Abstract
Rationale: Obstructive sleep apnea syndrome is due to upper airway obstruction and is associated with increased morbidity. Although continuous positive airway pressure efficaciously treats obstructive apneas and hypopneas, treatment is impeded by low adherence rates.
Objectives: To assess the efficacy on obstructive sleep apnea of a minimally intrusive method for delivering warm and humidified air through an open nasal cannula.
Methods: Eleven subjects (age, 49.7 ± 5.0 yr; body mass index, 30.5 ± 4.3 kg/m2), with obstructive apnea–hypopnea syndrome ranging from mild to severe (5 to 60 events/h), were administered warm and humidified air at 20 L/minute through an open nasal cannula.
Measurements and Main Results: Measurements were based on standard sleep-disordered breathing and arousal indices. In a subset of patients pharyngeal pressure and ventilation were assessed to determine the mechanism of action of treatment with nasal insufflation. Treatment with nasal insufflation reduced the mean apnea–hypopnea index from 28 ± 5 to 10 ± 3 events per hour (p < 0.01), and reduced the respiratory arousal index from 18 ± 2 to 8 ± 2 events per hour (p < 0.01). Treatment with nasal insufflation reduced the apnea–hypopnea index to fewer than 10 events per hour in 8 of 11 subjects, and to fewer than 5 events per hour in 4 subjects. The mechanism of action appears to be through an increase in end-expiratory pharyngeal pressure, which alleviated upper airway obstruction and improved ventilation.
Conclusions: Our findings demonstrate clinical proof of concept that a nasal cannula for insufflating high airflows can be used to treat a diverse group of patients with obstructive sleep apnea.
Keywords: treatment with nasal insufflation, TNI; pharyngeal pressure
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
High levels of continuous positive airway pressure (CPAP) are needed to alleviate obstructive apneas; low compliance with CPAP impedes its therapeutic effectiveness; and, because hypopneas can be treated with low levels of CPAP, nasal insufflation of air might effectively treat mild obstructive sleep apnea.
What This Study Adds to the Field
Nasal insufflation can provide distinct clinical advantages over CPAP for a substantial proportion of the patient population with sleep apnea.
Obstructive sleep apnea syndrome is due to upper airway obstruction leading to intermittent hypoxemia, sleep fragmentation, metabolic dysfunction (1, 2), and increased cardiovascular morbidity and mortality (3, 4). Current treatment options, including continuous positive airway pressure (5), oral appliances (6), and surgical procedures (7), are often intrusive or invasive, and not well tolerated, leaving a vast number of subjects untreated (8, 9). Therefore, improved therapeutic strategies are required to treat sleep apneas and hypopneas and their associated morbidity and mortality.
Upper airway obstruction is due to increased pharyngeal collapsibility (10–12), which decreases inspiratory airflow as manifested by snoring and obstructive hypopneas and apneas (13). This defect in upper airway collapsibility can be overcome by elevating nasal pressure. In fact, somewhat greater levels of nasal pressure are required to abolish apneas than hypopneas, and to restore normal levels of inspiratory airflow (12, 14). Thus, minimally intrusive methods for delivering low levels of airway pressure may be remarkably effective in treating hypopneas.
At present, continuous positive airway pressure (CPAP) is most effective in eliminating apneas and hypopneas, although long-term effectiveness is compromised by low adherence that is estimated at only 50 to 60% (15, 16). Poor adherence has been attributed to the side effects associated with nasal CPAP, including difficulty tolerating pressure and the nasal mask interface, nasal irritation, claustrophobia, and skin breakdown (17, 18). To address these issues, we developed a simplified method for increasing pharyngeal pressure by delivering warm and humidified air at a continuous high flow rate through an open nasal cannula. The present study was designed to determine whether treatment with nasal insufflation (TNI) alleviates obstructive sleep apnea and hypopnea across a spectrum of disease severity. Some of the results of these studies have been previously reported in the form of abstracts (19, 20).
METHODS
Participants
Subjects were recruited from the Johns Hopkins Sleep Disorders Center (Johns Hopkins University, Baltimore, MD) if they had more than five obstructive disordered breathing episodes per hour of sleep on a standard overnight polysomnogram. Patients were selected to provide a balanced range of disease severity encompassing a spectrum of mild (apnea–hypopnea index [AHI] ⩾ 5–15 events/h, n = 3), moderate (AHI, 15–30 events/h, n = 5), and severe (AHI ⩾ 30 events/h, n = 3) sleep apnea (Table 1), with a comparable mix of sex and body mass index. Seven patients were receiving CPAP, four of whom (subjects 3, 6, 9, and 10) participated in the study because they had difficulties tolerating CPAP, with compliance defined as CPAP use for 4 hours or more per night, for 70% or more of nights. Patients were excluded if they had central sleep apnea or serious medical conditions. Informed consent was obtained from all subjects, and the Johns Hopkins University Institutional Review Board approved the protocol.
TABLE 1.
ANTHROPOMETRICS AND SLEEP-DISORDERED BREATHING INDICES
| Disease Severity
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild
|
Moderate
|
Severe
|
|||||||||||
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Subject 7 | Subject 8 | Subject 9 | Subject 10 | Subject 11 | Mean | SEM | |
| Anthropometric data | |||||||||||||
| Sex | M | M | M | F | F | M | F | M | F | M | F | ||
| Age, yr | 33 | 24 | 49 | 39 | 31 | 56 | 42 | 53 | 70 | 77 | 56 | 49.7 | 5.0 |
| Height, cm | 168 | 190.5 | 180.0 | 152.4 | 160 | 182.9 | 154.9 | 172.7 | 157.5 | 182.9 | 172.7 | 170.7 | 4.1 |
| Weight, kg | 70.9 | 120.9 | 74 | 63.9 | 70.9 | 91.7 | 169.1 | 72.0 | 60.0 | 86.8 | 66.67 | 81.9 | 12.7 |
| BMI, kg/m2 | 25.2 | 33.2 | 22.6 | 27.4 | 27.6 | 27.4 | 70.3 | 24 | 24.1 | 25.9 | 22.35 | 30.5 | 4.3 |
| Sleep-disordered breathing | |||||||||||||
| AHI, events/h | 5 | 8 | 13 | 19 | 20 | 21 | 22 | 30 | 39 | 46 | 58 | 27.7 | 4.7 |
| HI, events/h | 4 | 7 | 12 | 19 | 20 | 20 | 19 | 26 | 4 | 29 | 23 | 17.9 | 2.4 |
| AI, events/h | 1 | 1 | 1 | 0 | 0 | 1 | 3 | 4 | 35 | 17 | 35 | 9.8 | 4.3 |
| Average base SaO2, % | 96.7 | 95.2 | 94.2 | 97.5 | 96.5 | 96 | 97.3 | 94.7 | 97.2 | 93.8 | 95.4 | 95.8 | 0.4 |
| Average low SaO2, % | 94.5 | 93.4 | 92.3 | 93.3 | 92.4 | 91.3 | 91.9 | 90.9 | 87.1 | 87.3 | 90.4 | 91.0 | 0.7 |
Definition of abbreviations: AHI = apnea–hypopnea index; AI = apnea index; BMI = body mass index; HI = hypopnea index; SaO2 = oxygen saturation.
Study Procedures
Polysomnography.
Polysomnography was performed with Somnologica biosignal recording and analysis software (Embla, Broomfield, CO). Signals included electroencephalograms (C3-A2, A2-O1), left and right electrooculograms, submental electromyogram, tibial electromyogram, electrocardiogram, oxyhemoglobin saturation, body position via infrared video camera, nasal cannula for measuring airflow (Nights 2 and 3), and thoracic and abdominal belts for measuring respiratory effort. On Night 1, a pneumotachometer (21) attached to a nasal CPAP mask (Respironics, Murraysville, PA) and a fluid-filled catheter (CooperSurgical, Trumbull, CT) were used to measure ventilation and supraglottic pressure on and off TNI.
Nasal insufflation.
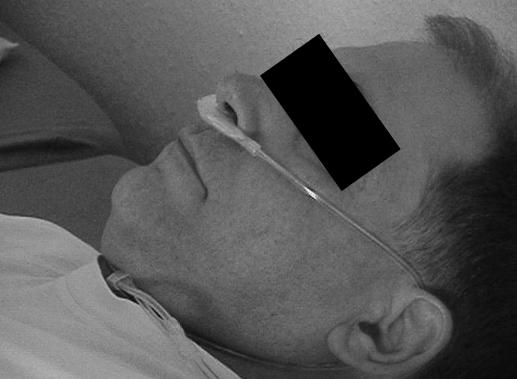
An air compressor (Seleon, Freiburg, Germany) delivered at the nose a constant flow rate of up to 20 L/minute, which was the upper limit of the current technology, given the dimensions of the cannula. A heater and humidifier regulated the temperature and humidity. A heated wire was incorporated into the lumen of the nasal cannula tubing to achieve a temperature of 30 to 33°C and relative humidity of approximately 80% at the nasal outlet (Figure 1). (For nasal cannula dimensions, see the caption to Figure 1).
Figure 1.
Nasal cannula for delivery of warm humidified air to a patient (treatment with nasal insufflation). As can be seen, the cannula is designed to leave the nose open, and thus a patient can expire freely through the nose. Dimensions of the cannula are as follows: length, 1,800 mm; outer diameter, 5 mm. Dimensions of the tube after the Y piece: length, 440 mm each; inner diameter, 3.4 mm; dimension of the prongs, 5 mm (outer diameter, each nostril). The cannula has been designed to decrease any potential noise caused by the high flow of air, minimizing noise-induced sleep disruption.
Study Protocols
On Night 1 (titration night), subjects initiated sleep on 5 L/minute on TNI for reasons of comfort. When subjects had established a stable period (> 10 min) of non–rapid eye movement (NREM) sleep, TNI was applied at 0, 10, or 20 L/minute for 5-minute intervals in random order. These trials were repeated a minimum of three times at each TNI level in the supine position during NREM sleep.
Subjects were then randomized to separate nights on and off TNI at 20 L/minute. Standard polysomnographic recording techniques were employed to characterize sleep and breathing patterns on these nights. On the basis of the findings in the TNI titration study, we anticipated that patients who had predominantly hypopneas would experience a greater effect than those who also had obstructive apneas.
Analysis
Polysomnography.
Standard polysomnographic scoring techniques were used to stage sleep (22), arousals (23), and respiratory events, which were scored according to the “Chicago criteria” (24).
Respiratory indices.
In brief, an apnea was defined as complete cessation of airflow for more than 10 seconds. Hypopnea was defined as a greater than 30% reduction of airflow. Flow-limited events were scored as hypopneas if airflow was reduced less than 30% compared with adjacent breaths and was associated with either an arousal from sleep or oxyhemoglobin desaturation equal to or greater than 3%. Each respiratory event (apnea and hypopnea) was subclassified as either central or obstructive on the basis of assessment of the respiratory flow and effort signals (supraglottic pressure catheter or abdominal and thoracic plethysmography) (24). Body position was carefully monitored during both the baseline and treatment nights, and an AHI for each individual was calculated for the supine and side positions separately. An overall AHI was then produced by weighting the time spent in each body position on the first night. On the second night, we applied a positional weighting factor from the first night to calculate an overall AHI.
Arousal analysis.
Arousals were scored as an abrupt shift in frequency that included θ, α, and β frequencies greater than or exceeding 16 Hz, but not spindles after a minimum of 10 consecutive seconds of stable sleep, and arousals in REM were scored only if accompanied by an increase in submental electromyogram amplitude (23). Assessment of interrater variability was performed by two board-certified sleep physicians in a subset of subjects (n = 9).
Breathing dynamics.
End-expiratory pharyngeal pressure, peak inspiratory airflow, and respiratory effort were measured on the basis of the 10 breaths immediately preceding and the last 10 breaths of each TNI trial.
Statistical Analysis
Data are reported as means ± SEM. A sign rank test was performed (Stata 8; StataCorp, College Station, TX) to compare (1) differences in polysomnographic indices between baseline diagnostic and clinical treatment night and (2) differences in breathing dynamics on and off TNI. p Values less than 0.05 were considered significant.
RESULTS
Subject Demographics
Eleven subjects (6 men and 5 women; age, 49.7 ± 5.0 yr; body mass index, 30.5 ± 4.3 kg/m2) completed the study. By design, our study population encompassed a wide spectrum from mild to severe disease severity (Table 1). In general, patients with milder disease severity had predominantly obstructive hypopneas (AHI, 5–15 events/h), whereas those with more severe sleep apnea (AHI, ⩾ 30 events/h) had many more obstructive apneas.
TNI Titration at 10 versus 20 L/Minute: Night 1
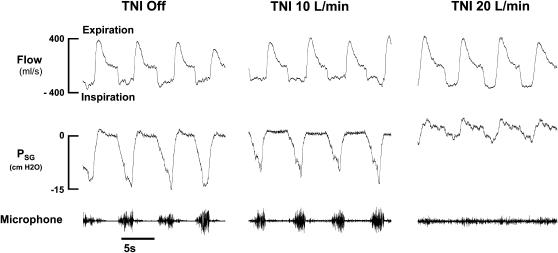
Figure 2 illustrates the effect of TNI at 10 and 20 L/minute on air flow dynamics and supraglottic pressure in one subject with predominantly obstructive hypopnea (subject 1, indicated by open circles in Figure 4). Breaths off TNI (Figure 2, left) during a hypopnea were characterized by a plateauing of inspiratory flow as supraglottic pressure continued to fall, and snoring (microphone signal). A TNI flow rate of 10 L/minute (Figure 2, middle) slightly increased end-expiratory supraglottic pressure and decreased inspiratory effort swings. Nevertheless, inspiratory flow limitation and snoring persisted. In contrast, breaths on TNI at 20 L/minute (Figure 2, right) were no longer flow limited as indicated by a rounded inspiratory flow contour, an increase in peak inspiratory airflow, a marked decline in supraglottic pressure swings, and the absence of snoring. Similar results were found in all our study participants.
Figure 2.
Airflow and supraglottic pressure (Psg) response to treatment with nasal insufflation (TNI) in one subject (subject 1). During baseline, with TNI off (left), large swings in supraglottic pressure and flattening of the inspiratory airflow contour occurred as supraglottic pressure continued to fall, indicating upper airway obstruction (left). Whereas TNI at 10 L/minute had no significant effect on airflow and supraglottic pressure swings (middle), TNI at 20 L/minute increased end-expiratory Psg from 0 to 2.2 cm H2O, which was associated with an increase in peak inspiratory airflow from 290 to 360 ml/second, and respiratory effort markedly declined as indicated by reductions of the supraglottic pressure swings from −15 to −3 cm H2O.
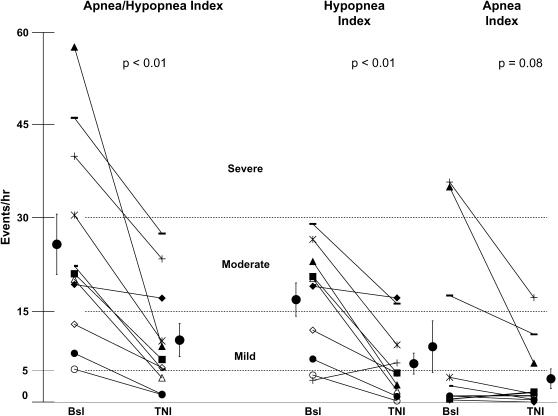
Figure 4.
Sleep-disordered breathing indices. Shown are apnea and hypopnea indices during the baseline (Bsl) diagnostic night and the clinical treatment night for individual subjects. Individual subject symbols are consistent between panels. TNI = treatment with nasal insufflation.
Effect on Sleep-disordered Breathing Indices
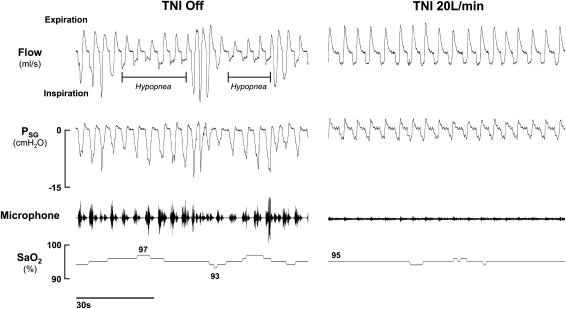
In Figure 3, the effect of TNI at 20 L/minute on the sleep-disordered breathing pattern is illustrated for one subject with obstructive hypopneas. Figure 3 (left) depicts two obstructive hypopneas (see horizontal bars) as indicated by decreased inspiratory airflow, progressively increasing respiratory effort (Psg), snoring (see microphone trace), and oxygen desaturations. When TNI was administered (Figure 3, right), sleep and breathing patterns stabilized, as reflected by the reduction in supraglottic pressure swings, and resolution of inspiratory flow limitation, snoring, and oxyhemoglobin desaturation.
Figure 3.
Effect of treatment with nasal insufflation (TNI) on obstructive hypopneas in one subject during non–rapid eye movement (NREM) sleep. Left: TNI off. Right: TNI 20 L/minute. Horizontal lines below the flow signal demarcate individual hypopnea events with oxyhemoglobin desaturations of 4 and 3%, respectively. Note the marked decline in the snoring signal on TNI compared with TNI off. Microphone = digitally displayed snoring auditory signal; Psg = supraglottic catheter pressure (cm H2O); SaO2 = oxygen saturation.
In Figure 4, the sleep-disordered breathing responses of subjects to TNI (20 L/min) are presented for the clinical treatment night (TNI on) and the baseline diagnostic night (TNI off). Two main effects can be discerned. First, TNI led to a reduction in the overall AHI (28 ± 5 to 10 ± 3 events/h, p < 0.01; Figure 4, left) and some improvement of the AHI was observed in each subject (Figure 4, left). In eight of these subjects, the AHI fell below 10 events/hour. Of the three remaining subjects, the nasal cannula dislodged for 2.5 hours in one subject (Figure 4, left, solid diamonds), and hence the AHI fell only minimally from 19 to 17, whereas more marked reductions in the AHI, from 46 to 27 and from 39 to 23 events/hour, were observed for the other two (Figure 4, left, bars and plus symbols).
Second, TNI responses in hypopneas and apneas are shown separately (Figure 4, middle and right, respectively). TNI decreased the hypopnea index (Figure 4, middle) from 18 ± 2 to 8 ± 2 events/hour (p < 0.01), and also reduced the number of obstructive apneas in the three subjects who had an apnea index greater than 10 events/hour during sleep (subjects 9, 10, and 11 in Table 1, and represented by plus, bar, and solid triangle symbols, respectively, in Figure 4, right). As can be seen, apneic subjects 9, 10, and 11 had a reduction in apnea index from 36 to 17, from 17 to 11, and from 35 to 6 events per hour of sleep, respectively, suggesting that TNI can decrease apneas as well as hypopneas. Assessment of interrater variability was performed with an intraclass correlation coefficient (ICC) for obstructive respiratory events (ICC, 1.0), respiratory arousals (ICC, 0.98), and spontaneous arousals (ICC, 0.8), indicating good agreement between reviewers in all categories; disagreements between reviewers were minor (Table 2).
TABLE 2.
INTERRATER RELIABILITY
| AHI
|
Respiratory Arousal Indices
|
Spontaneous Arousal Indices
|
||||
|---|---|---|---|---|---|---|
| Subject | Scorer 1 | Scorer 2 | Scorer 1 | Scorer 2 | Scorer 1 | Scorer 2 |
| C | 20 | 21 | 21 | 23 | 7 | 5 |
| D | 16 | 15 | 16 | 15 | 7 | 5 |
| E | 7 | 6 | 9 | 7 | 4 | 3 |
| F | 7 | 7 | 3 | 3 | 0 | 0 |
| G | 24 | 25 | 20 | 24 | 8 | 4 |
| H | 8 | 8 | 8 | 8 | 2 | 1 |
| I | 10 | 10 | 5 | 15 | 1 | 0 |
| J | 5 | 5 | 5 | 5 | 6 | 3 |
| K | 86 | 86 | 64 | 64 | 0 | 0 |
| Mean | 20 | 20 | 17 | 18 | 4 | 2 |
| SE | 26 | 26 | 19 | 19 | 3 | 2 |
| ICC | 1.00 | 0.98 | 0.80 | |||
Definition of abbreviations: AHI = apnea–hypopnea index; ICC = intraclass correlation coefficient.
Individual data are presented for a subset of patients (n = 9), scored by two experienced board-certified sleep medicine physicians, for the analysis of interscorer agreement for the apnea–hypopnea indices, respiratory and spontaneous arousal indices.
Mechanism of Action
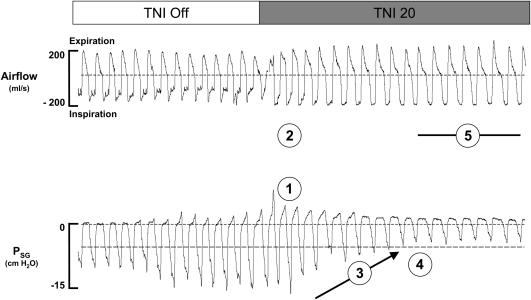
To explore the underlying mechanisms responsible for the effect of TNI on sleep-disordered breathing we assessed inspiratory airflow, end-expiratory supraglottic pressure, and respiratory effort in a subgroup of subjects (n = 7). In Figure 5, the immediate respiratory responses to TNI at a rate of 20 L/minute for one subject with obstructive hypopneas are demonstrated. As can be seen in Figure 5, breaths off TNI were characterized by inspiratory flow limitation indicated by a plateauing of inspiratory flow as supraglottic pressure continued to fall (see inspiratory flow limitation threshold marked by the horizontal dashed line). After TNI was initiated, there was an instantaneous increase in end-expiratory supraglottic pressure (Figure 5, circled 1) and mean inspiratory airflow (Figure 5, circled 2). Nevertheless, inspiratory flow limitation was still present over a short period of breaths in which supraglottic pressure swings declined gradually on a breath-by-breath basis (Figure 5, circled 3), indicating that improvements in airflow were associated with progressive reductions in respiratory drive. Once the supraglottic pressure swings no longer fell below the threshold for flow limitation (Figure 5, circled 4), the inspiratory airflow contour assumed a round, non–flow-limited pattern (Figure 5, circled 5).
Figure 5.
Mechanism of action. Airflow and supraglottic pressure are shown during the transition from flow-limited breathing with TNI off, to non–flow-limited breathing with TNI at 20 L/minute. Psg = supraglottic catheter pressure (cm H2O). Numbers in circles: 1, increase in end-expiratory Psg; 2, increase in mean inspiratory airflow; 3, decrease in supraglottic pressure swings on a breath-by-breath basis; 4, Psg threshold for inspiratory flow limitation; and 5, a round, non–flow-limited inspiratory pattern.
Pooled data for a subset of subjects (n = 7) demonstrate that TNI increased end-expiratory pharyngeal pressure from atmospheric to 1.8 ± 0.1 cm H2O (p = 0.04), increased inspiratory airflow from 255.1 ± 54.2 to 363.5 ± 26.7 ml/second (p = 0.04), and decreased supraglottic pressure swings from 11.3 ± 0.5 to 4.4 ± 0.6 cm H2O (p = 0.04). Thus, TNI alleviates upper airway obstruction through an immediate increase in pharyngeal pressure in combination with gradual reflexive reductions in ventilatory drive.
Sleep Characteristics and Arousal Indices
As shown in Table 3, TNI reduced the respiratory-related arousal frequency (18 ± 4 to 8 ± 2 events/h, p < 0.01), without a change in the spontaneous arousal frequency (3 ± 1 to 3 ± 1, p = 0.65). There was no overall change in total sleep time, sleep efficiency, or sleep stage distribution, perhaps as a result of our relatively small sample size. However, each patient exhibited an improvement in sleep stage distribution, with either a greater percentage of time in deeper stages of NREM sleep (subjects 3, 4, 5, 6, 8, and 11) or a greater percentage of total sleep time spent in REM sleep (subjects 1, 2, 7, 9, and 10), suggesting that TNI improved sleep quality.
TABLE 3.
SLEEP CHARACTERISTICS AND AROUSAL INDICES
| Baseline
|
TNI, 20 L/min
|
||||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | p Value | |
| TST, min | 317.9 | 26.0 | 326.7 | 12.3 | 0.64 |
| Sleep efficiency, % | 79.5 | 5.2 | 85.5 | 3.3 | 0.24 |
| NREM, % TST | 84.2 | 1.9 | 87.2 | 2.6 | 0.43 |
| Stage 1, % | 12.7 | 3.1 | 13.2 | 4.0 | 0.56 |
| Stage 2, % | 65.2 | 4.0 | 68.2 | 4.2 | 0.56 |
| Stage 1, % | 6.3 | 1.8 | 6.3 | 1.8 | 0.84 |
| REM, % TST | 14.1 | 2.2 | 12.8 | 2.6 | 0.87 |
| Arousal indices | |||||
| Respiratory | 18.3 | 3.7 | 8.3 | 1.5 | 0.005 |
| Spontaneous | 3.4 | 2.2 | 3.1 | 0.4 | 0.65 |
| Total | 21.6 | 3.6 | 11.4 | 1.5 | 0.007 |
Definition of abbreviation: NREM = non–rapid eye movement; TST = total sleep time.
Group data are presented for both the baseline and clinical treatment night with TNI at 20 L/minute.
DISCUSSION
Our study was designed to examine the effect of treatment with nasal insufflation (TNI) on obstructive sleep apnea. In a broad spectrum of patients, we found a significant reduction in inspiratory flow limitation severity on TNI at 20 versus 10 L/minute, and improvement in sleep apnea severity as reflected by a marked fall in both the apnea–hypopnea and arousal indices on TNI at 20 L/minute. The relief of upper airway obstruction was most likely due to small but consistent increases in pharyngeal pressure on TNI, which decreased the severity of inspiratory flow limitation.
Mechanism of Action of TNI
To determine the mechanism of action of TNI, we assessed airflow dynamics and supraglottic pressure responses to TNI at a low rate (10 L/min) and a high rate (20 L/min) during periods of hypopneas during NREM sleep. Whereas TNI at 10 L/minute had no effect on airflow dynamics, TNI at 20 L/minute increased peak inspiratory airflow and reduced supraglottic pressure swings. Although these changes were relatively modest, sleep and breathing patterns improved markedly in all subjects receiving TNI. These improvements can be attributed primarily to the increase in pharyngeal pressure while receiving TNI. Inspiratory airflow increases approximately 50 ml/second per cm H2O of CPAP pressure applied (25). TNI at a rate of 20 L/minute led to a similar increase in inspiratory airflow (45 ml/s per cm H2O). The peak inspiratory airflows of our patients during hypopneas were only mildly reduced to approximately 230 ml/second, and rose to approximately 300 ml/second, a level previously associated with the elimination of inspiratory flow limitation and stabilization of breathing patterns (25). Thus, improvements in peak inspiratory airflow were likely due to increases in pharyngeal pressure, which were of sufficient magnitude to treat hypopneas when inspiratory airflow levels are only mildly reduced.
Effect of TNI on Sleep-disordered Breathing
Although we expected marked improvements in the AHI primarily in patients with hypopneas rather than obstructive apneas, TNI lowered the AHI in all subjects, regardless of the apnea–hypopnea distribution. Although the primary mechanism of action appears to be related to increases in end-expiratory pharyngeal pressure, other factors may have further improved ventilation in addition to alleviating upper airway obstruction. First, even small increases in pharyngeal pressure may have increased lung volume. Increases in lung volume lead to improvements in both oxygen stores and upper airway patency (26–30), both of which may further stabilize breathing patterns during sleep. As ventilation improved in our patients during sleep, enhanced sleep continuity (decreased arousal frequency) may have also contributed to further reductions in the apnea–hypopnea indices (31, 32). Indeed, we found a trend toward improvement in sleep stage distribution in all subjects, with a reduction in respiratory arousals, and no change in spontaneous arousals. Additional benefits may have accrued from insufflating air directly into the nose, which may produce concomitant reductions in dead space ventilation. Therefore, improvements in oxygen stores, ventilation, and sleep continuity, along with enhanced upper airway patency, are likely responsible for the beneficial responses to TNI. We acknowledge that obstructive sleep apnea was not completely eliminated in all of our patients, and that nasal CPAP might still be more efficacious in reducing the AHI during treatment nights. Nevertheless, reduced compliance with CPAP can significantly compromise long-term therapeutic effectiveness, leaving a significant portion of patients untreated over time (33). Poor CPAP compliance has been attributed to cumbersome masks, and to difficulties in exhaling against a high backpressure (17). In contrast, TNI offers a simplified nasal interface for delivering relatively low levels of pharyngeal pressure, which may enhance long-term compliance, and overall therapeutic effectiveness, and thus might reduce long-term cardiovascular and metabolic complications of obstructive sleep apnea.
Limitations
There are several limitations in the current study. First, we used only flow rates of 10 and 20 L/minute in our study. It is possible that higher flow rates would have been even more effective in eliminating all respiratory events. However, we used relatively low flow rates to balance the comfort of nasal insufflation with efficacy. Indeed, there were no reports of significant discomfort or side effects after a full night of treatment with TNI at 20 L/minute, with the exception of reports that air temperatures were either too warm (n = 2) or cold (n = 1) for initiating sleep. Nevertheless, the majority of subjects did not have difficulty initiating or maintaining sleep as compared with baseline. None of the patients complained about noise related to the use of TNI, which we acknowledge might result from patient motivation, or perception relative to their previous experience with CPAP. Moreover, assessment of sleep architecture between nights on and off TNI indicates a trend toward improvement, without change in spontaneous arousal indices. Second, it is possible that the cannula may have dislodged during the night, accounting for the treatment failure in at least one patient. Although it is not yet clear how a minor dislodgement of the cannula can affect efficacy, the fact that the majority of our patients had a substantial reduction in sleep-disordered breathing indices suggests that the exact position of the nasal cannula is not critical. Third, the occurrence of apneas might be dependent on body position. We accounted for body position between the two nights, thus eliminating the impact of a change in position on the treatment effect. Fourth, TNI was used for only one night. Although patients did not report any discomfort when using it for one night, the response might be different when using TNI repeatedly over several nights. Further studies of TNI administered over several nights would be required to examine its effect relative to CPAP. Fifth, assessment of both spontaneous and respiratory arousals is potentially associated with poor agreement between scorers. All data in this study were reviewed by two experienced board-certified sleep physicians (H.S. and S.P.). To assess quality assurance of our scoring, the interrater reliability was analyzed for a subset of patients (n = 9), and was comparable to previous assessments of interrater reliability of both spontaneous and respiratory arousal indices (ICC, 0.72; 95% confidence interval: 0.44, 0.88) with experienced full-time scorers (34).
Implications
There are several clinical implications of our findings. First, our findings provide evidence that TNI may offer a viable treatment alternative to patients with obstructive hypopneas and apneas. The finding that TNI alleviated obstructive hypopneas in all but one patient predicts a high likelihood of treatment success in a similar patient population. A retrospective analysis of our patient database with 4,746 patients with obstructive sleep apnea–hypopnea syndrome studied between 1981 and 2000, whose AHI was greater than 10, showed that 28.4% of these patients had predominantly obstructive hypopneas (more than 90% of all events) and would meet the polysomnographic and anthropometric characteristics of our study population. Second, our findings that TNI also had an effect on obstructive apnea in our patients with an apnea index of greater than 15 implies that TNI may be beneficial in some patients with obstructive apneas as well. Further studies are required to elucidate the polysomnographic and/or clinical predictors of a TNI response. Third, we used a fixed flow rate and cannula size, which may obviate the need for titration studies. Indeed, it may be possible to offer an empiric, streamlined therapeutic approach with TNI for a large proportion of patients with sleep apnea.
In summary, our study provides clinical proof of concept for employing TNI as a novel treatment for patients with obstructive sleep apnea–hypopnea syndrome. Because one flow rate and cannula size was sufficient to stabilize breathing patterns in the majority of our subjects, titration may be obviated, thereby streamlining the initiation of treatment. Moreover, the minimally intrusive nasal interface of TNI may improve patient adherence, and may ultimately prove more effective in managing the long-term morbidity and mortality of sleep apnea. Further studies will be required to extend these findings and to determine the ultimate role of TNI in managing obstructive sleep apnea.
Acknowledgments
The authors thank Mr. Peter DeRosa and Mr. Christopher Smith for contributions to this study, which included technical support, data collection, and help in the preparation of tables and figures.
Supported by HL-72126, HL-50381, HL-37379, HL-077137, NHMRC-353705, and Seleon GmbH, Germany.
Originally Published in Press as DOI: 10.1164/rccm.200609-1336OC on March 15, 2007
Conflict of Interest Statement: B.M.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.P.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.P.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.L.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.R.S. received $18,000 in 2006, under a private licensing agreement between Dr. Schwartz and Seleon GmbH. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict of interest policies. H.S. received $78,000 from 2003 to 2006. Under a private licensing agreement between Dr. Schneider and Seleon GmbH, Dr. Schneider receives consulting fees (U.S. $18,000 in 2006) and is entitled to royalty payments on the future sales of products described in this article. Under a separate licensing agreement between Dr. Schneider and Seleon GmbH and Johns Hopkins University, Dr. Schneider is entitled to a share of royalty received by the university on sales of products described in this article. The terms of this arrangement are being managed by Johns Hopkins University in accordance with its conflict of interest policies. Funding for the study described in this article was partially provided by Seleon GmbH.
References
- 1.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med 2005;165:447–452. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM, Shahar E, Redline S, Golttlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521–530. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea–hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981;1(8225):862–865. [DOI] [PubMed] [Google Scholar]
- 6.Kushida CA, Morgenthaler TI, Littner MR, Alessi CA, Bailey D, Coleman J Jr, Friedman L, Hirshkowitz M, Kapen S, Kramer M, et al.; American Academy of Sleep. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances. American Sleep Disorders Association. Sleep 2005;18:511–513. [Google Scholar]
- 7.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996;19:156–177. [DOI] [PubMed] [Google Scholar]
- 8.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:887–895. [DOI] [PubMed] [Google Scholar]
- 9.McArdle N, Deveruex G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP Therapy for sleep apnea/hypopnea syndrome. Am Rev Respir Dis 1999;159:1108–1114. [DOI] [PubMed] [Google Scholar]
- 10.Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anesthesia and sleep. Lancet 2007;359:1207–1209. [DOI] [PubMed] [Google Scholar]
- 11.Mansour KF, Rowley JA, Meshenish AA, Shkoukani MA, Badr MS. A mathematical model to detect inspiratory flow limitation during sleep. J Appl Physiol 2002;93:1084–1092. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure–flow relationships. J Appl Physiol 1989;66:1626–1634. [DOI] [PubMed] [Google Scholar]
- 13.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 1991;143:1300–1303. [DOI] [PubMed] [Google Scholar]
- 14.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure–flow relationships in obstructive sleep apnea. J Appl Physiol 1988;64:789–795. [DOI] [PubMed] [Google Scholar]
- 15.Aloia MS, Stanchina M, Arnedt T, Malhotra A, Millman R. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest 2005;127:2085–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake CL, Day R, Hudgel D, Stefadu Y, Parks M, Syron ML, Roth T. Sleep during titration predicts continuous positive airway pressure compliance. Sleep 2003;26:308–311. [DOI] [PubMed] [Google Scholar]
- 17.Olson EJ, Moore WR, Morgenthaler TI, Gay PC, Staats BA. Obstructive sleep apnea–hypopnea syndrome. Mayo Clin Proc 2003;78:1545–1552. [DOI] [PubMed] [Google Scholar]
- 18.Weaver TE, Maislin G, Dinges DF, Younger J, Cantor C, McCloskey S, Pack AI. Self-efficacy in sleep apnea; instrument development and patient perceptions of obstructive sleep apnea risk, treatment benefit and volition to use continuous positive airway pressure. Sleep 2004;26:727–732. [DOI] [PubMed] [Google Scholar]
- 19.McGinley BM, Patil SP, Kirkness JP, Schwartz AR, Smith PL, Schneider H. Continuous nasal airflow (TNI) through a nasal cannula treats obstructive sleep hypopnea [abstract]. Proc Am Thorac Soc 2006;3:A869. [Google Scholar]
- 20.McGinley BM, DeRosa P, Schwartz AR, Kirkness JP, Patil SP, Smith PL, Schneider H. A novel strategy for treating upper airway obstruction (UAO) with transnasal insufflation [abstract]. Sleep 2005;28:A208. [Google Scholar]
- 21.Hager DN, Fuld M, Kaczka DW, Fessler HE, Brower RG, Simon BA. Four methods of measuring tidal volume during high-frequency oscillatory ventilation. Crit Care Med 2006;34:751–757. [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for deep states of human subjects. Bethesda, MD: National Institutes of Health; 1968. NIH Publication No. 204.
- 23.Sleep Disorders Task Force of the American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep 1992;15:174–184. [PubMed] [Google Scholar]
- 24.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999;22:667–689. [PubMed] [Google Scholar]
- 25.Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med 2004;170:89–93. [DOI] [PubMed] [Google Scholar]
- 26.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax 2006;61:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis 1984;130:175–178. [DOI] [PubMed] [Google Scholar]
- 28.Series F, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol 1990;98:2159–2164. [DOI] [PubMed] [Google Scholar]
- 29.Series F, Cormier Y, Lampron N, La Forge J. Increasing the functional residual capacity may reverse obstructive sleep apnea. Sleep 1988;11:349–353. [PubMed] [Google Scholar]
- 30.Stanchina M, Malhotra A, Fogel R, Trinder J, Edwards J, Schory K, White DP. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep 2003;26:851–856. [DOI] [PubMed] [Google Scholar]
- 31.Wellman A, Malhotra A, Fogel R, Edwards JK, Schory K, White DP. Respiratory system loop gain in normal men and women measured with proportional-assist ventilation. J Appl Physiol 2002;94:205–212. [DOI] [PubMed] [Google Scholar]
- 32.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004;169:623–633. [DOI] [PubMed] [Google Scholar]
- 33.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J 2006;16:921–927. [DOI] [PubMed] [Google Scholar]
- 34.Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep 2007;21:749–757. [DOI] [PubMed] [Google Scholar]