Abstract
We sought to determine whether wild-type hematopoietic cell transplantation directly into muscle could restore dystrophin expression in a relevant pre-clinical canine model of Duchenne muscular dystrophy (DMD). In recipients rendered tolerant to their dog leukocyte antigen (DLA)-matched unaffected littermates, through hematopoietic stem cell transplantation (HCT), intramuscular injection of donor marrow cells produced no evidence of dystrophin expression, and clonal analysis of satellite cells failed to reveal donor contribution.
Transdifferentiation of hematopoietic stem cells into muscle cells has been reported in animal models [1,2] and clinical practice [3,4]. This raised the potential for therapeutic application in ischemic and degenerative disorders of both skeletal and cardiac muscle. Limited success with clinical trials suggested the need for provocative signals or improved homing to permit relevant repopulation of affected muscle tissues. To overcome these barriers, we employed a canine model of Duchenne muscular dystrophy (DMD) [5,6], a degenerative, progressive, and ultimately fatal muscle disorder [7,8]. In humans, this disease affects 1 in 3500 male births, and results from a mutation in the gene encoding dystrophin [9], an essential component of striated muscle, leading to myofiber injury, necrosis and fibrosis [10].
We previously reported the failure of allogeneic hematopoietic cell transplantation (HCT) to result in reconstitution of dystrophin expression in striated or cardiac muscle in this canine model [11]. However, this strategy has provided immune tolerance, which permitted transplantation of allogeneic donor tissue that expressed dystrophin in the form of isolated donor muscle fibers [11] and vascularized muscle flaps (data not shown). To gain further insight, we asked whether direct injection of unmodified bone marrow from a healthy donor dog would yield dystrophin expression in affected DMD canine recipients.
Two littermate donor-recipient pairs with DLA-identity [12,13] were chosen where the recipients possessed the dystrophin mutation [14], and thus the DMD phenotype, while the donors were either wild-type or carrier littermates. HCT with myeloablative conditioning was performed as previously described [11] and resulted in full and sustained donor hematopoietic chimerism as assessed by microsatellite marker polymorphism analyses [15] (Figure 1). More than two years after HCT, skeletal muscle biopsies demonstrated no dystrophin expression above background. Four years after HCT donor bone marrow cells were collected by aspiration from donor humeri, filtered (100 μm), and an average of 2.19 × 106 nucleated cells (1% CD34+ cells) were injected directly under the aponeurosis of the supraspinatus forelimb muscles of the DMD affected littermates.
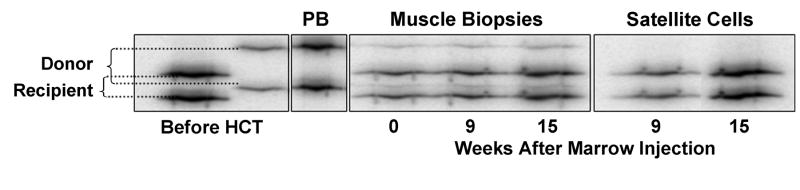
Figure 1.
Chimerism analyses from peripheral blood, muscle biopsies, and cultured muscle satellite cells from a DMD-affected dog given intra-muscular marrow injections from the unaffected HCT donor. Assays performed by PCR amplification of variable number tandem repeats [15] that differentiated donor and recipient. Muscle biopsies demonstrate contribution from both donor and recipient representative of the hematopoietic cells present in muscle tissue, while cultured satellite cells failed to show donor contribution. (PB; peripheral blood)
Excisional muscle biopsies were obtained under anesthesia before intra-muscular bone marrow injection and 5, 9, and 15 weeks following injection. Injection sites were clearly identified by placement of nonabsorbable monofilament sutures. Clonal satellite cell cultures were initiated from biopsies using myoblast culture techniques as previously described [11]; after 5 days and with the initiation of myotube formation, cultures were trypsinized and DNA extracted for analysis of donor contributions using a variable number of tandem repeats-PCR method [15]. Six replicates were examined for each biopsy, and no detectable donor contribution was observed (Figure 1). Consistent with these findings, immunofluorescence (data not shown) and reverse-transcription-PCR (Figure 2) analyses for dystrophin protein and mRNA expression, respectively, failed to demonstrate increases over background.
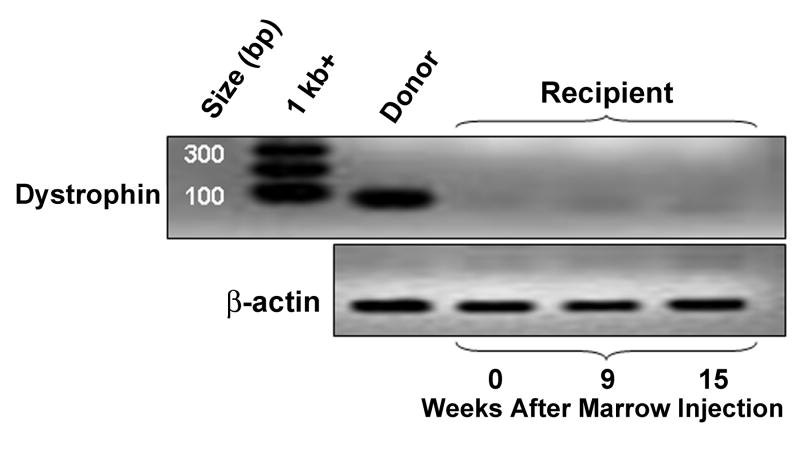
Figure 2.
Reverse-transcription-PCR reactions for dystrophin and β-actin in muscle biopsies from a DMD-affected dog demonstrate no dystrophin mRNA expression above the background after intramuscular marrow injections from the unaffected HCT donor.
Our results in a relevant large animal model of DMD, made tolerant to the dystrophin protein, demonstrate that, even in the absence of a requirement for muscle specific homing, hematopoietic cell transplantation does not contribute to muscle regeneration. These findings lessen the enthusiasm for strategies relying on hematopoietic stem cell transdifferentiation to repair or regenerate skeletal muscle.
Acknowledgments
This work was supported by National Institutes of Health grants CA78902, DK56465, CA15704, Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center grant HD47175, Parent Project Muscular Dystrophy, and Muscular Dystrophy Association – USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrari G, Mavilio F. Myogenic stem cells from the bone marrow: a therapeutic alternative for muscular dystrophy? (Review) Neuromuscular Disorders. 2002;12 1:S7–10. doi: 10.1016/s0960-8966(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 2.Muguruma Y, Reyes M, Nakamura Y, et al. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells. Exp Hematol. 2003;31:1323–1330. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Beeres SL, Bax JJ, Kaandorp TA, et al. Usefulness of intramyocardial injection of autologous bone marrow-derived mononuclear cells in patients with severe angina pectoris and stress-induced myocardial ischemia. Am J Cardiol. 2006;97:1326–1331. doi: 10.1016/j.amjcard.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 4.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 5.Partridge T. Animal models of muscular dystrophy--what can they teach us? (Review) Neuropathology & Applied Neurobiology. 1991;17:353–363. doi: 10.1111/j.1365-2990.1991.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper BJ, Winand NJ, Stedman H, et al. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- 7.Valentine BA, Cooper BJ, de Lahunta A, O'Quinn R, Blue JT. Canine X-linked muscular dystrophy. An animal model of Duchenne muscular dystrophy: clinical studies. J Neurol Sci. 1988;88:69–81. doi: 10.1016/0022-510x(88)90206-7. [DOI] [PubMed] [Google Scholar]
- 8.Valentine BA, Cooper BJ, Cummings JF, deLahunta A. Progressive muscular dystrophy in a golden retriever dog: light microscope and ultrastructural features at 4 and 8 months. Acta Neuropathologica. 1986;71:301–310. doi: 10.1007/BF00688053. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman EP, Brown RHJ, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 10.Emery AE. The muscular dystrophies (Review) Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 11.Dell'Agnola C, Wang Z, Storb R, et al. Hematopoietic stem cell transplantation does not restore dystrophin expression in Duchenne muscular dystrophy dogs. Blood. 2004;104:4311–4318. doi: 10.1182/blood-2004-06-2247. [DOI] [PubMed] [Google Scholar]
- 12.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 13.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharp NJ, Kornegay JN, Van Camp SD, et al. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- 15.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]