Abstract
Rationale: Previous studies have linked the development and severity of acute respiratory distress syndrome with a history of alcohol abuse. In clinical studies, this association has been centered on depletion of pulmonary glutathione and subsequent chronic oxidant stress.
Objectives: The impact on redox potential of the plasma or pulmonary pools, however, has never been reported.
Methods: Plasma and bronchoalveolar lavage fluid were collected from otherwise healthy alcohol-dependent subjects and control subjects matched by age, sex, and smoking history.
Measurements and Main Results: Redox potential was calculated from measured reduced and oxidized glutathione in plasma and lavage. Among subjects who did and did not smoke, lavage fluid glutathione redox potential was more oxidized in alcohol abusers by approximately 40 mV, which was not altered by dilution. This oxidation of the airway lining fluid associated with chronic alcohol abuse was independent of smoking history. A shift by 20 mV in plasma glutathione redox potential, however, was noted only in subjects who both abused alcohol and smoked.
Conclusions: Chronic alcoholism was associated with alveolar oxidation and, with smoking, systemic oxidation. However, systemic oxidation did not accurately reflect the dramatic alcohol-induced oxidant stress in the alveolar space. Although there was compensation for the oxidant stress caused by smoking in control groups, the capacity to maintain a reduced environment in the alveolar space was overwhelmed in those who abused alcohol. The significant alcohol-induced chronic oxidant stress in the alveolar space and the subsequent ramifications may be an important modulator of the increased incidence and severity of acute respiratory distress syndrome in this vulnerable population.
Keywords: glutathione, alcoholism, oxidative stress, pulmonary
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Chronic alcohol abuse is associated with an increased incidence and severity of acute lung injury. This has been linked with depletion of pulmonary glutathione and chronic oxidant stress, but the effects on redox potential in the lungs are unknown.
What This Study Adds to the Field
Chronic alcohol use induces a significant shift in lung redox potential toward a more oxidized state. This shift is not accurately predicted by changes in redox potential in the blood.
The lungs are constantly exposed to environmental and endogenous oxidants. The ability to neutralize these oxidants is essential to maintain pulmonary health. There are major differences between cellular and extracellular compartments in concentration of the thiol/disulfide systems and the relative redox states. Since Cantin and colleagues' report, published in 1987, on the high glutathione (GSH) concentration in the airspace (more than 20-fold than that in the plasma and over 90% in the reduced form), GSH has been considered a primary antioxidant in the alveolar space (1). Although the alveolar space is exposed to the oxidants present in cigarette smoke, the alveolar GSH pool is increased by 80% with no significant oxidant stress, as evidenced by the minimal shift toward the oxidized moiety oxidized glutathione (GSSG) (1). In the airspace, this high GSH concentration defends the lung against endogenously produced oxidants as well as environmental oxidants. Limited availability of GSH in the alveolar space is associated with a number of pulmonary diseases, including cystic fibrosis, acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, idiopathic interstitial pneumonia, and diffuse fibrosing alveolitis (2–4).
Previous studies reported a 2.7-fold elevation in the risk for ARDS in otherwise healthy alcohol abusers (5–7). Although the underlying cause remains unclear, recent research has elucidated a number of potential contributors, with one of the major causes being chronic oxidative stress. As outlined in a review by Guidot and Hart (8), the alcoholic lungs in ethanol-fed animal models are under increased oxidative stress, have altered nitric oxide metabolism, express abnormal amounts of transforming growth factor (TGF)-β, and are in a chronic proinflammatory state, with up-regulated expressions of IL-1β and tumor necrosis factor (TNF)-α. In this animal model, chronic ethanol ingestion depleted the GSH pool in the alveolar epithelial lining fluid (ELF) by as much as 80% (9). In clinical studies, chronic alcohol abuse resulted in a similar 80% decrease in the GSH pool of bronchoalveolar lavage fluid (BALF) (10). This decrease in GSH was accompanied by a fourfold increase in GSSG and was indicative of chronic oxidant stress. Altered homeostasis of the GSH/GSSG thiol pair was present even after 1 week of abstinence (11) and was independent of cirrhosis (10, 12). Therefore, these studies demonstrated that a history of alcohol abuse resulted in chronic oxidant stress in the alveolar space. Maccarrone and Ullrich suggested that the consideration of the redox state may be more reflective of the physiologically and pathological effects of oxidative stress than the mere absolute amounts of antioxidants and oxidants (13). Therefore, we examined the concentrations and redox potential of GSH and GSSG in the alveolar lining fluid and plasma pools of subjects stratified by both smoking and alcohol abuse. Some of these data were previously reported in abstract form (14).
METHODS
Subject Enrollment
Recruitment of subjects who have been chronically dependent on alcohol was made at the detoxification unit at the Veterans' Affairs Hospital in Atlanta, Georgia. Nonsmoking and smoking control subjects were enrolled from those who replied to postings at the different Emory University hospitals as well as from the community. The details of the recruitment process and selection criteria were previously reported (10). Basically, the alcoholic status was confirmed by a score of greater than 3 on the Short Michigan Alcohol Screening Test (SMAST) survey. Those with a score of 0 were considered as control subjects. Exclusion criteria included subjects with a prior history of cardiac disease, liver dysfunction, kidney disease, diabetes mellitus, lung disease, human immunodeficiency virus infection, gastrointestinal bleeding, or concomitant illicit drug use.
Collection and Processing of BALF
The lavage procedure on subjects with and without a history of alcohol abuse was performed as previously described (10). A flexible fiberoptic bronchoscope (model BF-1T20D; Olympus America, Inc., Melville, NY) was passed transnasally into a subsegmental bronchus of the right middle lobe in all subjects. Once wedged, 150 ml of sterile saline (three 50-ml aliquots) were injected and immediately aspirated into 50-ml suction traps under continuous low-pressure suction. Ten milliliters of blood were obtained from a peripheral vein within 10 minutes of the bronchoscopy. The BALF was immediately filtered through coarse gauze and centrifuged (750 × g for 10 min) to remove cellular elements. The acellular portion was stored at −80°C until future analysis.
Protein and pH Measurements of BALF
The pH of BALF samples was measured on 200 μl of aliquots using a Beckman microelectrode probe (Beckman Coulter Co., Fullerton, CA). The protein concentration in the samples was analyzed using the Pierce Better Bradford Coomassie assay (Pierce Chemical Co., Rockford, IL).
HPLC Measurement of Thiols
BALF and plasma samples were preserved immediately after collection in a 5% perchloric acid solution containing iodoacetic acid (6.7 μM) and boric acid (0.1 M). The preservation fluid also contained 5 μM of the internal standard γ-glutamyl-glutamate (γ-Glu-Glu). This step was completed within 30 minutes of collection to prevent degradation or oxidation of GSH. After protein removal, samples were derivatized with dansyl chloride and separated on a 10-μm Ultrasil amino column by HPLC (Waters 2690; Waters Corp., Milford, MA). Fluorescence detection was recorded by two detectors (Waters 474 and Gilson model 121 [Gilson, Inc., Middleton, WI]). The concentrations of GSH and GSSG were calculated by quantitating the integrated areas relative to that of γ-Glu-Glu. Dilution due to the lavage procedure was corrected using the urea method (15). The concentration of urea in all the samples was obtained with an adopted Berthelot method from Pointe Scientific, Inc. (Canton, MI) and has a sensitivity range of 0.05 to 150 mg/dl. The urea dilution factor is equal to [urea]plasma/[urea]BALF.
Redox Potential Calculations
The redox potential (Eh) of the GSH/GSSG thiol pair in the plasma and the BALF were calculated with the Nernst equation, Eh = Eo + RT/nF ln [disulfide]/([thiol1] [thiol2]). The Eo is the standard potential for the redox couple, R is the gas constant, T is the absolute temperature, n is 2 for the number of electrons transferred, and F is Faraday's constant. The standard potential Eo for the 2 GSH/GSSG couple was −264 mV at pH = 7.4. Adjustment for pH as appropriate was made by a +5.9-mV change in Eo with every 0.1 decrease in pH.
Statistical Analysis
Correlation between values for each subject was determined by Spearman or Pearson analysis depending on results of test for normality. Group mean or median comparisons were made with the Student t test or the nonparametric Mann-Whitney test, as appropriate. Statistical significance was obtained with p < 0.05. Statistics were presented either as mean ± SEM or as medians with the first and third quartiles.
RESULTS
Patient Demographics
As a group, subjects with alcohol dependence were slightly older than the control subjects, although control subjects were recruited by age matching. The distribution in sex did not differ between the groups (Table 1).
TABLE 1.
SUBJECT DEMOGRAPHICS
| Nonsmoking Control Subjects | Smoking Control Subjects | Alcoholic Nonsmokers | Alcoholic Smokers | |
|---|---|---|---|---|
| Number | 8 | 12 | 10 | 33 |
| Male, n (%) | 2 (75) | 9 (75) | 8 (80) | 31 (94) |
| Age, yr (SD) | 41 (4) | 46 (5) | 47 (5)* | 48 (5) |
p = 0.01 (compared with nonsmoking controls).
Basic Parameters of BALF Samples
As reported in Table 2, the median pH and corresponding ranges in the BALF of control subjects who did or did not smoke were 6.55 (6.45–6.83) and 6.51 (6.39–6.63), respectively. These were not different from BALF pH of the alcohol-dependent subjects who also did (6.37 [6.27–6.69]) or did not smoke (6.53 [6.44–6.68]). The urea concentrations in BALF or the dilution factor calculated as previously mentioned did not differ with smoking or alcohol status.
TABLE 2.
SUMMARY OF pH, UREA, AND PROTEIN PARAMETERS IN THE BRONCHOALVEOLAR LAVAGE FLUID
| BALF pH (log [H+]) | BAL Urea (mg/dl) | Urea Dilution Factor | |
|---|---|---|---|
| Nonsmoking Control Subjects | 6.59 ± 0.09 (6.26–6.88) | 0.54 ± 0.25 (0.17–2.28) | 36.0 ± 5.1 (7.50–51.6) |
| Smoking Control Subjects | 6.51 ± 0.06 (6.35–6.69) | 0.34 ± 0.05 (0.10–0.63) | 44.7 ± 10.4 (5.50–108.6) |
| Nonsmoking Alcoholics | 6.47 ± 0.12 (6.22–6.99) | 0.32 ± 0.09 (0.082–0.90) | 24.8 ± 5.3 (4.19–48.8) |
| Smoking Alcoholics | 6.55 ± 0.05 (6.05–7.05) | 0.18 ± 0.03 (0.021–0.70) | 66.5 ± 8.1 (4.91–160.2) |
| p Value | NS | NS | NS |
Definition of abbreviations: BALF = bronchoalveolar lavage fluid; NS = not significant.
Values are given as mean ± SEM, with ranges in parentheses.
GSH and GSSG Concentrations and Redox Potential in the Alveolar Fluids
Previous work in this group reported a decrease in total GSH concentration in the ELF with chronic alcoholism, from 576 to 79 μM (10). In this study, there was an alcohol-associated fourfold decrease in BALF total GSH regardless of smoking status (nonsmokers: 8.3 μM [4.7, 10.1] vs. 2.1 μM [1.2, 3.2]; smokers: 25.2 [7.84, 33.5] vs. 4.6 μM [2.18, 7.26], p < 0.05). The total glutathione pool (GSH + GSSG) in the ELF was also significantly depleted with chronic alcoholism, by eightfold among nonsmokers (400.4 [201.8, 556.7] vs. 53.4 μM [36.1, 68.5], p < 0.05) and twofold among smokers (546.3 [264.8, 818.8] vs. 253.1 μM [55.3, 517.1]).
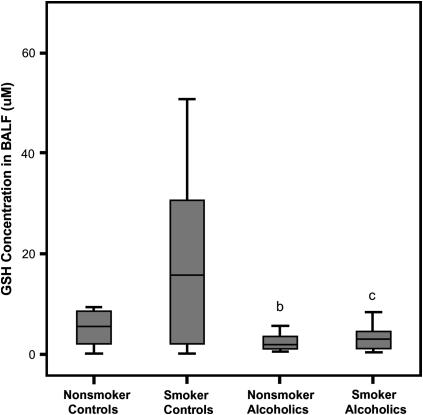
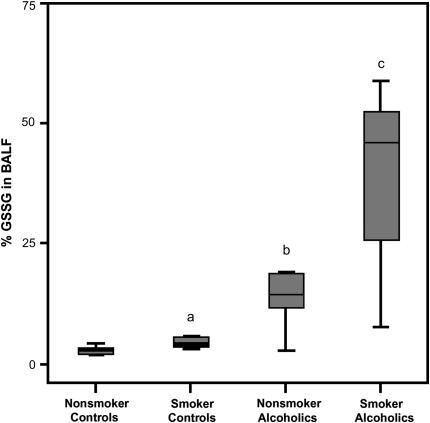
Chronic alcohol abuse was associated with a depletion of BALF GSH by more than 60% in both nonsmokers and smokers (p < 0.002) (Figure 1). The depletion was apparently alcohol dependent because stratifying by smoking status did not result in significant differences. A positive smoking history elevated GSSG by fivefold (0.11 [0.03–0.33] vs. 0.94 μM [0.15–2.05], p = 0.008). A similar increase in BALF GSSG concentration due to smoking was noted among subjects with chronic alcoholism (0.22 [0.05–0.49] vs. 1.02 μM [0.42–2.80]). Because the GSH concentrations decreased and the GSSG concentration increased, the percentage present as the oxidized moiety (%GSSG) was assessed and, in the control groups, found to be twofold higher in smokers than nonsmokers (p = 0.02). Among subjects who abused alcohol, %GSSG was approximately fourfold greater in smokers (Figure 2). Correspondingly, both groups of alcohol-dependent subjects were more oxidized by the %GSSG measure compared with the appropriate control population (with and without a smoking history; p < 0.003).
Figure 1.
Glutathione (GSH) concentrations in the bronchoalveolar lavage fluid (BALF). The GSH concentrations in the BALF of chronic alcoholics and control subjects with or without a smoking history were determined by HPLC. The data were presented as box plots with median lines, the lower and upper box edge for the first and third quartiles, respectively, and whiskers to indicate the smallest and largest values. bp < 0.002 when the nonsmoking control group was compared with nonsmoking subjects with a history of alcohol abuse; cp = 0.001 when the smoking control group was compared with the otherwise healthy alcoholics that smoked.
Figure 2.
Percentage of oxidized glutathione (%GSSG) in the bronchoalveolar lavage fluid (BALF). The percentage of the total GSH present as the oxidized moiety was determined in the BALF of chronic alcoholics and controls (%GSSG = [GSSG]/([GSH] + [GSSG]) × 100). Data are presented as box plots. ap= 0.02 when the smoking control group was compared with the nonsmoking control group; bp < 0.003 when the nonsmoking control group was compared with the nonsmoking group that abused alcohol; cp < 0.003 when the smoking control group was compared with the smoking group that abused alcohol.
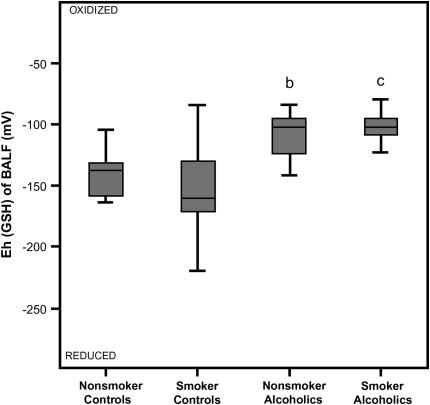
Despite the increased GSH concentration in the BALF of the smoking control group, the redox potential for the GSH/GSSG pair as calculated from the Nernst equation was not statistically different from values of nonsmoking control subjects (Figure 3). When the two groups of nonsmokers were compared, a history of alcohol abuse resulted in a significantly more oxidized alveolar microenvironment as evidenced by a shift in the GSH/GSSG redox potential of 40 mV in the BALF (p < 0.004). An even greater shift (50 mV) toward an oxidized state associated with alcohol dependence was apparent among smokers (p < 0.001). A history of smoking alone, however, did not alter GSH redox potential of subjects with chronic alcoholism.
Figure 3.
Redox potential values (Eh) for the reduced and oxidized (GSH/GSSG) thiol pair in the bronchoalveolar lavage fluid (BALF). The Eh values of the BALF were calculated from the GSH and GSSG concentrations using the Nernst equation with an E0 value of [GSSG]/[GSH]2 = −264 mV at a pH of 7.4. Data are presented as box plots. bp < 0.004 when the nonsmoking control group was compared with the nonsmoking group that abused alcohol; cp < 0.001 when the smoking control group was compared with the smoking group that abused alcohol.
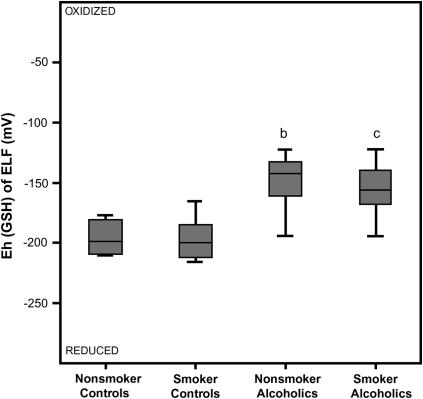
To consider conditions more representative of the ELF, redox potential for GSH was calculated after the dilution of the GSH and GSSG pools by the lavage procedure was corrected by the urea method. As observed in the BALF, the Eh for the GSH/GSSG pair in ELF was not statistically different in the control groups (i.e., not altered by a smoking history) (Figure 4). When the two nonsmoking groups were compared, a history of alcohol abuse resulted in a 50-mV shift in the ELF toward a more oxidized state (p < 0.01). A similar pattern was observed in the two smoking groups, with a 45-mV shift toward an oxidized state in those subjects who abused alcohol (p < 0.01). As observed in the BALF, there was minimal change in the redox pair within the alcohol-independent or alcohol-dependent groups when stratified according to their smoking history.
Figure 4.
Redox potential values (Eh) for the reduced and oxidized (GSH/GSSG) thiol pair in the epithelial lining fluid (ELF). The dilution of the GSH and GSSG concentrations by the lavage procedure was corrected using the urea method. The Eh values of the ELF were then calculated from the corrected GSH and GSSG concentrations using the Nernst equation with an E0 value of [GSSG]/[GSH]2 = −264 mV at a pH of 7.4. The data are presented as box plots. bp < 0.01 when the nonsmoking control group was compared with the nonsmoking group that abused alcohol; cp < 0.01 when the smoking control group was compared with the smoking group that abused alcohol.
If we assume that the pH of the ELF could be estimated by multiplying the BALF H+ ion (derived by BALF pH) with the urea dilution factor, then, indeed, the low ELF pH leads to a shift in redox potential toward the more oxidized state, from a median of −170 to −50 mV for nonsmoking control subjects. However, a similar shift applies to the other groups as well. Therefore, the differential in the redox potential values between alcoholics and their respective smoking control subjects remained the same and was independent of whether the dilution of pH was adjusted. The effect of alcoholism was still associated with an approximate 30-mV shift of redox potential toward the more oxidized state: 1.0 versus −51.9 mV among nonsmokers and −10.1 versus −39.1 mV among smokers.
GSH and GSSG Concentrations and Redox Potential in Plasma
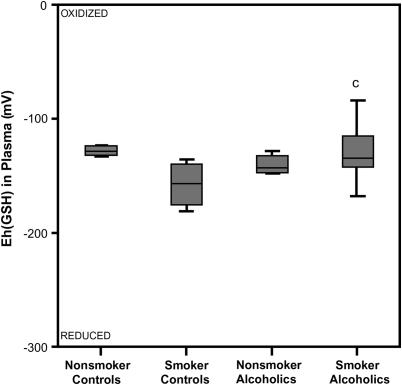
The redox potential of plasma GSH/GSSG was calculated by the Nernst equation (16) using the concentrations of GSH and GSSG. The median (quartile) GSH Eh value for the nonsmoking and smoking control groups was −129.1 mV (−145.8, −124.5] and −155.8 mV (−176.6, −139.5), respectively, and not statistically different (Figure 5). Plasma GSH Eh did not differ by alcohol abuse in nonsmoker subjects. But, among smokers, chronic alcoholism was associated with a shift in plasma GSH/GSSG redox toward the more oxidized state by nearly 30 mV (p = 0.01). There was no statistical difference in the plasma redox potential when stratified according to smoking history.
Figure 5.
Redox potential values (Eh) for the reduced and oxidized GSH/GSSG thiol pair in plasma. The Eh values of the plasma were calculated from the GSH and GSSG concentrations using the Nernst equation with an E0 value of [GSSG]/[GSH]2 = −264 mV at a pH of 7.4. A more negative Eh value corresponded to a more reduced redox state; likewise the more positive the Eh value, the more oxidized the redox state. cp = 0.01 when the smoking control group was compared with the smoking group that abused alcohol.
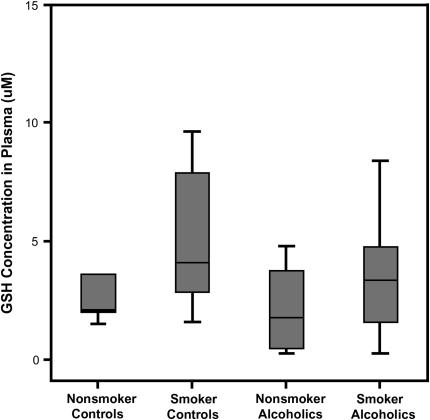
This oxidative shift was not detected by merely comparing plasma GSH concentration (Figure 6). Median plasma GSH concentrations were not statistically different at 2.9 (0.5–4.8) and 3.6 μM (2.0–6.4), respectively, for subjects who did or did not abuse alcohol. Even among smokers, chronic alcohol dependence did not significantly decrease plasma GSH (2.4 [1.4–4.6] vs. 4.1 μM [3.4–6.8]). Alcohol abusers, regardless of smoking status, had similar plasma GSH levels.
Figure 6.
Plasma glutathione (GSH). The GSH concentrations in the plasma of chronic alcoholics and control subjects with or without a smoking history were determined by HPLC.
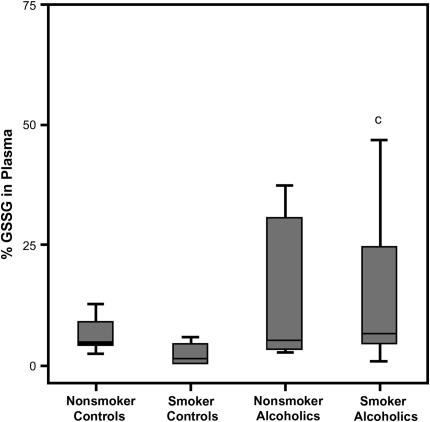
Systemic GSH oxidant status was also evaluated in terms of %GSSG, calculated as the percentage of the total plasma GSH present as GSSG. A history of alcohol abuse was associated with increased %GSSG, by approximately 1% among nonsmokers, and aggravated by the combination of smoking and alcohol dependence to more than threefold that of smokers (p = 0.02) (Figure 7). As was the case for both Eh (GSH) and GSH concentration, %GSSG did not differ by smoking status among subjects with a history of alcoholism.
Figure 7.
The percentage of oxidized glutathione (%GSSG) in plasma. The percentage of the total GSH present as the oxidized moiety was determined in the plasma of chronic alcoholics and control subjects (%GSSG = [GSSG]/([GSH] + [GSSG]) × 100). The data are presented as box plots. cp = 0.025 when the smoking control group was compared with the smoking group that abused alcohol.
DISCUSSION
Alcohol is the most frequently abused drug in the world, claiming 100,000 lives annually and costing more than $100 billion per year in alcohol-related problems (17). In the United States, 15 to 20 million individuals are classified as alcoholics, and in patients admitted to general hospitals, 20 to 40% have alcohol-related disorders (17). In trauma patients, the risk of pulmonary complications, such as respiratory failure requiring mechanical ventilation, is higher if there is a history of alcohol abuse (7). In septic shock patients, 70% of alcohol-dependent patients developed ARDS compared with 31% among others. After controlling for potential confounding variables, the relative risk of ARDS associated with chronic alcohol abuse was 3.7 (6). In these studies, a history of alcohol abuse alone did not result in acute lung injury. Rather, alcohol abuse increased the incidence and severity of ARDS when superimposed on a second injury, such as trauma, sepsis, or aspiration.
Several studies indicated that changes in plasma GSH and GSSG concentrations or deviation from a redox potential of approximately −140 mV provided a measure of systemic oxidant stress (18–20). In the current study, the plasma GSH, %GSSG, and the redox potential for the GSH/GSSG thiol pair of nonsmoking control subjects were similar to those reported elsewhere. Studies by Jones and coworkers (21) demonstrated a 11-mV shift in the redox potential toward a more oxidized state in subjects who had a smoking history. That oxidation of the plasma pool in subjects who smoke was not observed in the current study as measured by decreased GSH, increased GSSG, or changes in the GSH/GSSSG redox potential. It is unclear why different results were obtained. However, it may due to sample size because the current study was not powered to detect significance in an 11-mV change in the redox potential. In addition, the study by Samiec and colleagues (20) reported an age-related increase in the GSH/GSSG redox potential of 0.7 mV per year after age 45. In the current study, the subjects ranged in age from 34 to 56 years, a factor that could also contribute to variance in the redox potential.
In a study by Loguercio and coworkers (22) that included 10 subjects with chronic alcohol abuse, depletion of plasma GSH has previously been reported. The current study did not display the same effects of alcohol on plasma GSH levels. Concurrent alcohol abuse and smoking significantly increased the percentage of the glutathione pool present as GSSG. This increase in plasma %GSSG was mirrored in smoking alcoholics by a similar shift in the redox potential of the GSH/GSSG pair toward an oxidized state (∼ 17 mV). Therefore, the significantly more oxidized redox potential of the GSH/GSSG pair and the greater %GSSG suggested a systemic oxidative stress, particularly in alcohol abusers who also smoked, a behavior characteristic of 80% of all alcoholics (23).
As observed by others (1), the GSH concentration in the ELF was 140-fold greater than that present in the plasma, with less than 5% of the ELF pool present as the oxidized moiety. After correction for dilution by the lavage procedure, the redox potential of the ELF was approximately −190 mV, a redox potential that approaches that of tissues (−185 to −258 mV) (24). When the GSH/GSSG redox potential of the plasma and the ELF were compared, the ELF was 30 mV more reduced than plasma.
In the BALF, a smoking history is associated with a significant increase in the GSH pool, with 98% remaining in the reduced form (1). In the current study, a similar increase in lavage fluid GSH concentration was suggested in smoking control subjects. However, this smoking-associated increase in GSH was accompanied by an increase in GSSG. As a result, smoking was not associated with a significant shift in the GSH/GSSG redox potential in the BALF or in the ELF of the control groups.
In previous studies by this research group, a history of alcohol abuse was associated with an 80% decreased in the GSH content of the lavage fluid and a fourfold increase in the %GSSG (10, 11). In the current study, a similar decrease in the GSH pool was observed in those subjects who abused alcohol. Furthermore, this decrease in GSH associated with alcohol abuse was independent of their smoking history. When the GSSG pool was assessed, alcohol abuse resulted in a three- to fourfold increase in those subjects, independent of their smoking history. When the GSH/GSSG redox potential of the BALF or the ELF was determined, the alveolar lining fluid was approximately 40 mV more oxidized in subjects with a history of alcoholism. This dramatic increase in alveolar oxidant stress was independent of their smoking histories. Therefore, the ELF redox potential of subjects who abused alcohol was only 15 mV more reduced than the corresponding plasma sample. This was in sharp contrast to a 30-mV ELF-to-plasma potential difference observed in control subjects.
In the alveolar space, GSH protects by neutralizing environmentally or endogenously derived free radical species. As a major antioxidant in the alveolar space, it is used not only to eliminate peroxides but to also maintain vitamins C and E in their reduced and functional forms (25). Alveolar epithelial cells and airspace cells are dependent on the availability of GSH in the ELF and dramatic decreases could promote intracellular oxidant stress and negatively impact cellular functions and cell viability. Oxidant stress can promote lipid peroxidation, protein cross-linking, protein fragmentation, and DNA damage and strand breaks. The effects of the extracellular GSH redox state on cell apoptosis, nuclear factor (NF)-κB activation, activator protein (AP)-1 activation, and cell signaling are areas of recent research in pulmonary pathological processes (26–28). Downstream of NF-κB and AP-1 activation are the up-regulation of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β (29, 30). Other potential sites sensitive to the redox status are thiol sites critical for protein activity. The capacity of α1-protease inhibitor to limit the proteolytic damage from neutrophil-derived elastase is attenuated by chronic oxidant stress (31, 32). This inactivation of the α1-protease inhibitor is modulated by GSH availability (33). A 40-mV change in the redox potential would be sufficient to cause a ninefold change in the ratio of reduced to oxidized forms of proteins with vicinal dithiols (34). Such proteins include protein disulfide isomerase (35), thioredoxin (36), αlIbβ3 integrin (37), and the Na(+)-H+ exchanger (38). Priming of all of these different parameters by alcohol-induced chronic oxidant stress superimposed on the reactive oxygen species generated during the influx of neutrophils, eosinophils, and leukocytes may contribute to the increased incidence and severity of ARDS associated with alcohol abuse (10).
The redox potential of GSH in plasma was approximately 18 mV more oxidized in subjects with chronic alcoholism compared with age-, sex-, and smoking-matched control subjects. This suggested that a history of alcohol abuse was associated with systemic oxidant stress, which was not detectable in GSH measurements alone. The shift in redox potential toward the more oxidized state was significantly greater in the BALF and the ELF. Thus, the changes in the redox potential of the plasma underestimated the alcohol-induced changes in the redox potential of the alveolar space. When comparing GSH or GSSG concentrations in the BALF, there is a need to establish a valid normalization for lavage dilution. Similarly, the determination of the actual redox potential of the GSH/GSSG pair in the ELF is dependent on correction for the dilution due to the lavage procedure. However, the GSH/GSSG redox potential of the BALF and the ELF both shifted by 40 mV in the otherwise healthy subjects with a history of alcoholism. In other words, a normalization factor was not needed to determine the degree by which decreases in GSH and increases in GSSG translated into oxidant stress in the alveolar space. Rather, determination of the redox state of the GSH/GSSG thiol pair provided a dynamic and quantitative measure of oxidant stress in the alveolar space (16).
There are undoubtedly multiple mechanisms that could potentially contribute to the altered balance between GSH and GSSG, including increased cellular uptake and utilization. Our data represent only the free GSH and GSSG, and decreased GSH could be partially explained by a shift from free to protein-bound GSH. Of course, chronic alcoholism may alter the activity of enzymes that affect GSH and GSSG concentration and balance (e.g., glutathione reductase and glutathione peroxidase). In addition, increased utilization for the detoxification of other oxidants could account for this decrease in the GSH pool.
Human studies undoubtedly require consideration of a large number of factors that may contribute to the observed findings. In these subjects, there are a number of dietary factors that may alter plasma thiol state, including sulfur amino acid intake, availability of glutamine, dietary antioxidants, inducers of GSH synthesis, and redox active micronutrients such as selenium, flavin, and niacin (39). Chronic alcoholism has been associated with deficiency in folate and other B vitamins that may participate in methionine and cysteine synthesis (40, 41). Specific transporters move extracellular amino acids into the cells, and although studies in alcoholic micropig models have suggested down-regulation of transporters involved in folate and B-vitamin absorption, the effects of alcohol on these mechanisms in the lung remain unstudied. Evaluations of these numerous contributors in human studies would better explain the observations and reveal effective targets to decrease the risk of ARDS in this vulnerable population.
In summary, individuals with a history of chronic alcohol abuse manifest significant oxidant stress in the alveolar space as defined by decreased GSH, increased GSSG, and an oxidative shift in the redox potential. The degree of alcohol-induced oxidant stress in the alveolar space was not accurately reflected systemically. Because the oxidant stress in the ELF was not exacerbated by a smoking history, alcohol abuse was suggested as the driving factor in alveolar GSH depletion and oxidant stress. In the smoking control groups, there was compensation for the oxidant stress caused by smoking and the redox potential in the alveolar space was not altered. However, the capacity to maintain a reduced GSH pool was overwhelmed in the alveolar space of subjects with long-term alcohol abuse and suggested that history of alcohol abuse resulted in a greater oxidant burden than a history of smoking. Therefore, GSH deficiency and the subsequent ramifications of significant chronic oxidant stress in the alveolar space may explain the increased incidence and severity of ARDS in this vulnerable population.
Supported by the National Institute of Alcohol Abuse and Addiction grants R21 AA015335 (L.A.S.B. and M.M.), R01 AA-014435 (M.M.), and 1 P50 AA135757 (L.A.S.B., M.M., and E.L.B.).
Originally Published in Press as DOI: 10.1164/rccm.200611-1722OC on May 16, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol 1987;63:152–157. [DOI] [PubMed] [Google Scholar]
- 2.Rahman I, Kilty I. Antioxidant therapeutic targets in COPD. Curr Drug Targets 2006;7:707–720. [DOI] [PubMed] [Google Scholar]
- 3.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 1993;75:2419–2424. [DOI] [PubMed] [Google Scholar]
- 4.Beeh KM, Beier J, Haas IC, Kornmann O, Micke P, Buhl R. Glutathione deficiency of the lower respiratory tract in patients with idiopathic pulmonary fibrosis. Eur Respir J 2002;19:1119–1123. [DOI] [PubMed] [Google Scholar]
- 5.Moss M, Steinberg KP, Guidot DM, Duhon GF, Treece P, Wolken R, Hudson LD, Parsons PE. The effect of chronic alcohol abuse on the incidence of ARDS and the severity of the multiple organ dysfunction syndrome in adults with septic shock: an interim and multivariate analysis. Chest 1999;116:97S–98S. [PubMed] [Google Scholar]
- 6.Moss M, Parsons P, Steinberg K, Hudson L, Guidot D, Burnham E, Cotsonis G. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003;31:869–877. [DOI] [PubMed] [Google Scholar]
- 7.Moss M, Gillespie MK, Ackerson L, Moore FA, Moore EE, Parsons PE. Endothelial cell activity varies in patients at risk for the adult respiratory distress syndrome. Crit Care Med 1996;24:1782–1786. [DOI] [PubMed] [Google Scholar]
- 8.Guidot DM, Hart CM. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Investig Med 2005;53:235–245. [DOI] [PubMed] [Google Scholar]
- 9.Brown LA, Harris FL, Bechara R, Guidot DM. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alcohol Clin Exp Res 2001;25:1078–1085. [PubMed] [Google Scholar]
- 10.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 2000;161:414–419. [DOI] [PubMed] [Google Scholar]
- 11.Burnham EL, Brown LA, Halls L, Moss M. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcohol Clin Exp Res 2003;27:1167–1172. [DOI] [PubMed] [Google Scholar]
- 12.Foreman MG, Hoor TT, Brown LA, Moss M. Effects of chronic hepatic dysfunction on pulmonary glutathione homeostasis. Alcohol Clin Exp Res 2002;26:1840–1845. [DOI] [PubMed] [Google Scholar]
- 13.Maccarrone M, Ullrich V. Redox regulation in disease and ageing. Cell Death Differ 2004;11:949–951. [DOI] [PubMed] [Google Scholar]
- 14.Yeh M, Burnham EL, Moss M, Sisson J, Brown L. Monitoring of the bronchoalveolar oxidant status in chronic alcoholics by exhaled breath condensate and segmental lavage [abstract]. Proc Am Thorac Soc 2006;3:A428. [Google Scholar]
- 15.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 1986;60:532–538. [DOI] [PubMed] [Google Scholar]
- 16.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, et al. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med 2003;35:1582–1588. [DOI] [PubMed] [Google Scholar]
- 17.Lieber CS. Medical disorders of alcoholism. N Engl J Med 1995;333:1058–1065. [DOI] [PubMed] [Google Scholar]
- 18.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med 2000;28:625–635. [DOI] [PubMed] [Google Scholar]
- 19.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 2002;348:93–112. [DOI] [PubMed] [Google Scholar]
- 20.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P Jr, Reed RL, Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med 1998;24:699–704. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP, Carlson JL, Samiec PS, Sternberg P Jr, Mody VC Jr, Reed RL, Brown LA. Glutathione measurement in human plasma: evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta 1998;275:175–184. [DOI] [PubMed] [Google Scholar]
- 22.Loguercio C, Clot P, Albano E, Argenzio F, Grella A, De Girolamo V, Delle Cave M, Del Vecchio Bianco C, Nardi G. Free radicals and not acetaldehyde influence the circulating levels of glutathione after acute or chronic alcohol abuse: in vivo and in vitro studies. Ital J Gastroenterol Hepatol 1997;29:168–173. [PubMed] [Google Scholar]
- 23.DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol 1990;51:130–135. [DOI] [PubMed] [Google Scholar]
- 24.Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med 1999;27:1208–1218. [DOI] [PubMed] [Google Scholar]
- 25.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001;30:1191–1212. [DOI] [PubMed] [Google Scholar]
- 26.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219–242. [DOI] [PubMed] [Google Scholar]
- 27.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation 2005;111:2973–2980. [DOI] [PubMed] [Google Scholar]
- 28.Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res 2006;9:169–181. [DOI] [PubMed] [Google Scholar]
- 29.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 2004;18:1897–1899. [DOI] [PubMed] [Google Scholar]
- 30.Haddad JJ. Glutathione depletion is associated with augmenting a proinflammatory signal: evidence for an antioxidant/pro-oxidant mechanism regulating cytokines in the alveolar epithelium. Cytokines Cell Mol Ther 2000;6:177–187. [DOI] [PubMed] [Google Scholar]
- 31.Mohsenin V. Lipid peroxidation and antielastase activity in the lung under oxidant stress: role of antioxidant defenses. J Appl Physiol 1991;70:1456–1462. [DOI] [PubMed] [Google Scholar]
- 32.Wallaert B, Aerts C, Gressier B, Gosset P, Voisin C. Oxidative inactivation of alpha 1-proteinase inhibitor by alveolar epithelial type II cells. J Appl Physiol 1993;75:2376–2382. [DOI] [PubMed] [Google Scholar]
- 33.Gressier B, Lebegue S, Gosset P, Brunet C, Luyckx M, Dine T, Cazin M, Cazin JC, Wallaert B. Protective role of glutathione on alpha 1 proteinase inhibitor inactivation by the myeloperoxidase system: hypothetic study for therapeutic strategy in the management of smokers' emphysema. Fundam Clin Pharmacol 1994;8:518–524. [DOI] [PubMed] [Google Scholar]
- 34.Jones DP, Mody VC Jr, Carlson JL, Lynn MJ, Sternberg P Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med 2002;33:1290–1300. [DOI] [PubMed] [Google Scholar]
- 35.Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem Res Toxicol 2005;18:1324–1331. [DOI] [PubMed] [Google Scholar]
- 36.Patel-King RS, Benashki SE, Harrison A, King SM. Two functional thioredoxins containg redox-senesitive vicinal dithiols from the Chlamydomonas outer dynein arm. J Biol Chem 1996;271:6283–6291. [DOI] [PubMed] [Google Scholar]
- 37.Essex DW, Li M. Redox control of platelet aggregation. Biochemistry 2003;42:129–136. [DOI] [PubMed] [Google Scholar]
- 38.Kulanthaivel P, Simon BJ, Leibach FH, Mahesh VB, Ganapathy V. An essential role for vicinal dithiol groups in the catalytic activity of the human placental Na(+)-H+ exchanger. Biochim Biophys Acta 1990;1024:385–389. [DOI] [PubMed] [Google Scholar]
- 39.van de Poll MC, Dejong CH, Soeters PB. Adequate range for sulfur-containing amino acids and biomarkers for their excess: lessons from enteral and parenteral nutrition. J Nutr 2006;136:1694S–1700S. [DOI] [PubMed] [Google Scholar]
- 40.Halsted CH, Villanueva JA, Devlin AM, James SJ. Interactions of ethanol and folate deficiency in development of alcoholic liver disease in the micropig. Trans Am Clin Climatol Assoc 2002;113:151–162. (discussion 62-3). [PMC free article] [PubMed] [Google Scholar]
- 41.Halsted CH, Villanueva JA, Devlin AM, Chandler CJ. Metabolic interactions of alcohol and folate. J Nutr 2002;132:2367S–2372S. [DOI] [PubMed] [Google Scholar]