Abstract
Rationale: Metabolic syndrome (MetS) affects 4 to 10% of adolescents. Risk factors include overweight, male sex, and Hispanic ethnicity. Although sleep-disordered breathing (SDB) has been implicated as a risk factor for MetS in adults, its association with SDB in adolescents is unknown.
Objectives: To define the association of SDB with MetS in adolescents.
Methods: Standardized measurements of SDB, anthropometry and bioassays, were made in 270 adolescents, aged 13.6 ± 0.7 years. MetS was identified if threshold levels were exceeded in three of five areas: waist circumference, blood pressure, triglyceride level, high-density lipoprotein cholesterol level, and glucose levels.
Measurements and Main Results: Although 70% of children with SDB (apnea–hypopnea index ⩾ 5) were overweight and 59% had MetS, 16% of children without SDB had MetS. Twenty-five percent of those with MetS had SDB. After adjusting for age, race, sex, and preterm status, children with SDB had a 6.49 (95% confidence interval, 2.52, 16.70) increased odds of MetS compared with children without SDB. Indices of SDB stress associated with MetS included respiratory event frequency, degree of oxygen desaturation, and sleep efficiency. Analyses of individual metabolic parameters showed that, after adjustment for body mass index, SDB was associated with systolic and diastolic blood pressure, low-density lipoprotein cholesterol, and fasting insulin levels.
Conclusions: A majority of adolescents with SDB are overweight and meet criteria for MetS. The close association between MetS and SDB and their putative interacting pathophysiologies suggests a need to develop screening, prevention, and treatment strategies for both disorders in high-risk, overweight adolescents.
Keywords: sleep apnea, metabolic syndrome, obesity
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Sleep apnea has been associated with diabetes and glucose impairment in adults. It has not yet been established whether sleep apnea is also associated with metabolic syndrome in children.
What This Study Adds to the Field
Obesity and metabolic syndrome are prevalent in adolescents with modest sleep apnea. Associations of metabolic dysfunction with sleep apnea persisted after considering weight, suggesting that sleep apnea contributes to metabolic dysfunction in children.
Metabolic syndrome (MetS) refers to a clustering of abnormalities in glucose and lipid metabolism, believed to result from insulin resistance and/or central obesity. MetS, defined according to criteria established by the Adult Treatment Panel III (ATP III), is estimated to affect more than 20% of United States' adults, who frequently progress to diabetes and are at increased risk for premature cardiovascular disease (1). Approximately 95% of adults with MetS are overweight or obese (2).
MetS is also estimated to afflict 4 to 10% of adolescents (3, 4). Similar to adults, overweight children have greater degrees of metabolic dysfunction than normal-weight children, and such abnormalities appear to track over time and predict metabolic dysfunction and cardiovascular risk profiles in adulthood (5, 6). The rising prevalence of overweight among children and adolescents has been implicated in the 10-fold estimated increased rate of noninsulin diabetes in adolescents, and has raised concerns over long-term cardiovascular health sequelae in the population (7, 8). In addition to increased weight and waist circumference, childhood risk factors for MetS include male sex and Mexican-American ethnicity (4).
Sleep-disordered breathing (SDB), characterized by loud snoring, sleep fragmentation, and sleep-associated intermittent hypoxemia, occurs in more than 4% of adults (9) and 1 to 6% of children (10). Recent cross-sectional and longitudinal data have shown strong and consistent associations between indices of SDB with glucose impairment and/or diabetes in adults (11, 12). Causal relationships are supported by data showing that treatment of SDB is associated with improvement in indices of glucose impairment (13) or diabetes control (14). However, research that addresses the role of SDB as a risk factor for MetS in children is more limited. The few available reports have provided conflicting data, and have been restricted to reports of children referred for evaluation at a sleep clinic where various referral biases may operate.
In this report, we quantified the association between MetS and SDB in a community-based cohort of adolescents who had undergone standardized assessments of SDB and measurement of key components of MetS. We addressed the hypothesis that MetS is more prevalent among adolescents with SDB than in those without SDB. In addition, we evaluated whether the association between MetS and SDB is independent of socioeconomic class, race, and sex, and addressed whether MetS is associated with specific components of SDB, including indices of overnight hypoxemia, sleep fragmentation, or insufficient sleep. Finally, we explored whether individual measures of metabolic dysfunction were associated with SDB independently of body mass index (BMI).
METHODS
Study Population
The study sample was derived from an ongoing longitudinal cohort study, the Cleveland Children's Sleep and Health Study (CCSHS). The CCSHS is an urban, community-based cohort of 907 children recruited between 1998 and 2002 to participate in a study of sleep and health in children. Children were recruited from the 1988 and 1993 birth rosters of three area hospitals; sampling was designed to overrepresent preterm and African-American children and included 644 children who had participated in the Low Birthweight–Maternal Employment Study at ages 4 to 6 years, as described previously (10). The first CCSHS examination, conducted when children were 8 to 11 years old, included in-home assessment of sleep patterns, SDB, blood pressure, anthropometry, and neuropsychological function. A second exam was conducted in 2002–2006, when the children were 13 to 16 years of age. This examination included an overnight stay in a general clinical research center and assessment of sleep, anthropometric, blood pressure, and metabolic parameters. Recruitment for this second examination aimed to enroll at least 250 CCSHS participants, targeting all snorers and SDB cases identified at the time of the first CCSHS examination, and a stratified (sex, race, term) random sample of the remaining cohort (n = 389). Of those 389, 75.1% (n = 292) agreed to participate, 14.9% refused, 10.0% could not be located, and less than 1% were ineligible due to severe illnesses (e.g., renal failure, cancer). Of the families who refused to participate, the most common reasons were as follows: passive refusal (e.g., agreed to participate but did not make appointments) (17.2%), too busy (19.0%), reluctance to participant in hospital-based tests (13.8%), and miscellaneous reasons (50.0%). A comparison of participants to nonparticipants targeted for this exam revealed no significant differences in the age, race, sex, preterm status, maternal educational level, or sleep characteristics (apnea–hypopnea index [AHI], average and minimal saturation) measured at the first CCSHS visit. Exclusion of two children with known diabetes and 20 children with incomplete data yielded an analytic sample of 270 participants.
Study Protocol
As part of a standardized research protocol, each child was studied in a dedicated clinical research facility at a time when free of acute illness, and underwent overnight polysomnography, venipuncture both before (at 10:00 p.m.) and after sleep and overnight fasting (7:00 a.m.), an oral glucose tolerance test (oral administration of 75 g of anhydrous glucose and venipuncture after 2 h), and physiological and anthropometric assessments. Informed consent was obtained from the child's parent or legal guardian and written assent from the child. The study was approved by the governing institutional review board.
Measurements
Height was measured using a rigid stadiometer, and weight with a calibrated digital scale. BMI was calculated by dividing the weight in kilograms by height in meters squared and converted into age- and sex-adjusted percentiles based on population data from NHANES (National Health and Nutrition Examination Survey) 2000 (15). The minimal waist circumference was measured by trained research staff in duplicate and averaged. Three blood pressure readings at each of three measurement times (10:00 p.m., 7:00 a.m., and 1:00 p.m.) were obtained following standardized guidelines using a calibrated sphygmomanometer (16). Information on health habits and demographic variables was obtained from a standardized questionnaire (17). Indices of socioeconomic status included parent report of family income and educational level as well as median income of the census tract of residence of the family at the time of enrollment in the CCSHS. The latter was derived by matching each child's address to the corresponding 2000 U.S. Census Bureau tract database (18). Measures of sleep duration were based on a 5- to 7-day sleep diary (75.5% of the sample had sleep diary data available for all 7 d), requesting the participant to record on a daily basis bed time, wake time, and approximate time to fall asleep (19). Pubertal status was ascertained by Tanner staging (20, 21) performed by a physician. Preterm status was defined gestational age of less than 36 weeks. For full-term children, birth weight was obtained by parent report, and for preterm children, by extraction from the neonatal birth record.
Glucose and fasting lipids were measured by enzymatic methods under Centers for Disease Control and Prevention guidelines (22). Insulin was measured by radioimmunoassay. The homeostatic model assessment (HOMA) was calculated as a product of fasting insulin and glucose (23).
The polysomnography recording (Compumedics E-series; Compumedics, Abbotsford, Australia) consisted of the following: two electroencephalograms (C3/C2 and C4/C1), bilateral electrooculograms, a bipolar submental electromyogram, thoracic and abdominal respiratory inductance plethysmography (Compumedics Summit IP), airflow (by a Protec nasal–oral thermocouple [Protec, Wordsville, WA] and nasal pressure recording), finger pulse oximetry (Nonin model 320 pulse oximeter [Nonin Medical, Plymouth, MA] with displayed waveform), electrocardiogram, body position, and monitoring bilateral leg movements by piezoelectric sensors. Studies were scored by certified research technologists (24). Obstructive apneas were scored when there was a complete or nearly complete absence of airflow on the thermistry channel for 8 seconds or more and the duration of two breaths, in association with respiratory effort. Hypopneas were identified as a clear (> 30%) decline in airflow (from the thermocouple or nasal pressure signals) or respiratory effort (from inductive respiratory bands) for 8 seconds or more associated with an oxygen desaturation of 3% or more. Sleep staging and arousals were based on standard criteria (25, 26). The AHI was calculated as the average number of obstructive apneas and hypopneas per hour of sleep. Our primary exposure variable was SDB, based on an AHI of 5 or greater, a value associated with mild SDB in adult studies, and one we considered to conservatively identify this disorder in adolescents. Levels above an AHI threshold of 5 have been found to be associated with elevated C-reactive protein levels in adolescents (27). However, secondary analyses examined a lower AHI threshold (⩾ 1), as well as examined as continuous variables the AHI, and measures of sleep hypoxemia (average and nadir levels of oxygen saturation during sleep and oxygen saturation < 90%), percentage of time in slow-wave sleep, sleep efficiency (percentage of the sleep period spent asleep), and the arousal index (number of cortical arousals per hour of sleep).
Definition of MetS
Our primary definition of MetS was based on criteria outlined in a report by de Ferranti and colleagues, who adapted ATP III adult criteria for a pediatric population (4). Adolescents who met at least three of the following five criteria were classified as having MetS: (1) waist circumference greater than the 75th percentile for age and sex (28); (2) mean systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height (29) or current blood pressure medication use (n = 3); (3) triglyceride levels of 97.35 mg/dl or greater; (4) low high-density lipoprotein (HDL) (for boys aged 15–17, HDL < 45.17 mg/dl; all others, HDL < 50.19 mg/dl); (5) fasting glucose of 100 mg/dl or greater (30), or an oral glucose tolerance test result of 140 or more. Secondary analyses used the criteria proposed by Cook and colleagues (3), which differed from de Ferranti as follows: central obesity (waist circumference ⩾ 90th percentile for age and sex) (28), elevated triglyceride (triglyceride ⩾ 110 mg/dl), and low HDL (HDL ⩽ 40 mg/dl).
Statistical Analysis
Bivariate comparisons were evaluated using the two-sample t test, the Wilcoxon rank sum test, and the Pearson χ2 test for normally distributed, skewed, and categorical data, respectively. Logistic regression was used to assess the relationship between SDB (AHI ⩾ 5) and MetS. Models were fit without covariate adjustment as well as adjusted for age, sex, and race, covariates associated with MetS in prior work (4). Although preliminary analyses showed no association between preterm history and MetS, models were also adjusted for preterm status as a design variable, because this covariate had been used in constructing the sample. Secondary analyses assessed the associations of alternative polysomnographic measures with MetS, as well as the association between individual measures of metabolic dysfunction with SDB status adjusted for BMI percentile. The results are summarized using odds ratios (ORs) and 95% confidence intervals (95% CIs). Analyses were performed using SAS version 9.1.3 (SAS Institute, Inc., Cary, NC).
RESULTS
The sample comprised children with a mean age of 13.7 (± 0.7) years, with approximately equal proportions of African Americans and European Americans, and of males and females (Table 1). Approximately 25% of the sample was overweight and 19% met criteria for MetS. A greater proportion of adolescents with MetS compared with unaffected children was male and overweight. No significant group (MetS vs. no MetS) differences were observed with respect to age, preterm history, race, Tanner stage, or any index of socioeconomic status (Table 1).
TABLE 1.
CLEVELAND CHILDREN'S SLEEP AND HEALTH STUDY SAMPLE: PARTICIPANT, FAMILY, AND SLEEP CHARACTERISTICS, STRATIFIED BY METABOLIC SYNDROME AND SLEEP-DISORDERED BREATHING (AHI ⩾ 5)
Table 1 also shows the distribution of sleep characteristics of adolescents with and without MetS. SDB (AHI ⩾ 5) was observed in 25% of children with MetS, but only in 4% of adolescents without MetS (p < 0.001). Compared with children without MetS, those with MetS had more severe nocturnal desaturation and poorer sleep efficiency, but do not differ in their arousal index, percentage time in slow-wave sleep, average sleep time on weekdays, or habitual snoring. Although weekend sleep duration was reported to be less in those with MetS, the difference between weekend and weekday sleep, a measure used to approximate “sleep debt,” was not different between groups.
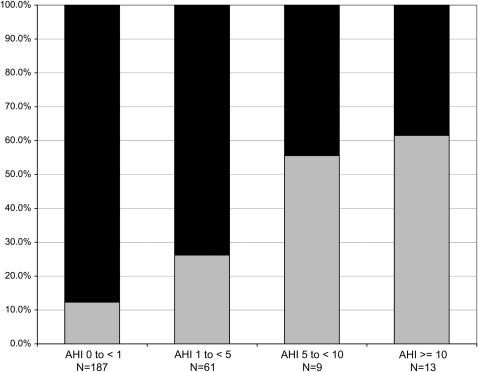
Figure 1 further demonstrates that, as AHI severity category increases, there was a progressive increase in the proportion of children classified with MetS (p < 0.001).
Figure 1.
Prevalence of the metabolic syndrome (MetS) in increasingly severe categories of sleep-disordered breathing, as defined by increasing levels of the apnea–hypopnea index (AHI). A linear association (p < 0.001) was demonstrated between MetS and AHI category. Black bars, non-MetS; gray bars, MetS.
To further understand the association between SDB and MetS, we compared the distribution of subject characteristics in adolescents with and without SDB (Table 1, right columns). Both overweight and MetS were more prevalent in the SDB compared with the non-SDB group. Overweight and MetS, respectively, were observed in 73 and 59% of children with SDB, compared with 21 and 16% of children without SDB (< 0.001). A greater proportion of adolescents with SDB was male. However, age, race, preterm status, and indices of socioeconomic status did not vary by SDB. Self-reported sleep duration on weekdays was almost identical in the SDB and non-SDB groups (8.67 ± 1.06 vs. 8.67 ± 0.66 h for non-SDB and SDB, respectively), but weekend sleep duration was shorter in the SDB group (10.20 ± 1.59 vs. 8.75 ± 2.11 h, p < 0.001).
Logistic regression analysis, with MetS as the outcome, showed that adolescents with SDB had more than a sevenfold increased odds of MetS compared with those without SDB (OR, 7.74; 95% CI, 3.10, 19.35) (Table 2). Adjusting for sex, age, race, and preterm status did not appreciably alter the magnitude of this association (adjusted OR, 6.49; 95% CI, 2.52, 16.70). When redefining MetS using similar criteria as Cook and colleagues (3), the adjusted odds for SDB was even higher, 9.02 (95% CI, 3.17, 25.66). Secondary analyses conducted on the entire sample, substituting alternative measures of SDB severity, showed that the odds of MetS varied with level of overnight desaturation, sleep efficiency, and AHI as a continuous variable (Table 3). Although slow-wave sleep was marginally associated with MetS, no significant association was observed with the arousal index. Finally, we explored the relationship between SDB, defined using a lower threshold value of AHI (⩾ 1) and MetS. This definition classified 82 children with SDB. The adjusted OR for MetS using even this low threshold AHI level was significant, 3.54 (95% CI, 1.83, 6.84).
TABLE 2.
UNADJUSTED AND ADJUSTED ODDS OF METABOLIC SYNDROME FOR SLEEP-DISORDERED BREATHING*
| Unadjusted
|
Adjusted
|
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| SDB (AHI ⩾ 5) | 7.74 (3.10, 19.35) | < 0.001 | 6.49 (2.52, 16.70) | < 0.001 |
| Age (per 1-yr increase) | 0.99 (0.62, 1.56) | 0.95 | ||
| Male sex | 2.62 (1.30, 5.27) | 0.007 | ||
| African-American race/ethnicity | 0.86 (0.45, 1.66) | 0.66 | ||
| Preterm status | 0.90 (0.47, 1.74) | 0.76 | ||
Definition of abbreviations: AHI = apnea–hypopnea index; CI = confidence interval; OR = odds ratio; SDB = sleep-disordered breathing.
Based on logistic regression analyses with metabolic syndrome as the outcome. Each covariate in the adjusted model was adjusted for all other covariates.
TABLE 3.
LOGISTIC REGRESSION ANALYSES: ODDS OF METABOLIC SYNDROME FOR ALTERNATIVE MEASURES OF SLEEP-DISORDERED BREATHING*
| Adjusted OR (95% CI) | p Value | |
|---|---|---|
| ln(AHI) [per unit increase in ln(AHI)] | 1.75 (1.37, 2.24) | < 0.001 |
| Arousal index | 1.01 (0.92, 1.11) | 0.83 |
| > 0% Time < 90% saturation | 4.26 (1.06, 17.10) | 0.041 |
| Average O2 saturation | 0.59 (0.41, 0.85) | 0.005 |
| Minimum O2 saturation | 0.92 (0.86, 0.98) | 0.006 |
| Stage 3–4, % time | 0.97 (0.94, 1.00) | 0.085 |
| Sleep efficiency | 0.94 (0.90, 0.97) | < 0.001 |
Definition of abbreviations: AHI = apnea–hypopnea index; CI = confidence interval; OR = odds ratio.
Each OR is adjusted for age, sex, race, and preterm status, and derived by separate logistic regression models.
Additional exploratory analyses were conducted to ascertain the pattern of metabolic dysfunction in this sample and to identify differences in metabolic parameters between the SDB and non-SDB group, and to explore which metabolic parameters were associated with SDB independently of BMI (percentile) (Table 4). Because precise cutoffs for abnormality were not needed for these analyses, we assessed the variation among continuous indices of blood pressure, lipid and glucose levels, and insulin indices. These results showed that even after adjusting for BMI percentile, the SDB group was observed to have significantly higher levels of systolic and diastolic blood pressure, fasting insulin and HOMA levels, and low-density lipoprotein cholesterol.
TABLE 4.
ASSOCIATION OF SLEEP-DISORDERED BREATHING WITH INDIVIDUAL METABOLIC PARAMETERS, UNADJUSTED AND ADJUSTED FOR BODY MASS INDEX PERCENTILE AND SEX
| Non-SDB, Mean (SE) (n = 248) | SDB, Mean (SE) (n = 22) | Non-SDB, Adjusted Mean (SE) | SDB, Adjusted Mean (SE) | p Value* | |
|---|---|---|---|---|---|
| Blood pressure | |||||
| Systolic blood pressure, mm Hg | 113.74 (0.53) | 122.23 (2.68) | 113.90 (0.52) | 119.78 (1.78) | 0.0015 |
| Diastolic blood pressure, mm Hg | 66.32 (0.41) | 70.04 (2.33) | 66.35 (0.44) | 69.68 (1.51) | 0.0345 |
| Lipids | |||||
| Cholesterol, mg/dl | 149.1 (1.77) | 165.9 (6.21) | 149.4 (1.73) | 161.3 (5.91) | 0.0537 |
| Triglycerides, mg/dl | 74.9 (2.35) | 102.0 (10.60) | 75.6 (2.264) | 91.1 (7.744) | 0.0556 |
| HDL, mg/dl | 48.6 (0.76) | 44.8 (1.92) | 48.4 (0.715) | 46.6 (2.426) | 0.4780 |
| LDL, mg/dl | 85.5 (1.55) | 100.8 (5.55) | 85.8 (1.505) | 96.3 (5.094) | 0.0489 |
| Glucose metabolism | |||||
| Fasting plasma glucose, mg/dl | 92.5 (0.68) | 96.6 (1.47) | 92.5 (0.644) | 94.9 (2.218) | 0.3099 |
| Glucose after oral glucose load, mg/dl† | 104.8 (1.25) | 120.5 (11.27) | 103.2 (1.301) | 112.0 (4.894) | 0.0740 |
| Fasting insulin, μIU/ml† | 13.71 (0.79) | 26.37 (3.69) | 11.0 (0.388) | 17.0 (2.052) | 0.0005 |
| HOMA, μIU/ml† | 3.22 (0.22) | 6.36 (0.93) | 2.49 (0.093) | 3.98 (0.506) | 0.0004 |
Definition of abbreviations: HDL = high-density lipoprotein; HOMA = homeostatic model assessment; LDL = low-density lipoprotein; SDB = sleep-disordered breathing.
p Value from linear regression model using SDB to predict metabolic component, adjusting for sex and body mass index percentile.
For regression modeling, highly-skewed outcome variables were log-transformed.
DISCUSSION
Two recent analyses of population-based data from the NHANES have estimated the overall prevalence of MetS among adolescents to range from 4 to 10% (3, 4), with differences attributable to variations in the definitions of MetS used in each study. These analyses, as well as those of clinic-based samples, indicate that MetS occurs in approximately 25 to 30% of overweight children, and in as many as 50% of severely obese children (31, 32). Although prevalence of individual components of MetS vary among subgroups of children, MetS appears to be slightly more common in boys, and especially prevalent in Mexican Americans (4). Our data for the first time address SDB as a risk factor for MetS in a community sample of adolescents and demonstrate that adolescents with SDB are at an approximately sevenfold increased odds of MetS than unaffected children (found in almost 60% of our sample with SDB), and this association is not explained by sex, race, or socioeconomic status. Furthermore, secondary analyses suggested that, even after adjusting for sex and BMI percentile, adolescents with SDB have elevated levels of blood pressure, insulin, and low-density lipoprotein cholesterol (i.e., indices associated with MetS).
SDB is increasingly recognized as contributing to a substantive public health burden in both adults and children. In adults, even after adjusting for the effects of obesity, SDB has been associated with a 40 to 200% increased odds of cardiovascular disease and glucose intolerance (12, 33, 34). In the Sleep Heart Health Study, increasing sleep apnea severity, as measured by the AHI or nocturnal oxygen saturation levels, was associated with glycemic status (12). Relative to individuals with an AHI of less than 5.0, glucose intolerance was 30 to 50% more prevalent in those with mild and moderate SDB, respectively (12). Longitudinal data also indicate that individuals with SDB are at increased risk for incident diabetes (11, 35). In a 10-year prospective evaluation performed in the Nurses' Health Study, habitual snorers had an age- and BMI-adjusted relative risk for diabetes of 2.3 (11). A study in adult patients with SDB showed an improvement of insulin resistance by 32% after sleep apnea treatment (13).
Relatively little research has addressed the association of metabolic dysfunction with SDB in children, and no study has yet explicitly studied MetS in relationship to childhood SDB. An early Australian study of obese snoring children referred to a sleep laboratory reported a significant association between fasting insulin levels and the AHI, arousal index, and desaturation time that appeared to be unrelated to BMI (36). However, an uncontrolled follow-up study from this group failed to confirm an independent association between SDB and fasting insulin levels, nor did it show consistent associations between treatment of SDB and improvement in metabolic indices (37). Two other reports from pediatric sleep laboratories of young, prepubertal children, including one study that excluded obese children (38), also did not show significant associations between fasting insulin levels and indices of SDB (38, 39). In contrast, in our community-based sample of predominantly postpubertal adolescents, we have demonstrated strong associations of SDB with MetS as well as with individual metabolic parameters commonly associated with MetS, including fasting insulin and the HOMA index. A longitudinal study of children has demonstrated that many components of the MetS become more prevalent between the ages of 10 and 19 years (40). Thus, it is possible that study differences reflect a potential age dependency of the expression of MetS. Stronger findings in adolescents could be due to effects associated with the chronicity of SDB, modifying influences related to puberty or other age-related changes in metabolism, or to differences in the pathogenesis of SDB in younger compared with older children (including a possibly stronger influence of obesity on SDB pathogenesis in adolescents compared with younger children). For example, whereas our prior analysis of the CCSHS cohort showed a modest and nonsignificant association between BMI and SDB in children aged 8 to 11 years (10), in the subgroup followed at ages 13 to 16 years, a stronger association between obesity and SDB was observed. Because an analysis by Tauman and colleagues, restricted to obese children, failed to demonstrate an association between indices of glucose homeostasis and SDB (39), study differences are unlikely to be due solely to differences in obesity prevalence in the study populations.
In children, standardized definitions of MetS do not yet exist. We chose as our primary definition one that was adapted from the ATP III recommendations (4) and repeated our analyses using a more conservative definition (3). Although the prevalence of MetS was lower using the latter definition, the overall magnitude of associations between SDB and MetS was similar.
In adults, MetS is most commonly defined on the basis of a clustering of abnormalities in blood pressure, glucose–insulin homeostasis, lipids, and/or evidence of central obesity. A pivotal role for central obesity in this syndrome is supported by data showing an increased incidence of MetS in children with persistent elevations in waist circumference during childhood (40), as well as other data showing strong correlations between indices of obesity and other components of the MetS (5). This appears to be due to a role of adipose tissue in mediating cytokine, growth factor, and sex steroid levels, which interactively may modify the actions and secretion of insulin and the metabolism of fatty acids (41). Thus, epidemiologic studies of MetS do not usually attempt to separate out the influence of obesity, which is integral to the syndrome. Accordingly, our primary analyses quantified the association between MetS and SDB, acknowledging that central obesity is an integral component of MetS.
Among adolescents, the prevalence of obesity has tripled from 5 to 15% over the last 30 years, with an additional 30% of adolescents currently considered overweight (42). Childhood obesity is associated with a wide range of pediatric health effects, including insulin resistance and childhood diabetes (43, 44), hypertension, and psychosocial morbidity, and also predicts the development of chronic health conditions in adulthood (45, 46). Obesity is also a strong risk factor for adult SDB. Although data in children are more equivocal, obesity has been reported to be a risk factor for snoring or SDB in samples of older children and adolescents (47, 48). Our data are consistent with this and underscore the importance of overweight as an SDB risk factor in adolescents: 72% of children with SDB in our sample were overweight and 77% had an increased waist circumference.
The cross-sectional study design and high prevalence of obesity in the SDB group prevent precise assessment of the causal role of SDB in the pathogenesis of MetS. The biological plausibility of SDB as a contributor to the pathogenesis of MetS is supported by data from adult studies showing that SDB-associated sympathetic activation and hypoxemia may lead to the generation of reactive oxygen species, stimulation of various inflammatory cytokines, and endothelial dysfunction (49–51). Such effects appear to be relevant to pediatric patients with SDB as well, as evidenced by studies showing elevations in inflammatory mediators in children with SDB (27, 52, 53). To explore the potential impact of SDB that may be secondary to effects of obesity and directly related to SDB stresses, we performed a series of secondary analyses. These suggested that individual parameters associated with MetS, such as levels of blood pressure and fasting insulin and HOMA, vary with SDB status independently of sex and BMI percentile. Furthermore, we showed that MetS was strongly associated with indices of sleep-related oxygen desaturation and low sleep efficiency, but not arousal frequency. Use of a more liberal criterion of SDB (AHI ⩾ 1) was associated with a weaker association with MetS than a higher threshold value, and snoring history was not associated with MetS. Studies in adults also have shown that, of the conventionally measured indices of SDB, measures of hypoxic stress were the strongest correlates of glucose intolerance and insulin resistance (12, 54). In aggregate, these findings point to SDB, possibly associated with hypoxic stress, as a contributing cause of MetS in SDB, and suggest the need for further research aimed both at elucidating the potential causal pathways that may link SDB and metabolic dysfunction, and whether treatment of SDB improves metabolic dysfunction in adolescents. Regardless of its potential causal role, the high prevalence of central obesity and MetS in an urban community sample of adolescents suggests the importance of screening for MetS in adolescents with SDB.
Sleep deprivation also has been implicated as a risk factor for glucose intolerance and/or diabetes, with risk as high as 250% for those getting fewer than 5 hours of sleep (55). Effects have been attributed to sympathetic nervous system overactivity as well as hypothalamic pituitary–adrenal axis dysfunction occurring secondary to sleep deprivation (56). In the current study, average sleep duration on weekdays did not significantly differ in children with or without SDB, or with or without MetS. Although weekend sleep duration was less in children with MetS, it is unclear how isolated differences in weekend sleep duration may have contributed to the study findings. It is possible that the prevalence of severely curtailed sleep duration was too low to detect a significant relationship in this sample.
Study strengths include the community-based sample and use of standardized assessments of MetS and SDB. Study limitations include the low representation of Hispanics. Because the initial cohort was originally designed to compare differences in health outcomes between full and preterm children, our sample had a higher proportion of former preterm children than in the general population; this group has been postulated to be at increased risk for the development of MetS (57). However, we observed no relationship between preterm status or birth weight with MetS, suggesting that oversampling former preterm children was unlikely to have biased our estimates; in addition, final primary analyses were adjusted for preterm status. Adjusted and unadjusted ORs were only modestly different, again emphasizing that age, sex, race, or premature history did not substantively influenced the associations between SDB and MetS. Because this was a community sample, the number of children with moderate to severe SDB was relatively small and it is possible that findings may differ in a clinical sample with greater morbidity. Because our sample was recruited from an urban Midwestern city and had a higher prevalence of overweight than reported in national surveys (58), our findings may be less generalizable to other samples with lower rates of obesity. Analyses were cross-sectional and, because the definition of MetS includes a measure of central obesity, primary analyses did not adjust for obesity; it is unclear the extent to which MetS was independently influenced by the obesity of the SDB sample, as compared with the direct effects of overnight SDB stresses. Nonetheless, the high prevalence of MetS in the SDB sample was even greater than what has been reported in morbidly obese children (32).
Conclusions
These results underscore a high prevalence of MetS in adolescents with SDB and the potential importance of overnight hypoxemia as a central mediator of metabolic perturbations. The aggregation of MetS, overweight, and SDB in adolescents suggests the need to screen adolescents with SDB for MetS, and to screen children with MetS and overweight for SDB. The high prevalence of central obesity in SDB also emphasizes the importance of weight management interventions in groups at risk for MetS due to both obesity and to SDB-associated nocturnal stresses. The association between many components of the MetS with SDB, observed even after adjusting for BMI, suggests that SDB may contribute to metabolic dysfunction over and beyond effects of overweight alone. Because weight management is often unsuccessful, additional research is required to address whether treatment of SDB may improve metabolic function in young populations.
Supported by National Institutes of Health grants HL07567, HL60957, K23 HL04426, RO1 NR02707, M01 RR00080, and 1 U54CA116867.
Originally Published in Press as DOI: 10.1164/rccm.200703-375OC on May 31, 2007
Conflict of Interest Statement: S.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.S.-I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.L.R. received 10% effort salary support in 2005 and 2006 from Cephalon as research grants for participating in a multicenter clinical trial. She also received 10% effort salary support in 2006 and currently from Advanced Brain Monitoring as a subcontract investigator in an SBIR research grant. N.L.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.L.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.M.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Circulation 2002;106:3143. [PubMed] [Google Scholar]
- 2.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med 2003;163:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 2003;157:821–827. [DOI] [PubMed] [Google Scholar]
- 4.de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation 2004;110:2494–2497. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood: the Bogalusa Heart Study. Diabetes 2002;51:204–209. [DOI] [PubMed] [Google Scholar]
- 6.Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med 1998;338:1650–1656. [DOI] [PubMed] [Google Scholar]
- 7.Kimm SYS, Barton BA, Obarzanek E, McMahon RP, Kronsberg SS, Waclawiw MA, Morrison JA, Schreiber GB, Sabry ZI, Daniels SR. Obesity development during adolescence in a biracial cohort: the NHLBI Growth and Health Study. Pediatrics 2002;110:e54. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 2002;288:1728–1732. [DOI] [PubMed] [Google Scholar]
- 9.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 10.Rosen CL, Larkin EK, Kirchner HL, Emancipator JL, Bivins SF, Surovec SA, Martin RJ, Redline S. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr 2003;142:383–389. [DOI] [PubMed] [Google Scholar]
- 11.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol 2002;155:387–393. [DOI] [PubMed] [Google Scholar]
- 12.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521–530. [DOI] [PubMed] [Google Scholar]
- 13.Harsch IA, Schahin SP, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004;169:156–162. [DOI] [PubMed] [Google Scholar]
- 14.Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med 2005;165:447–452. [DOI] [PubMed] [Google Scholar]
- 15.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data 2000;314:1–27. [PubMed] [Google Scholar]
- 16.American Heart Association. Recommendations for human blood pressure determination by sphygmomanometers: report of a subcommittee of the Postgraduate Education Committee. Dallas, TX: American Heart Association; 1980.
- 17.Kump K, Whalen C, Tishler PV, Browner I, Ferrette V, Strohl KP, Rosenberg C, Redline S. Assessment of the validity and utility of a sleep-symptom questionnaire. Am J Respir Crit Care Med 1994;150:735–741. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Census Bureau. Census CD neighborhood change database: 1970–2000 tract data: selected variables for the US census tracts.
- 19.Spilsbury J, Storfer-Isser A, Drotar D, Rosen CL, Kirchner HL, Benham H, Redline S. Sleep behavior in an urban US sample of school-aged children. Arch Pediatr Adolesc Med 2004;158:988–994. [DOI] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–270. [PubMed] [Google Scholar]
- 23.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;23:57–63. [DOI] [PubMed] [Google Scholar]
- 24.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep 1998;21:759–767. [PubMed] [Google Scholar]
- 25.American Sleep Disorders Association (ASDA). ASDA Report. EEG arousals: scoring rules and examples. Sleep 1992;15:173–184. [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A manual of standardized techniques and scoring system for sleep stages of human subjects. Washington, DC: U.S. Government Printing Office; 1968. NIH Publication No. 204.
- 27.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, Zambito AM, Tracy RP, Jenny NS, Redline S. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation 2005;111:1978–1984. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 2004;145:439–444. [DOI] [PubMed] [Google Scholar]
- 29.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–576. [PubMed] [Google Scholar]
- 30.American Diabetes Association. American Diabetes Association clinical practice recommendations 2001. Diabetes Care 2001;24:S1–S24. [PubMed] [Google Scholar]
- 31.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–2374. [DOI] [PubMed] [Google Scholar]
- 32.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002;346:802–810. [DOI] [PubMed] [Google Scholar]
- 33.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 34.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 35.Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Intern Med 2000;248:13–20. [DOI] [PubMed] [Google Scholar]
- 36.de la Eva RC, Baur LA, Donaghue KC, Waters KA. Metabolic correlates with obstructive sleep apnea in obese subjects. J Pediatr 2002;140:654–659. [DOI] [PubMed] [Google Scholar]
- 37.Waters KA, Sitha S, O'Brien LM, Bibby S, de Torres C, Vella S, de la Eva R. Follow-up on metabolic markers in children treated for obstructive sleep apnea. Am J Respir Crit Care Med 2006;174:455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaditis AG, Alexopoulos EI, Damani E, Karadonta I, Kostadima E, Tsolakidou A, Gourgoulianis K, Syrogiannopoulos GA. Obstructive sleep-disordered breathing and fasting insulin levels in nonobese children. Pediatr Pulmonol 2005;40:515–523. [DOI] [PubMed] [Google Scholar]
- 39.Tauman R, O'Brien LM, Ivanenko A, Gozal D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics 2005;116:e66–e73. [DOI] [PubMed] [Google Scholar]
- 40.Morrison JA, Friedman LA, Harlan WR, Harlan LC, Barton BA, Schreiber GB, Klein DJ. Development of the metabolic syndrome in black and white adolescent girls: a longitudinal assessment. Pediatrics 2005;116:1178–1182. [DOI] [PubMed] [Google Scholar]
- 41.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 2003;27:S53–S55. [DOI] [PubMed] [Google Scholar]
- 42.Troiano RP, Flegal KM. Overweight children and adolescents: description, epidemiology, and demographics. Pediatrics 1998;101:497–504. [PubMed] [Google Scholar]
- 43.Freeman DS, Burke GL, Harsha DW, Srinivasan SR, Cresanta JL. Relationship of changes in obesity to serum lipid and lipoprotein changes in childhood and adolescence. JAMA 1985;254:515–520. [PubMed] [Google Scholar]
- 44.Rosenbaum M, Leibel RL. Pathophysiology of childhood obesity. Adv Pediatr 1988;35:73–137. [PubMed] [Google Scholar]
- 45.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents: a follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992;327:1350–1355. [DOI] [PubMed] [Google Scholar]
- 46.Slyper AH. Childhood obesity, adipose tissue distribution, and the pediatric practitioner. Pediatrics 1998;102:e4. [DOI] [PubMed] [Google Scholar]
- 47.Corbo GM, Forastiere F, Agabiti N, Pistelli R, Dell'Orco V, Perucci CA, Valente S. Snoring in 9- to 15-year-old children: risk factors and clinical relevance. Pediatrics 2001;108:1149–1154. [DOI] [PubMed] [Google Scholar]
- 48.Wing YK, Hui SH, Pak WM, Ho CK, Cheung A, Li AM, Fok TF. A controlled study of sleep related disordered breathing in obese children. Arch Dis Child 2003;88:1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 2002;165:934–939. [DOI] [PubMed] [Google Scholar]
- 50.Ip MS, Lam B, Chan LY, Zheng L, Tsang KW, Fung PC, Lam WK. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med 2000;162:2166–2171. [DOI] [PubMed] [Google Scholar]
- 51.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep 2004;27:1113–1120. [DOI] [PubMed] [Google Scholar]
- 52.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics 2004;113:e564–e569. [DOI] [PubMed] [Google Scholar]
- 53.Gozal D, Lipton AJ, Jones KL. Circulating vascular endothelial growth factor levels in patients with obstructive sleep apnea. Sleep 2002;25:59–65. [DOI] [PubMed] [Google Scholar]
- 54.Sulit L, Storfer-Isser A, Kirchner HL, Redline S. Differences in polysomnographic predictors of hypertension and impaired glucose tolerance. Sleep 2006;29:777–783. [DOI] [PubMed] [Google Scholar]
- 55.Gottlieb DG, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ. Short sleep time is associated with diabetes mellitus and impaired glucose tolerance. Arch Intern Med 2005;165:863–867. [DOI] [PubMed] [Google Scholar]
- 56.Van Cauter E, Plat L, Copinschi G. Interrelations between sleep and the somatotropic axis. Sleep 1998;21:553–566. [PubMed] [Google Scholar]
- 57.Barker DJP, Martyn CN. The maternal and fetal origins of cardiovascular disease. J Epidemiol Community Health 1992;46:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;29:1549–1555. [DOI] [PubMed] [Google Scholar]