described rickets on the basis of clinical and postmortem experience with the condition. Glisson’s work, which remains a classic, should be credited with highlighting the importance of morbid anatomy in the description of a clinical disorder.39
Little progress in the understanding of rickets was made for the next two and a half centuries.28,30 At the dawn of the 20th century, the expansive industrialization and urban migration in the major cities of western Europe and the northern United States set the stage for the high prevalence of rickets among infants residing in those polluted and “sunless” cities.36,38 Overcrowded living conditions in the big-city slums and tenements and the sunlight deprivation precipitated by atmospheric pollution from smoke and smog were responsible for a rickets epidemic. Increased ozone concentration from industrial pollution and the haze and clouds from atmospheric pollution compromise vitamin D production by absorbing the UV-B photons essential for its synthesis.42–44
The first two and a half decades of the 20th century saw phenomenal advances in the understanding of rickets: the induction of experimental rickets in animal models and the understanding of histological changes in rickets, the delineation of the antirachitic properties of cod liver oil and ultraviolet irradiation, and the use of biochemical and radiological tests in the study of rickets.38 Alfred F. Hess, a New York pediatrician and pioneering nutritional researcher in the early 20th century, fondly referred to this period as the “second great chapter” in the history of rickets and its renaissance period.36(p37)
The medical benefits of cod liver oil, although not as a specific antirachitic agent, were recognized in the folklore of the coastal residents of northern Europe.45 Use of cod liver oil specifically to prevent rickets was first reported by D. Schutte in 1824,46 and German and French physicians recommended cod liver oil for this purpose during the rest of the century.38,45,47 Unfortunately, during the rickets epidemic in Europe and the northern United States at the turn of the 20th century, the specific antirachitic properties of cod liver oil were not universally acknowledged and the medical establishment had become skeptical about its usefulness,45,47 perhaps because prescribed cod liver oil was often of poor quality or contained impurities.36 Classic animal experiments by Edward Mellanby and Elmer McCollum established irrevocably the antirachitic properties of cod liver oil.48,49 They ascribed the antirachitic function of cod liver oil to “fat soluble A” (or vitamin A, which is present in high concentrations in cod liver oil) or a similar substance.38,48 In 1922, McCollum demonstrated that heated cod liver oil lost its protectiveness against vitamin A deficiency (dryness of the conjunctiva and cornea [xerophthalmia] and softening of the cornea [keratomalacia]) but still retained its anti-rachitic function. He coined the term “vitamin D” to refer to the antirachitic factor in cod liver oil, as it was fourth in the sequence of vitamins discovered.38,50
As faith in cod liver oil as an antirachitic agent was being restored, the role of ultraviolet radiation as a therapeutic agent in rickets was established. In 1919, Kurt Huldschinsky cured rickets in infants by exposing them to light rays from a mercury vapor lamp.51 As early as 1822, Jedrzej Sniadecki, a Polish physician, had observed that rates of rickets were higher among infants residing in the polluted, sunless tenements of the inner-city areas of Warsaw than in rural areas; he identified lack of exposure to sunlight as the etiologic factor for rickets.52,53
In 1890, Theobald Palm observed the negative relationship between latitude and occurrence of rickets.54 In the sunny tropics, despite poverty and poor sanitation, rickets among infants was rarer than in temperate climates, where living conditions and diet were better. Palm recognized the benefits of sunlight in the prevention and treatment of rickets and recommended the “systematic use of sun-baths as a preventive and therapeutic measure in rickets.”54(p342) He also suggested that infants and children afflicted with rickets be moved “as early as possible from large towns to a locality where sunshine abounds and the air is dry and bracing.”54(p342)
Establishment of the fact that cod liver oil and sunlight were distinct but similar in their ability to prevent and treat rickets was a significant advance in the study of rickets during the early 20th century.55,56 In 1923, Martha May Eliot, a faculty pediatrician at Yale School of Medicine and a member of the United States Children’s Bureau, began a pioneering 3-year communitywide demonstration project in New Haven, Conn, to explore the efficacy of cod liver oil and sunlight in the prevention of rickets.57 Infants born in the study district during the first 2 years of the study were enrolled and underwent monthly clinical and radiological assessments for rickets. Eliot showed that prophylactic cod liver oil and sunlight therapy were effective in preventing or reversing the disease’s progression. This discovery led to a public health campaign for sensible sun exposure and food fortification with vitamin D2, a major step in the eradication of the rickets epidemic.13 Vitamin D2 (ergocalciferol) is derived from ultraviolet radiation of ergosterol, a vitamin D precursor naturally found in yeast and fungi (ergot) and was the first photosynthesized vitamin D used for food fortification.
SUNLIGHT AND RICKETS
The medical benefits of sunlight were recognized as early as 1822 by Sniadecki.52 In 1903, Auguste Rollier established a natural heliotherapy center at Leysin in the Swiss Alps to treat patients with symptomatic tuberculosis by exposure to sunlight.58 Rollier empirically recognized the benefit of heliotherapy for the cure of rickets as early as 1916.36 In his book on heliotherapy published in 1923, he acknowledged Huldschinsky’s discovery of curing rickets with ultraviolet rays as “the beginnings of a scientific foundation for our own purely empirical conclusions. Sun and ultraviolet rays bear much the same relation to one another as crude drugs do to their synthetically prepared chemical substitutes.”58(p270)
Hess had critically appraised the seasonal variation in the occurrence of rickets among his pediatric patients in the context of their diet.59 In 1922, as a practicing pediatrician in New York City, he was aware that rickets was most prevalent among his patients at the end of March, when it was present in nearly 50% of the breastfed infants despite adequate maternal nutrition.59 Hess noted that “breastmilk, although valuable, is provided with but a scant factor of safety against rickets, and . . . additional protective influence is needed—namely, light.”59(p14) As Sniadecki had done 100 years earlier, Hess identified lack of sunlight as the dominant etiologic agent in the rickets epidemic observed in temperate climates since the time of Glisson.
In the development of experimental or clinical rickets, sunlight has a reciprocal relationship to diet, rate of growth, and the degree of skin pigmentation.60 Animals protected from rickets by exposure to a constant dose of sunlight become vulnerable to rickets if their rate of growth increases, because rickets is a metabolic state of defective mineralization of the growing bones. Compared with a normally growing infant, a marasmic infant with growth failure is relatively protected against rickets and may need less sunlight to stave it off. The degree of skin pigmentation determines the efficacy of sunlight.22,60 In 1917, Hess and Lester Unger observed rickets in nearly 90% of Black infants residing in the Columbus Hill District, a Black neighborhood in New York City.61
Race, diet, rate of growth, geographic latitude of residence, customs, religion, culture, and environmental pollution are factors that modify the influence of sunlight on rickets. Religion and culture play a role in the development of rickets and vitamin D deficiency among breastfed infants from sunny locales such as the Middle East62–64 or first-generation infants from Middle Eastern immigrant families residing in North America, Europe, or Australia.65,66 The custom of wearing traditional clothing that covers most of the body leads to sunlight deprivation in the mother and clinical or subclinical vitamin D deficiency in the nursing infant.67
Hess and several other investigators were able to establish that mere exposure to sunlight could cure rickets.68,69 Establishing the chemical basis of heliotherapy in the treatment of rickets was the next step. John Howland and Benjamin Kramer observed consistently low serum phosphorus levels in infants with active rickets and noted that cod liver oil therapy cured the rickets and normalized the serum phosphorus.70 Hess and Margaret Gutman observed the impact of direct sunlight on rickets and phosphorus levels in a cohort of 7 infants (aged 7 to 37 months) from June through September 1921.71 They found that sunlight therapy improved the clinical and radiological status of rickets and increased mean phosphorus levels from 3.11 mg/dL (low) to 4.02 mg/dL (normal). Hess and Gutman thus established the chemical basis of heliotherapy and showed that the curative processes of sunlight and cod liver oil therapy were similar.71 They claimed that their results “furnish the first definitive evidence of metabolic change in the animal body brought about by the solar rays.”71(p31)
ULTRAVIOLET RADIATION AND RICKETS
Understanding that the antirachitic potency of sunlight and artificial light was limited to a narrow band of ultraviolet radiation was the next major advance in the history of vitamin D3. Hess showed that exposure to a mercury vapor lamp (spectral range=230–595 nm) for 3 minutes from a distance of 3 feet prevented the onset of rickets in rats maintained on a standard diet.59 Interposing a Corning window glass filter (2.6mm thickness) rendered the mercury vapor lamplight ineffective at preventing rickets.59 The filter blocked ultraviolet rays of less than 334 nm, suggesting that these wavelengths were responsible for the antirachitic effect.59
Hess and Mildred Weinstock further characterized the antirachitic spectra of ultraviolet radiation.72 They studied the prevention of rickets in 4-week-old rats on a rachitogenic diet by exposing them to various spectra and intensities of ultraviolet radiation generated from a mercury vapor lamp with special filters (Table 1 ▶). The animals were X-rayed after 21 days and were screened for the histological presence of rickets after 28 days. They concluded that the spectra of ultraviolet rays protective against rickets “have a wave length not longer than 302 or possibly 313 millimicrons [nanometers].”72(p689)
Table 1—
Prevention of Rickets by Filtered Mecury Vapor Lamp Rays
| Filter | Lower Limit of Ultraviolet Spectra, nm | Exposure Time, Min. | Distance, In | X-Ray Evidence of Ricketsa | Ricketsa |
| G38 H 4.6mm thickness | 475 | 10–60 | 9–12 | + | + |
| Window glass 2.6 mm | 334 | 15–60 | 9–36 | + | + |
| G 586A 4.3 mm | 302 | 60 | 9 | − | − |
| Pyrex 0.8 mm | 289 | 6–15 | 18 | − | − |
Source. Adapted from Hess and Weinstock.72
Note. G 38 H and G 586 A were glass filters manufactured by Corning Glass Works, Corning, NY.
aPlus sign denotes moderate or marked rickets; minus sign denotes absence of rickets.
Ultraviolet radiation from the sun constitutes less than 1% of total solar radiation, and the shortest waves of sunlight reaching the surface of the earth are 290 nm.73 It is therefore only a narrow band of the sun’s ultraviolet radiation (290–315 nm) that influences the vitamin D3 status, bone health, and calcium economy of humans.74 According to Hess, the quantity and quality of the antirachitic ultraviolet spectrum of sunlight is “circumscribed by nature and furthermore limited by natural and artificial meteorological conditions.”36 Seasonal variation in the antirachitic ultraviolet spectrum of sunlight was the dominant factor in the excessive prevalence of rickets during winter among infants residing in the temperate climes during the early part of the 20th century.36(p114) Variation in the ultraviolet spectrum of sunlight reaching the earth’s surface hinges on altitude and latitude, season of the year, time of day, and atmospheric pollution; however, Hess wrote, “the dominant factor in regard to the antirachitic activity of solar rays is not so much the number of hours of sunshine as its quality and intensity.”36(p114)
Activation of Vitamin D in Foods by Ultraviolet Radiation
The quest to trap the sun’s radiant energy in foods to render them antirachitic soon followed. The role of sunlight in rickets was shrouded in mystery.75 It was evident that direct ultraviolet irradiation could promote growth in rats maintained on a vitamin D–deficient diet containing a high calcium and low phosphorus content. Ultraviolet irradiation from mercury vapor lamps promoted growth in rats failing to thrive on diets deficient in fat-soluble vitamin A, despite the rats’ overt vitamin A deficiency (progressive xerophthalmia).76 Heated and oxidized cod liver oil (devoid of vitamin A) was comparable to ultraviolet irradiation in growth promotion, suggesting that the antirachitic agent in ultraviolet radiation and cod liver oil were identical in function.76
In 1923, Eleanor Hume and Hannah Smith reported from England that rats transferred to “empty” jars that were previously exposed to ultraviolet radiation grew as well as rats that were irradiated directly; they concluded that “irradiated air” was imparted with a growth-promoting property.77 Attempts to corroborate their study, however, were unsuccessful.78,79 E.M. Nelson and Harry Steenbock of the University of Wisconsin at Madison were aware of Hume and Smith’s report and speculated that the protection noted in the “empty” irradiated jars was perhaps because of the activation of residual sawdust or foods that were not removed prior to the irradiation of the jars.79 They were intrigued by the results of their own irradiation experiments. Much to their amazement, rats maintained on a rachitogenic diet began to grow when irradiated rats were introduced into their cage.79 They attributed the growth promotion in the nonirradiated rats to the ingestion of the “photochemically activated” excreta of the irradiated rats.79,80
Steenbock and Archie Black irradiated foods to see if they could be rendered antirachitic. They presumed that foods that caused growth failure in rats that did not show signs of overt vitamin A deficiency were deficient in the antirachitic factor. When they exposed such foods to a mercury vapor lamp and fed them to rachitic rats, the foods promoted growth and calcium assimilation in the rats, similar to what happened when the rats were irradiated directly.81 Steenbock realized the potential of his discovery and chose to patent it to prevent the misuse of the irradiation process and to monitor the advertisements, claims, and quality of irradiated products.82,83
Soon after Steenbock published his findings, Hess and Weinstock reported similar results. Cottonseed oil, linseed oil, wheat germ, and lettuce deemed inert and ineffective for treating rickets were made potent antirachitic agents by ultraviolet irradiation.84 They speculated that if the antirachitic factor in the irradiated foods was similar to cod liver oil, it should be considered a vitamin, and their results would “constitute the first demonstration of the production of vitamin in vitro.”84(p143) Hess did not foresee the commercial potential for activation of foods by ultraviolet irradiation and suggested that “the therapeutic value of this procedure is of secondary importance”84(p143) and perhaps of value only in the event of cod liver oil shortage.
The discovery that ultraviolet irradiation of foods could render them antirachitic was a major breakthrough. It became possible to enhance the vitamin D content of common infant foods such as milk and cereal in an inexpensive and palatable way. Consumption of such vitamin D–enhanced foods led to the eradication of “epidemic” rickets. People were advised to take their “daily dose of sunshine”85(p129) in their diet. Within 2 decades, a wide variety of foods and beverages were fortified with vitamin D, including bread, custard, soda, hot dogs, and even beer.53
Steenbock Patents
Steenbock wanted to patent his irradiation process to ensure the quality of the commercially produced vitamin D–enhanced irradiated foods and to protect the Wisconsin dairy industry from the oleomargarine industry.83,86 Unlike butter, margarine, a cheap butter substitute, lacked vitamins A and D; however, margarine could be fortified with vitamin A. Steenbock was convinced that the Wisconsin dairy industry would suffer if the oleomargarine manufacturers had access to a process for vitamin D enrichment.83,86
Steenbock asked the University of Wisconsin to manage his patents. The response from the Board of Regents was tentative and lukewarm, as there was no precedence for patent management.83,86 Realizing the prospects of Steenbock’s discovery, Harry L. Russell, dean of the College of Agriculture, and Charles S. Slichter, dean of the Graduate School, convinced several alumni to create an independent organization to handle the patents.83,86,87 Thus, the Wisconsin Alumni Research Foundation (WARF) was founded on November 14, 1925, to administer Steenbock’s patents.87 WARF granted the licenses for using the irradiation process and functioned as an intermediary between the university and commerce.86 It ensured the quality of irradiated products and monitored the appropriateness of the manufacturers’ advertising claims. WARF was able to deny licensing to the oleomargarine industry, thereby ensuring the commercial viability of Wisconsin’s dairy industry, the driving force of the state’s agricultural economy.83 The monies generated from the licenses were used exclusively for the promotion of research at the University of Wisconsin.83
Quaker Oats received the first license from WARF in February 1927 to manufacture vitamin D–enriched breakfast cereal.87 Licenses were issued to pharmaceutical companies (Abbott Laboratories, Mead Johnson, Parke Davis, Winthrop Chemical Co, and Squibb) to manufacture a medicinal vitamin D product called Viosterol (irradiated ergosterol).83 By 1934, the irradiation process was extended to produce vitamin D–fortified milk.83 Soon, vitamin D fortification was achieved inexpensively by adding vitamin D directly to milk. The advent and consumption of vitamin D–fortified foods led to the eradication of rickets. Vitamin D food fortification was a public health triumph. Photosynthesis of vitamin D in foods by ultraviolet irradiation, made feasible by the seminal discoveries of Steenbock and Hess, was instrumental in this success.
THE NATURE OF SUNSHINE VITAMIN D
The chemical nature of vitamin D was yet to be discerned. The vitamin D precursor substrate, activated by irradiation, was traced to the “sterol” fraction of foods, phytosterol in vegetable foods and to cholesterol in animal foods.88 Hess et al. demonstrated that phytosterol obtained from cottonseed oil and cholesterol obtained from brain tissue could be rendered antirachitic by ultraviolet irradiation.88 With intuition, they hypothesized that ultraviolet irradiation from solar rays and artificial sources activates the cholesterol in the skin to render it antirachitic and suggested that the proposed mechanism “presupposes not only formation of active cholesterol within the skin but its further transport by way of circulation.”88,89 Huldschinsky showed that irradiation of 1 arm could cure the rickets in the other, suggesting that something made in the skin had to enter the circulation to impart the cure.51,55
Chemical purification of the sterols was attempted to help further delineate the exact nature of the vitamin D precursor. It was realized that the vitamin D precursor in cholesterol rendered antirachitic by ultraviolet irradiation was a contaminant and not a part of the purified cholesterol.90 An international collaborative effort between Hess (New York, NY), Adolf Windaus (Gottingen, Germany), and Otto Rosenheim (London, England) was responsible for identifying and clarifying the nature of the vitamin D precursor “contaminant” in cholesterol.89 Spectroscopic absorption studies highlighted that the vitamin D precursor fraction of cholesterol exhibited 3 absorption peaks (269, 280, 293 nm).91 From these data, they identified the vitamin D precursor fraction as ergosterol, which is typically found in yeast and fungi (ergot). Irradiated ergosterol (also known as ergocalciferol, calciferol, viosterol, and vitamin D2) was the first photosynthesized antirachitic agent to be discovered.
The vitamin D precursor factor in animals or humans that, through exposure to ultraviolet irradiation, became antirachitic had to be different because ergosterol is distributed only in plants and fungi. In 1934, J. Waddell demonstrated that the vitamin D precursor in cod liver oil and irradiated nonpurified cholesterol was different from ergosterol.92 Windaus and F. Bock identified the vitamin D precursor in animal skin as 7-dehydrocholesterol.89,93 Irradiated 7-dehydrocholesterol was the photosynthesized antirachitic agent in the skin and in foods of animal origin; it came to be called vitamin D3, or cholecalciferol.89
CONCLUSION
We have reviewed the story of the discovery of photosynthesized vitamin D. Edwards Park states, “But for rickets vitamin D would not have been discovered. Its discovery was the secret to rickets; its use is essentially the therapy of that disease.”94 The discovery of vitamin D led to the eradication of the epidemic rickets of the early 20th century. Pioneering advances were made in the understanding of vitamin D and rickets from 1915 to 1935. The discovery of the synthesis of vitamin D by the irradiation of foods was the “jewel in the crown” of vitamin D discoveries. This discovery was a catalyst for the public health triumph against rickets. It became feasible to fortify and enrich milk and other foods with vitamin D to ensure that the general population was likely to consume sufficient vitamin D. The fortification of milk with vitamin D was also adopted in Europe; however, the process was not closely monitored, and in Great Britain it caused an outbreak of vitamin D intoxication, or hypercalcemia, the clinical manifestations of which are loss of appetite, lethargy, excessive thirst and polyuria, nausea and vomiting, constipation, and muscle weakness, and renal failure if the hypercalcemic state is unrecognized and prolonged.95 This outbreak led to the banning of the vitamin D fortification of milk in most of Europe.96
In most of present-day Europe, margarine and some cereals are the commonly available vitamin D–enriched foods. Recognizing the excessive prevalence of wintertime vitamin D deficiency, the Finnish government reinstituted the vitamin D3 fortification of milk in 2002.97–99 In the United States, vitamin D fortification of milk was carefully monitored for its vitamin D content through the WARF antirachitic line-test assay, which prevented the occurrence of hypercalcemia as a consequence of vitamin D fortification. The promotion and consumption of vitamin D–fortified milk, vitamin D supplementation, and sensible sun exposure were the factors responsible for the near eradication of the epidemic rickets of the early 20th century.
The current reemergence of nutritional rickets among vulnerable groups of infants warrants public health initiatives and strategies. Campaigns need to highlight the relevance of vitamin D nutrition for the skeletal and general health of all age groups. Screening strategies for rickets and vitamin D deficiency—such as assessment of vitamin D status by measuring the concentration of serum 25(OH)D—among at-risk infants need to be developed and evaluated for feasibility and cost-effectiveness. From the perspective of prevention of nutritional rickets, the focus has to rely on maintaining adequate vitamin D status for both mother and infant. Sensible sun exposure, adequate vitamin D supplementation, and the availability and consumption of vitamin D–fortified foods during pregnancy and lactation are relevant for ensuring an adequate vitamin D concentration in breast-milk. A targeted public health campaign to guarantee that all breastfed infants are receiving adequate vitamin D supplementation and are screened and monitored for rickets during infancy will ensure the eradication of reemerging rickets.
Figure 1.

Alfred F. Hess (1875–1933).
Source. Courtesy of National Library of Medicine
Figure 2.
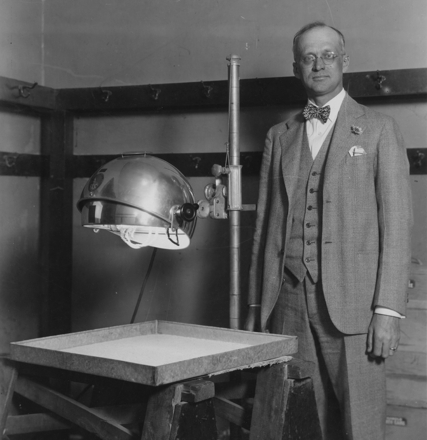
Harry Steenbock (1886–1967) in an undated photograph showing Steenbock conducting a food irradiation experiment.
Source. Courtesy of University of Wisconsin–Madison Archives.
Acknowledgments
This work was supported in part by the National Center on Minority Health and Health Disparities, National Institutes of Health (grant P60 MD000207).
We acknowledge the University of Wisconsin–Madison Archives and the National Library of Medicine for the photographs.
Peer Reviewed
Contributors K. Rajakumar originated the idea for this project and developed it into an article, with direct contributions from the coauthors. The coauthors directly contributed and edited the article at various stages of its development and revision.
REFERENCES
- 1.Severe malnutrition among young children—Georgia, January 1997–June 1999. MMWR Morb Mortal Wkly Rep. 2001;50:224–227. [PubMed] [Google Scholar]
- 2.Tomashek KM, Nesby S, Scanlon KS, et al. Nutritional rickets in Georgia. Pediatrics. 2001;107:E45. Available at: http://www.pediatrics.org/cgi/content/full/107/4/e45. Accessed July 15, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Kreiter SR, Schwartz RP, Kirkman HN Jr, Charlton PA, Calikoglu AS, Davenport ML. Nutritional rickets in African-American breast-fed infants. J Pediatr. 2000;137:153–157. [DOI] [PubMed] [Google Scholar]
- 4.Biser-Rohrbaugh A, Hadley-Miller N. Vitamin D deficiency in breast-fed toddlers. J Pediatr Orthop. 2001;21: 508–511. [PubMed] [Google Scholar]
- 5.Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004;80:1697S–1705S. [DOI] [PubMed] [Google Scholar]
- 6.Ladhani S, Srinivasan L, Buchanan C, Allgrove J. Presentation of vitamin D deficiency. Arch Dis Child. 2004;89: 781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eugster EA, Sane KS, Brown DM. Minnesota rickets: need for policy changes to support vitamin D supplementation. Minn Med. 1996;79:29–32. [PubMed] [Google Scholar]
- 8.Binet A, Kooh SW. Persistence of vitamin D deficiency rickets in Toronto in the 1990s. Can J Public Health. 1996; 87:227–230. [PubMed] [Google Scholar]
- 9.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academy Press; 1997;251–287. [PubMed]
- 11.Vitamins: vitamin D. In: Kleinman RE, ed. Pediatric Nutrition Handbook. 4th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 1998:275–277.
- 12.Hollis BW, Wagner CL. Vitamin D requirements during lactation: high-dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and nursing infant. Am J Clin Nutr. 2004;80:1752S–1758S. [DOI] [PubMed] [Google Scholar]
- 13.Rajakumar K, Thomas SB. Reemerging nutritional rickets: a historical perspective. Arch Pediatr Adolesc Med. 2005;159:335–341. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D: the under-appreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes. 2002;9: 87–98. [Google Scholar]
- 15.Holick MF. McCollum Award Lecture, 1994: vitamin D—new horizons for the 21st century. Am J Clin Nutr. 1994; 60:619–630. [DOI] [PubMed] [Google Scholar]
- 16.Stamp TCB, Round JM. Seasonal changes in human plasma levels of 25 (OH) vitamin D. Nature. 1974;247: 563–565. [DOI] [PubMed] [Google Scholar]
- 17.McLauglin M, Fairney A, Lester E, et al. Seasonal variations in serum 25-hydroxycholecalciferol in healthy people. Lancet. 1974;i:536–537. [DOI] [PubMed] [Google Scholar]
- 18.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:1108–1110. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, MacLaughlin JA, Clark MB, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210: 203–205. [DOI] [PubMed] [Google Scholar]
- 20.Norman AW. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxy vitamin D: integral component of vitamin D endocrine system. Am J Clin Nutr. 1998;67:1108–1110. [DOI] [PubMed] [Google Scholar]
- 21.Harris SS, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations in young American black and white women. Am J Clin Nutr. 1998;67:1232–1236. [DOI] [PubMed] [Google Scholar]
- 22.Clemens TL, Henderson SL, Adams JS, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. [DOI] [PubMed] [Google Scholar]
- 23.Nesby-O’Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. [DOI] [PubMed] [Google Scholar]
- 24.Moore CE, Murphy MM, Holick MF. Vitamin D intakes by children and adults in the United States differ among ethnic groups. J Nutr. 2005;135: 2478–2485. [DOI] [PubMed] [Google Scholar]
- 25.American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111:908–910. [DOI] [PubMed] [Google Scholar]
- 26.Vitamin D supplementation of breastfed infants—2004 Health Canada recommendation. Available at: http://www.hc-sc.gc.ca/fn-an/nutrition/child-enfant/infant-nourisson/vita_d_supp_e.html. Accessed July 26, 2006.
- 27.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81: 353–373. [DOI] [PubMed] [Google Scholar]
- 28.El-Hajj Fuleihan G, Nabulsi M, Choucair M, et al. Hypovitaminosis D in healthy school children. Pediatrics. 2001; 107:E53. [DOI] [PubMed] [Google Scholar]
- 29.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. [DOI] [PubMed] [Google Scholar]
- 30.Gordon CM, DePeter KC, Feldman HA, et al. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158: 531–537. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan SS, Rosen CJ, Halteman WA, et al. Adolescent girls in Maine are at risk for vitamin D insufficiency. J Am Diet Assoc. 2005;105:971–974. [DOI] [PubMed] [Google Scholar]
- 32.Marwaha RK, Tandon N, Reddy DR, et al. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005; 82:477–482. [DOI] [PubMed] [Google Scholar]
- 33.Sachan A, Gupta R, Das V, et al. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060–1064. [DOI] [PubMed] [Google Scholar]
- 34.Al Faraj S, Al Mutairi K. Vitamin D deficiency and chronic low back pain in Saudi Arabia. Spine. 2003;28:177–179. [DOI] [PubMed] [Google Scholar]
- 35.Gaugris S, Heaney RP, Boonen S, et al. Vitamin D inadequacy among post-menopausal women: a systematic review. QJM. 2005;98:667–676. [DOI] [PubMed] [Google Scholar]
- 36.Hess AF. Rickets Including Osteomalacia and Tetany. Philadelphia, Pa: Lea & Febiger; 1929.
- 37.Ruhrah J. Pediatrics of the Past. New York, NY: Paul B. Hoeber Inc; 1925.
- 38.Rajakumar K. Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective. Pediatrics. 2003; 112: e132–e135. Available at: http://www.pediatrics.org/cgi/content/full/112/e132. Accessed July 15, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Still GF. The History of Pediatrics. The Progress of the Study of Disease of Children up to the End of XVIIIth Century. London, England: Oxford University Press, Humphrey Milford; 1931.
- 40.Polyandri a Kerchoven J, Whistler D. Disputatio Medica Inaugurales de Morbo Puerili Anglorum Quem Patrio Idiômate Indiginae Vocant The Rickets: Quam Deo Suppetias Ferente. [Inaugural Medical Disputation on the Disease of English Children Which Is Properly Termed the Rickets.] London: Ex Typis Thomæ Flesher; 1684. Reprint, originally published: Lugduni Batavorum: Ex Officinâ Wilhemi Christiani Boxii, 1645.
- 41.Glisson F, Bate G, Regemorter A. De Rachitide, Sive, Morbo Puerili: Qui Vulgo The Rickets Dicitur, Tractatus /Operâ Primò ac Potissimum Francisci Glissonii: Adscitis in Operis Societatem Georgio Bate & Ahasuero Regemorto. [A Treatise of the Rickets, Being a Disease Common to Children.] London: Typis Guil. Dugardi, Impensis Laurentii Sadler, & Roberti Beaumont; 1650.
- 42.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61: 638S–645S. [DOI] [PubMed] [Google Scholar]
- 43.Engelsen O, Brustad M, Aksnes L, et al. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol. 2005;81:1287–1290. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal KS, Mughal MZ, Upadhyay P, et al. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002;87:11–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hide AJ. Studies on the history of rickets, II: the roles of cod liver oil and light. Pharm Hist. 1975;17:13–20. [PubMed] [Google Scholar]
- 46.Schutte D. Beobachtungen Uber den Nutzen des Berger Leberthrans (Oleum jecoris Aseli, von Gadus asellus L). (First report on the use of cod liver oil in the treatment of rickets.) Arch Med Erfahr. 1824;2:79–92. [Google Scholar]
- 47.Guy RA. The history of cod liver oil as a remedy. Am J Dis Child. 1923; 26:112–116. [Google Scholar]
- 48.Mellanby E. An experimental investigation on rickets. Lancet. 1919;1: 407–412. [Google Scholar]
- 49.Shipley PG, Park EA, McCollum EV, Simmonds N, Parsons HT. Studies on experimental rickets, II: the effect of cod liver oil administered to rats with experimental rickets. J Biol Chem. 1921;45: 343–348. [Google Scholar]
- 50.McCollum EV, Simmonds N, Becker JE, Shipley PG. Studies on experimental rickets, XXI: an experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem. 1922;53:293–312. [PubMed] [Google Scholar]
- 51.Huldschinsky K. Heilung von Rachitis durch Kunstliche Hohensonne. [Rickets Cured by Ultraviolet Irradiation.] Dtsch Med Wochenschr 1919;45: 712–713. [Google Scholar]
- 52.Mozolowski W. Jedrzej Sniadecki (1768–1838) on the cure of rickets. Nature. 1939;143:121–124. [Google Scholar]
- 53.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular diseases. Am J Clin Nutr. 2004; 80(suppl):1678S–1688S. [DOI] [PubMed] [Google Scholar]
- 54.Palm T. The geographical distribution and etiology of rickets. Practitioner. 1890;45:270–279,321–342. [Google Scholar]
- 55.Park EA. The etiology of rickets. Physiol Rev. 1923;3:106–163. [Google Scholar]
- 56.Park EA, Guy RA, Powers GF. A proof of the regulatory influence of cod liver oil on calcium and phosphorus metabolism. Am J Dis Child. 1923;26: 103–111. [Google Scholar]
- 57.Eliot MM. The control of rickets. JAMA. 1926;85:656–663. [Google Scholar]
- 58.Rollier A. Heliotherapy. London, England: Henry Frowde and Hodder & Stoughton; 1923.
- 59.Hess AF. Influence of light in the prevention and cure of rickets. Lancet. 1922;ii:367. Reprinted in: Hess AF. Collected Writings. Vol 2. Springfield, Ill: Charles C. Thomas; 1936:5–14. [Google Scholar]
- 60.Hess AF. Newer aspects of the rickets problem. JAMA. 1922;78:1177–1183. [Google Scholar]
- 61.Hess AF, Unger LJ. Prophylactic therapy of rickets in a Negro community. JAMA. 1918;70:900. Reprinted in: Hess AF. Collected Writings. Vol 1. Springfield, Ill: Charles C. Thomas; 1936:487–494. [Google Scholar]
- 62.Elidrissy AT, Sedrani SH, Lawson DE. Vitamin D deficiency in mothers of rachitic infants. Calcif Tissue Int. 1984; 36:266–268. [DOI] [PubMed] [Google Scholar]
- 63.Karrar ZA. Vitamin D deficiency rickets in developing countries. Ann Trop Paediatr. 1998;18:S89–S92. [DOI] [PubMed] [Google Scholar]
- 64.Andiran N, Yordam N, Ozon A. Risk factors for vitamin D deficiency in breast-fed newborns and their mothers. Nutrition. 2002;18:47–50. [DOI] [PubMed] [Google Scholar]
- 65.Robinson PD, Hogler W, Craig ME, et al. The re-emerging burden of rickets: a decade of experience from Sydney. Arch Dis Child. 2006;91:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thatcher T, Fischer P, Strand M, et al. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr. 2006;26:1–16. [DOI] [PubMed] [Google Scholar]
- 67.DeLucia MC, Carpenter TO. Rickets in the sunshine? Nutrition. 2002;18: 97–99. [DOI] [PubMed] [Google Scholar]
- 68.Chick H. Study of rickets in Vienna 1919–1922. Med Hist. 1976;20:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hess AF, Unger LJ. The cure of infantile rickets by artificial light and by sunlight. Proc Soc Exper Biol Med. 1921; 18:298. [Google Scholar]
- 70.Howland J, Kramer B. Calcium and phosphorus in the serum in relation to rickets. Am J Dis Child. 1921;22:105. [Google Scholar]
- 71.Hess AF, Gutman MB. The cure of infantile rickets by sunlight accompanied by an increase in the inorganic phosphate of the blood. JAMA. 1922;78: 29–31. [Google Scholar]
- 72.Hess AF, Weinstock M. A study of light waves in their relation to rickets. JAMA. 1923;80:687–690. [Google Scholar]
- 73.Hess AF. The ultraviolet rays of the sun. JAMA. 1925;84:1033. Reprinted in: Hess AF. Collected Writings. Vol 2. Springfield, Ill: Charles C. Thomas; 1936:167–178. [Google Scholar]
- 74.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–1003. [DOI] [PubMed] [Google Scholar]
- 75.Carpenter KJ, Zhao L. Forgotten mysteries in the early history of vitamin D. J Nutr. 1999;129:923–927. [DOI] [PubMed] [Google Scholar]
- 76.Steenbock H, Nelson EM. Fat-soluble vitamine, XIII: light in its relation to ophthalmia and growth. J Biol Chem. 1923;56:355–373. [Google Scholar]
- 77.Hume EM, Smith HH. The effect of air, which has been exposed to the radiations of the mercury-vapor quartz lamp, in promoting the growth of rats, fed on a diet deficient in fat-soluble vitamins. Biochem J. 1923;17:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Webster TA, Hill L. The supposed influence of irradiated air on growth. Biochem J. 1924;18:340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nelson EM, Steenbock H. Fat-soluble vitamins, XXI: observations bearing on the alleged induction of growth-promoting properties in air by irradiation with ultra-violet light. J Biol Chem. 1925; 62:575–593. [Google Scholar]
- 80.Nelson EM, Steenbock H. Fat-soluble vitamins, XXV: further observations on the anti-rachitic action of irradiated animals on the non-irradiated when placed in the same cage. Am J Physiol. 1925;73:341–345. [Google Scholar]
- 81.Steenbock H, Black A. Fat-soluble vitamins: the induction of growth-promoting and calcifying properties in a ration by exposure to ultra-violet light. J Biol Chem. 1924;61:405–422. [DOI] [PubMed] [Google Scholar]
- 82.Steenbock H. The induction of growth-promoting and calcifying properties in a ration by exposure to light. Science. 1924;60:224–225. [DOI] [PubMed] [Google Scholar]
- 83.Apple RD. Patenting university research: Harry Steenbock and the Wisconsin Alumni Research Foundation. Isis. 1989;80:375–394. [PubMed] [Google Scholar]
- 84.Hess AF, Weinstock M. Antirachitic properties imparted to inert fluids and to green vegetables by ultraviolet radiation. J Biol Chem. 1924;62: 301–313. Reprinted in: Hess AF. Collected Writings. Vol 2. Springfield, Ill: Charles C. Thomas; 1936:136–146. [Google Scholar]
- 85.Blunt K, Cowan R. Ultraviolet Light and Vitamin D in Nutrition. Chicago, Ill: University of Chicago Press; 1930.
- 86.Apple RD. “To protect the interest of the public”: vitamins, marketing, and research. In: Vitamania: Vitamins in American Culture. New Brunswick, NJ: Rutgers University Press; 1996:33–53.
- 87.Wisconsin Alumni Research Foundation (WARF). Steenbock and WARF’s founding. Available at: http://www.warf.ws/about/index.jsp?cid=26&scid. Accessed August 27, 2007.
- 88.Hess AF, Weinstock M, Helman FD. The antirachitic value of irradiated phytosterol and cholesterol. I. J Biol Chem. 1925;63:305–309. [Google Scholar]
- 89.Wolf G. The discovery of vitamin D: the contribution of Adolf Windaus. J Nutr. 2004;134:1299–1302. [DOI] [PubMed] [Google Scholar]
- 90.Hess AF, Windaus A. Contaminating substances as a factor in the activation of cholesterol by irradiation. Proc Soc Exper Biol Med. 1927;26:369–370. [Google Scholar]
- 91.Heilbron IM, Kamm ED, Morton RA. The absorption spectrum of cholesterol and its biological significance with reference to vitamin D. Part 1. Biochem J. 1927;21:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waddell J. The provitamin D of cholesterol, I: the antirachitic efficacy of irradiated cholesterol. J Biol Chem. 1934; 105:711–739. [Google Scholar]
- 93.Macintyre I, Evans IMA, Larkins RG. Vitamin D. Clin Endocrinol. 1977;6: 65–79. [DOI] [PubMed] [Google Scholar]
- 94.Park EA. The use of vitamin D preparations in the prevention and treatment of disease. JAMA. 1938;111: 1179–1187. [Google Scholar]
- 95.Stewart WK, Mitchell RG, Morgan HG, et al. The changing incidence of rickets and infantile hypercalcemia as seen in Dundee. Lancet. 1964;15: 679–682. [DOI] [PubMed] [Google Scholar]
- 96.British Pediatric Association. Hypercalcemia in infants and vitamin D. Br Med J. 1956;2:149. [Google Scholar]
- 97.Ovesen L, Andersen R, Jakobsen J. Geographic differences in vitamin D status, with particular reference to European countries. Proc Nutr Soc. 2003;62: 813–821. [DOI] [PubMed] [Google Scholar]
- 98.Laaksi IT, Ruohola JPS, Auvinen A, et al. Vitamin D fortification as public health policy: significant improvement in vitamin D status in young Finnish men. Eur J Clin Nutr. 2006;60:1035–1038. [DOI] [PubMed] [Google Scholar]
- 99.Tylavsky FA, Cheng S, Lyytikainen A, et al. Strategies to improve vitamin D status in northern European children: exploring the merits of vitamin D fortification and supplementation. J Nutr. 2006; 136:1130–1134. [DOI] [PubMed] [Google Scholar]


