Abstract
Rationale: Inhaled nitric oxide (NO) has been used to prevent bronchopulmonary dysplasia, but with variable results. Ethyl nitrite (ENO) forms S-nitrosothiols more readily than does NO, and resists higher-order nitrogen oxide formation. Because S-nitrosylation is a key pathway mediating many NO biological effects, treatment with inhaled ENO may better protect postnatal lung development from oxidative stress than NO.
Objectives: To compare inhaled NO and ENO on hyperoxia-impaired postnatal lung development.
Methods: We treated newborn rats beginning at birth to air or 95% O2 ± 0.2–20.0 ppm ENO for 8 days, or to 10 ppm NO for 8 days. Pups treated with the optimum ENO dose, 10 ppm, and pups treated with 10 ppm NO were recovered in room air for 6 more days.
Measurements and Main Results: ENO and NO partly prevented 95% O2–induced airway neutrophil influx in lavage, but ENO had a greater effect than did NO in prevention of lung myeloperoxidase accumulation, and in expression of cytokine-induced neutrophil chemoattractant-1. Treatment with 10 ppm ENO, but not NO, for 8 days followed by recovery in air for 6 days prevented 95% O2–induced impairments of body weight, lung compliance, and alveolar development.
Conclusions: Inhaled ENO conferred protection superior to inhaled NO against hyperoxia-induced inflammation. ENO prevented hyperoxia impairments of lung compliance and postnatal alveolar development in newborn rats.
Keywords: bronchopulmonary dysplasia, O-nitrosoethanol, S-nitrosylation
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
S-nitrosylation may affect a broad number of regulatory cell responses, such as inflammation. Inhaled agents that promote S-nitrosylation may provide targeted protection from inflammation.
What This Study Adds to the Field
Inhaled ethyl nitrite dose-dependently prevents hyperoxia-induced lung inflammation in newborn rats, and, at an equivalent dose, afforded better protection of postnatal lung function and development than nitric oxide.
Inhaled nitric oxide (NO) has been used to prevent and treat bronchopulmonary dysplasia (BPD) in premature newborns, with mixed success (1–4). The rationale underlying clinical trials has included improvement of ventilation–perfusion matching through classical effects on guanylyl cylase–mediated pulmonary vascular tone and remodeling (5), alveolar capillary development (6), and reducing inflammation (7). However, many, if not most NO biological effects are mediated through reversible S-nitrosylation of critical thiols that govern multiple biological pathways (8–10).
Inhaled agents preferentially forming S-nitrosothiols may “deliver” NO biological effects more efficiently, which may be of particular benefit in treating patients subjected to high levels of inhaled oxygen, as NO tends to rapidly form peroxynitrite in the presence of superoxide. Inhaled ethyl nitrite (ENO) has been used to treat newborns with pulmonary hypertension (11), and efficiently achieves S-nitrosation of glutathione compared with NO, without releasing significant amounts of reactive •NO species (12), so it should be less susceptible to forming higher-order nitrogen oxides.
We therefore compared the effects of inhaled NO and ENO in hyperoxia-exposed newborn rats, which we have previously shown to develop an acute inflammatory response followed by alveolar hypoplasia, both features of clinical BPD (13). To determine if inhaled ENO could prevent pulmonary inflammation and protect against hyperoxia-impaired alveolar development in vivo, we treated newborn rat pups with 95% O2 with or without inhaled ENO at 0.2–20 ppm, and measured effects on S-nitroso (SNO) accumulation in bronchoalveolar lavage (BAL), and on pulmonary leukocyte influx, inflammatory chemokine expression, nuclear factor (NF)-κB activation, antioxidant enzyme activity, 3-nitrotyrosine, static lung compliance, and alveolar development, and compared effects with groups treated with inhaled NO at 10 ppm, a dose previously used in clinical trials to prevent BPD (14). We have previously reported effects of ENO on in vitro NF-κB activation in the form of an abstract (15).
METHODS
Materials
Time-mated pregnant Sprague-Dawley rats were obtained from Charles River Laboratories (Raleigh, NC). Reagents were from Sigma-Aldrich (St. Louis, MO), unless otherwise specified. Gas sources and descriptions are in the online supplement.
Animal Exposures
Procedures were approved by the institutional animal care and use committee. Exposure conditions and details are in the online supplement. Animals were exposed, beginning on the day of birth, to air, 95% O2+ 5% N2, 95% O2 + 0.2, 1.0, 10, and 20 ppm ENO, or 10 ppm NO (balance gas: N2). Exposures were continued for 8 days, body weights were recorded, and pups were killed with sodium pentobarbital. Additional litters were exposed to air or 95% O2 ± ENO or NO, both at 10 ppm, for 8 days, recovered in air for 6 more days (total = 14 d), and then killed.
Methemoglobin
Methemoglobin was measured in blood samples obtained by cardiac puncture at Day 8 in six pups per treatment group (Model 482 co-oximeter; Instrumentation Laboratories, Lexington, MA).
Pulmonary Leukocyte Influx
BAL was performed at 8 days, as previously described (13). After lavage and pulmonary artery perfusion with 0.9% NaCl, lungs from Day-8 pups were snap frozen in liquid nitrogen. Lung extracts were reacted with o-dianisidine to detect myeloperoxidase (MPO) activity (13).
SNO Accumulation
BAL fluid (BALF) samples were snap frozen, and NO was measured in thawed samples by chemiluminescense after UV-photolysis before and after reduction of SNO by HgCl2, as previously described, using an S-nitrosoglutathione standard curve (16). Equal volumes of BALF were analyzed from each pup.
Cytokine-induced Neutrophil Chemoattractant-1 Expression
Lung extracts from Day-8 pups in each group were analyzed in duplicate by ELISA for cytokine-induced neutrophil chemoattractant-1 (CINC-1), as previously described (13). Random sections from four animals per treatment group were immunostained with anti–CINC-1 (see details in the online supplement). Real-time polymerase chain reaction measurements of CINC-1 and L32 cyclin (loading control) mRNA were performed, as previously described, (17) (see details in the online supplement). Mean CINC-1/L32 crossing-point ratios from hyperoxia-exposed groups were expressed as a proportion of the mean CINC-1/L32 ratio in the air-exposed group.
3-Nitrotyrosine
Lung homogenates, 0.4 mg total protein/lung from pups in each treatment group (n = 6 or 7/group) at Day 8 were analyzed in duplicate for 3-nitrotyrosine by ELISA (Northwest Life Science Specialties, Vancouver, WA). Results below the limit of detection for the assay were assigned a value of one half of the detection limit for the purposes of statistical comparisons.
NF-κB Activation
Nuclear proteins were extracted from Day-8 pups, as described in the online supplement. Nuclear protein from tumor necrosis factor- α–activated Jurkat cells was supplied with the kit as a positive control, along with nuclear protein extracted from lungs from LPS (Escherichia coli serotype 055, 6 mg/kg intraperitoneally)-treated adult rats (18), with vehicle-treated adult rats as additional negative controls. Activated NF-κB was detected using a commercial kit (TransAM NFκB; Active Motif, Carlsbad CA), binding nuclear protein extracts to NF-κB–binding DNA sequence covalently bound to an ELISA plate (19). Samples (10 μg/pup) were assayed in duplicate from three pups per group.
Activated NF-κB was detected in histologic sections from each treatment group by immunohistochemistry using an antibody directed against the nuclear localization epitope of the p65 subunit, which is exposed during activation. Details are in the online supplement.
Antioxidant Enzyme Expression
Whole-lung homogenates from pups at 8 days (n = 4/group) were analyzed in triplicate for total superoxide dismutase (SOD) (WST-1; Dojindo, Molecular Technologies, Gaithersburg, MD) and catalase (AmplexRed; Invitrogen, Carlsbad, CA) activities, according to the manufacturers' directions, using bovine SOD and catalase as standards. SOD1, −2, and −3 isoforms and catalase expression were detected by immunoblot and quantified by image analysis, normalizing to β-actin signal, as previously described (17). Details are in the online supplement.
Lung Mechanics
Pups exposed to air or 95% O2 ± ENO or NO, 10 ppm, for 8 days, then recovered in air for 6 days (total, 14 postnatal days) were anesthetized and connected to a small animal ventilator. Pressures and flows at specified tidal volumes were measured using forced oscillatory maneuvers with a small animal ventilator (FlexiVent; SCIREQ, Montreal, PQ, Canada) to generate pressure–volume loops, and to calculate compliance, as previously described in detail (20).
Lung Morphometry
Random sections of lungs from pups exposed to air or 95% O2 ± ENO or NO, 10 ppm for 8 days, then recovered in air, as described above (n = 6/group), were stained with malachite green according to the manufacturer's directions (Sigma), and with Hart's elastin, as previously described (21). Random images were obtained from each section for measurements of alveolar volume density (estimates alveolar number), alveolar surface density (estimates surface area), and secondary crest density normalized to alveolar volume density to account for inflation differences. Details are provided in the online supplement.
Data Analysis
Data are expressed as the mean ± SEM, except where noted. Significant differences between treatment groups were determined by analysis of variance. Post hoc differences were identified by Tukey-Kramer tests. Significance was accepted for comparisons at p less than 0.05, and calculations were made using statistical analysis software (SPSS version 14; SPSS, Inc., Chicago, IL).
RESULTS
Survival, Weight Gain, Methemoglobin
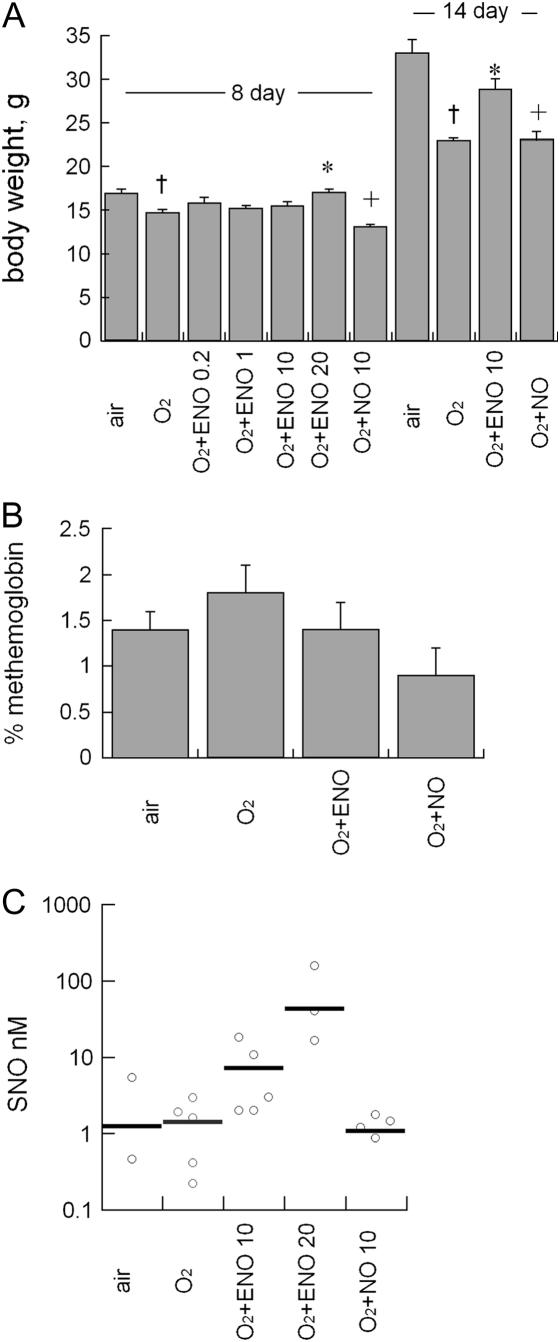
Survival was slightly less in hyperoxia-exposed groups (95%) compared with air-exposed groups (97%), but was not statistically different between hyperoxia and hyperoxia + ENO– or NO-exposed groups at Days 8 and 14. No gross abnormalities in behavior or appearance were seen by inspection in any treatment group. As expected, hyperoxia exposure impaired postnatal weight gain, measured as body weight at Days 8 and 14 (Figure 1), but this effect was prevented in pups treated with hyperoxia + 20 ppm ENO at 8 days, and in pups treated with 10 ppm ENO at 8 or 14 days. Inhaled NO + hyperoxia–treated pups were smaller than hyperoxia-treated pups at 8 days, but not at 14 days. Whole-blood methemoglobin measured at Day 8 was not significantly affected by exposure to hyperoxia or to hyperoxia + ENO or NO (Figure 1). No gross abnormalities were observed by visual inspection in thoracic or abdominal organs in any treatment group.
Figure 1.
(A) Effect of air or 95% oxygen (O2) ± ethyl nitrite (ENO; 0.2–20 ppm) × 8 days, or 10 ppm nitric oxide (NO) × 8 days on body weight (left-hand bars: n = 16/group; †p < 0.05 vs. 8-day air; *p < 0.01 vs. 8-day O2 control group; +p < 0.05 vs. 95% O2 + 10 ppm ENO). Effect of air × 14 days or 95% O2 ± 10 ppm ENO or NO × 8 days, followed by recovery in air for 6 days, on body weight (right-hand bars: †p < 0.05 vs. air control group; *p < 0.005 vs. 14 days O2 control; +p < 0.05 vs. 95% O2 + 10 ppm ENO; mean + SEM). (B) Effect of air or 95% O2 ± 10 ppm ENO or NO × 8 days on whole-blood methemoglobin; mean + SEM; n = 6/group. (C) Effect of air or 95% O2 ± 10 or 20 ppm ENO or 10 ppm NO on S-nitroso concentration in lavage. Bars represent means of average values from four or five pups per treatment group.
SNO Accumulation
We measured SNO in lavage from 4 or 5 pups per group treated with air, 95% O2, or 95% O2 + 10 or 20 ppm ENO for 8 days, or 10 ppm NO for 8 days. ENO treatment showed a trend toward increased SNO concentrations, but the differences were not statistically significant, as shown in Figure 1.
Effects on Inflammatory Responses
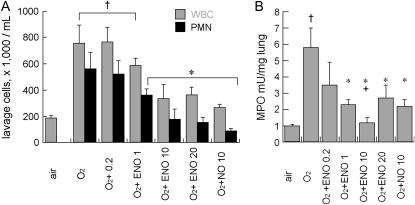
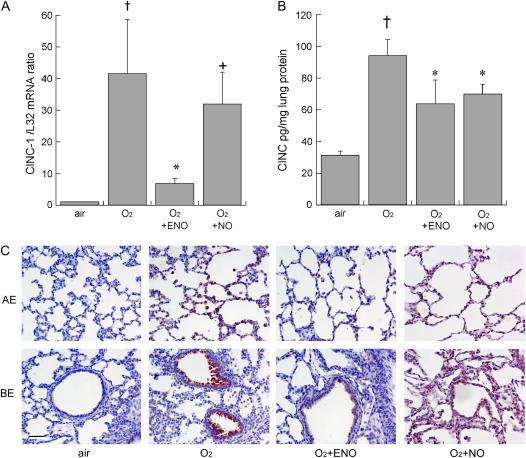
As shown in Figure 2, treatment with ENO for 8 days dose-dependently decreased 95% O2–induced BALF leukocyte and neutrophil accumulation and tissue MPO activity (n = 8/group). NO at 10 ppm also prevented BALF leukocyte and neutrophil influx, but was less effective at preventing MPO accumulation. There were no significant differences between ENO and NO effects on BALF neutrophils. ENO, but not NO, at 10 ppm, prevented hyperoxia-induced total lung CINC-1 mRNA accumulation at Day 8, and both ENO and NO partly prevented CINC-1 protein accumulation in whole lung, as measured by ELISA (Figure 3). ENO treatment partly prevented hyperoxia-induced alveolar epithelial and bronchiolar epithelial CINC-1 immunostaining, whereas NO treatment only reduced immunostaining in bronchiolar epithelium.
Figure 2.
(A) Effect of air or 95% O2 ± ENO at 0.2, 1, 10, or 20 ppm, or NO at 10 ppm, × 8 days, on bronchoalveolar lavage leukocytes (WBC) and neutrophils (PMN); n = 8/group; mean ± SEM; †p < 0.05 versus air; *p < 0.05 versus O2 control group. (B) Effect of air or 95% O2 ± ENO at 0.2, 1, 10, or 20 ppm, or NO at 10 ppm, × 8 days, on myeloperoxidase (MPO) activity in perfused, lavaged whole lung; n = 8/group; mean ± SEM; †p < 0.05 versus air; *p < 0.05 versus O2 control group; +p < 0.05 versus NO.
Figure 3.
(A) Effect of air or 95% O2 ± ENO or NO, 10 ppm × 8 days on whole lung cytokine-induced neutrophil chemoattractant-1 (CINC-1) mRNA (normalized to L32 cyclin) expressed as fold difference versus air control group (n = 4/group) and (B) CINC-1 protein measured by ELISA (n = 8/group); mean + SEM; †p < 0.05 versus air; *p < 0.05 versus O2 control group; and +p < 0.05 versus O2 ENO group. (C) CINC-1 immunolabeling in alveolar and bronchiolar epithelium (AE and BE, respectively); scale bar = 50 μm.
3-Nitrotyrosine
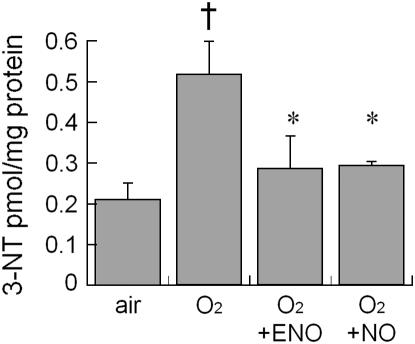
Hyperoxia induced a statistically significant increase in whole-lung 3-nitrotyrosine, compared with air-exposed animals at Day 8 (Figure 4). In contrast, hyperoxia did not significantly increase 3-nitrotyrosine in animals treated with ENO or NO at 10 ppm.
Figure 4.
Effect of air or 95% O2 ± 10 ppm ENO or NO, × 8 days on 3-nitrotyrosine detected by ELISA; mean + SEM; n = 6 or 7/group; †p < 0.05 versus air; *p < 0.05 versus O2 control group.
NF-κB Activation
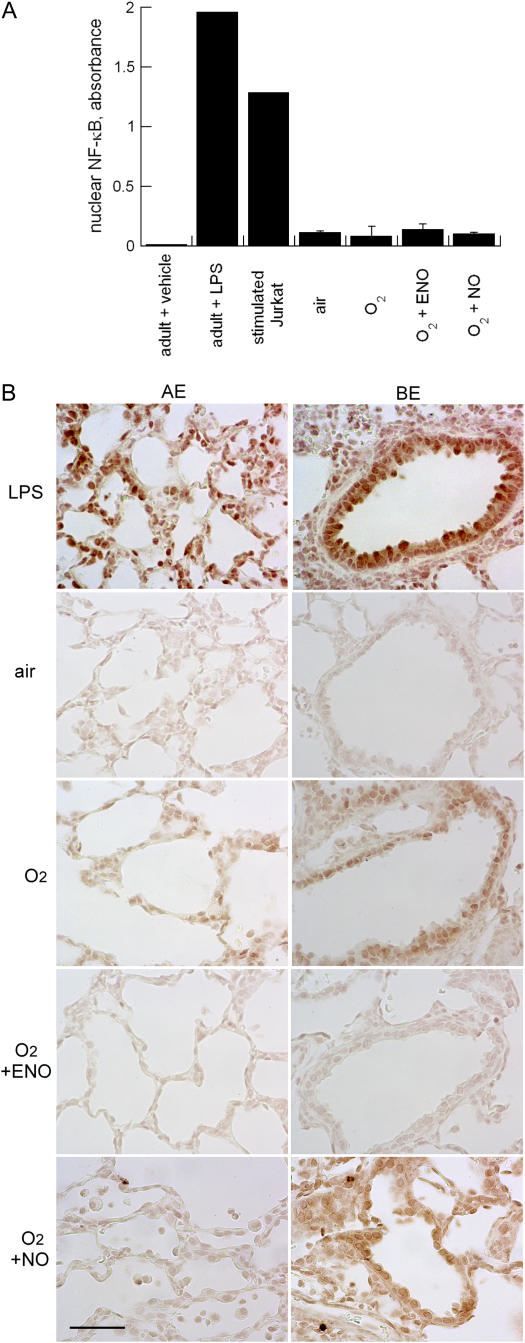
Hyperoxia activated NF-κB in a subpopulation of cells, mainly in bronchiolar epithelial cells (Figure 5), but also in alveolar epithelium, as detected by immunohistochemistry. This was in marked contrast to the widespread activation observed in lungs from LPS-treated adult rat. Treatment with ENO, but not NO, decreased bronchiolar epithelial NF-κB activation. Effects on alveolar epithelium were modest and heterogeneous in all hyperoxia-exposed groups at 8 days. Hyperoxia did not significantly increase NF-κB activation in whole lung compared with air-exposed newborn pups, and this was unaffected by ENO or NO. The overall activation in lungs from each of the neonatal exposure groups was small compared with the activation in LPS-treated adults (or in stimulated Jurkat cells), in accord with the immunohistochemical studies (Figure 5).
Figure 5.
(A) Nuclear factor (NF)-κB activation in air (negative control group)-exposed adult rat lung, LPS-treated adult rat lung, or tissue plasminogen activator–stimulated Jurkat cells (positive control group), 8-day-old rat lung from air, 95% O2–exposed, 95% O2 + ENO, and 95% O2 + NO treatment groups; mean + SD; n = 3/group. (B) Immunohistochemical localization of activated (nuclear localization signal) NF-κB in representative sections from each treatment group. AE = alveolar epithelium; BE = bronchiolar epithelium.Scale bar = 50 μm.
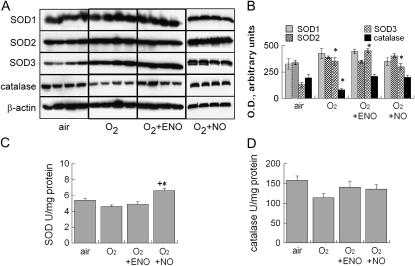
Lung Antioxidant Enzyme Expression
Whole-lung SOD activity was unaffected by hyperoxia or hyperoxia + 10 ppm ENO (Figure 6), but was modestly increased in NO-treated pups. Catalase activity was decreased in hyperoxia-exposed pups, but this was not statistically significant (p = 0.08). There was no significant effect of hyperoxia + ENO or NO on catalase activity. Whole-lung SOD1 and SOD2 abundance in immunoblots normalized to β-actin were unaffected by 95% O2 ± ENO or NO at 8 days. SOD3 was increased in all hyperoxia-exposed groups compared with air-exposed pups, but with no differences among hyperoxia-exposed treatment groups. Parallel to the effects on activity, catalase abundance was decreased in 95% O2–exposed pups, but not in 95% O2 + ENO– or NO-exposed pups compared with air-exposed pups.
Figure 6.
(A) Effect of air or 95% O2, or 95% O2 + 10 ppm inhaled ENO or NO, × 8 days on superoxide dismutase (SOD)-1, −2, and −3, catalase, and β-actin immunoblot expression in whole-lung homogenates, 20 μg/lane; n = 4/group. (B) Blots were quantified by image analysis, mean optical density ± SEM. (C) Effects on total lung SOD activity (*p < 0.05 vs. air control group; +p < 0.05 vs. ENO) and (D) catalase activity; n = 4/group; mean + SEM.
Lung Mechanics and Alveolar Development after Recovery in Air
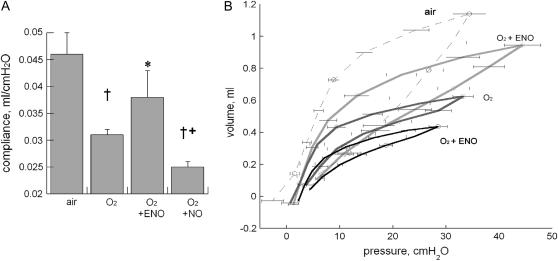
To determine longer-term effects of ENO and NO on hyperoxia-impaired postnatal lung function and development, pups were exposed to air, 95% O2, 95% O2 + 10 ppm ENO, or 95% O2 + 10 ppm NO for 8 days, followed by recovery in air for 6 days. Hyperoxia significantly decreased lung compliance measured at Day 14, and this was prevented in ENO-treated pups, but not in NO-treated pups, which showed worse compliance than O2-exposed control animals (Figure 7).
Figure 7.
Effect of air or 95% O2 ± 10 ppm ENO or NO for 8 days followed by recovery in air for 6 days on (A) static compliance (†p < 0.05 vs. air; *p < 0.05 vs. O2; +p < 0.05 vs. O2 + ENO and vs. O2) and (B) pressure–volume loops; n = 5 or 6/group; mean ± SEM.
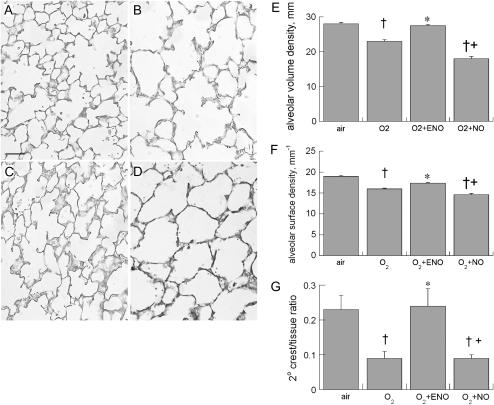
Effects on alveolar development were parallel to effects on static lung compliance. Using lung morphometry in random sections from each treatment group, we found parallel effects of hyperoxia, ENO, and NO treatment on alveolar surface density, alveolar volume density, and secondary alveolar septal crest density (elastin-stained) normalized to septal tissue. Hyperoxia exposure impaired alveolar volume density, alveolar surface density, and secondary septal crest densities, as expected. Treatment with 10 ppm ENO for 8 days significantly ameliorated these effects. NO treatment did not prevent hyperoxia-impaired alveolar development (Figure 8).
Figure 8.
Representative lung photomicrographs of the effects of (A) air, (B) 95% O2, (C) 95% O2 + ENO, or (D) 95% O2 + NO × 8 days followed by recovery for 6 days; scale bar = 50 μm. Effects of air, O2→air recovery, O2+ENO→air recovery, and O2+NO→air recovery on (E) alveolar volume density, (F) alveolar surface density, and (G) 2° septal crest:tissue ratios; n = 6 or 7 pups/group. †p < 0.05 versus air; *p < 0.05 versus O2; +p < 0.05 versus O2 + ENO.
DISCUSSION
Increasing evidence points to S-nitrosylation as a key pathway that mediates many of the physiologic responses to endogenous and exogenous NO (8). Our rationale for treatment with inhaled ENO was based on its superior ability to achieve in vitro S-nitrosylation of glutathione (12), abundant in alveolar lining fluid (22), compared with NO treatment. A trend toward higher concentrations of SNO were detected in BALF from ENO-treated pups in the small number of samples analyzed, consistent with earlier in vitro comparisons of ENO and NO gases (12). In preliminary studies, we attempted to measure SNO in lung homogenates, but were unable to consistently detect SNO groups, possibly due to rapid intracellular SNO group metabolism via transnitrosation. Alternatively, SNOs are avidly taken up by hemoglobin, and it may be that residual blood in the perfused lungs interfered with detection in lung homogenates (23), but this was not directly addressed.
We found that both inhaled ENO and NO partly prevented hyperoxia-induced leukocyte influx in BALF in newborn rats. In contrast, 10 ppm ENO treatment was superior to 10 ppm NO at preventing hyperoxia-induced accumulation of MPO. Because the lungs were lavaged and perfused, the tissue MPO should reflect both adherent intravascular as well as interstitial leukocytes. We did not perform detailed analysis of interstitial inflammation. ENO treatment prevented hyperoxia induction of CINC-1 mRNA, a neutrophil chemokine, and both ENO and NO partly prevented hyperoxia-induced CINC-1 protein in whole lung. CINC-1 immunostaining was more widespread, including alveolar septal tissue in NO-treated pups compared with ENO-treated pups. These differences in local chemokine accumulation may have contributed to the differing effects on leukocyte adhesion reflected in the MPO activities. Effects of inhaled ENO and NO on inflammation may differ because of disparate effects on adhesion molecules that govern leukocyte trafficking. NO has been shown to inhibit leukocyte adhesion (24) and viability (25). S-nitrosoglutathione inhibits monocyte adhesion (26), and S-nitrosoacetylpenicillamine, another NO donor, downregulates expression of CXCR2, a neutrophil chemokine receptor (27). No comparable studies have been done with ENO, as far as we know. Differences between ENO and NO effects may depend on the dosing strategies, which can be further defined in future studies.
We focused our assessments of inflammatory cytokines on CINC-1 because of the importance of the CINC-1/CXCR2 pathway to hyperoxia-impaired postnatal lung development (13, 21, 28). Although other neutrophil chemokines that we did not measure could have contributed to inflammation, our previous studies using this model system showed that treatment with neutralizing anti–CINC-1 (13), or with an antagonist to its receptor, CXCR2 (21), were sufficient to substantially protect against hyperoxia-impaired postnatal lung development.
Localized differences in cellular uptake of inhaled NO or ENO could also account for some of the observed differences in inflammation and local CINC-1 expression. Because the ease of formation of S-nitrosothiol is a key distinction between ENO and NO (12), differences in pulmonary S-nitrosothiol uptake from airway-formed SNOs could be important as an explanation for differing biological effects. The role of metabolism of airway-formed SNOs and its contribution to lung biology is incompletely understood, as are differences in diffusion from the airway to the interior of lung epithelial cells, and downstream metabolism and trafficking. Extracellularly formed S-nitrosoglutathione appears to require importation via amino acid transporters to achieve intracellular S-nitrosylation in some cell types (29). If true in lung epithelium, this may partly explain the more robust effect of ENO, as it forms S-nitrosothiol more avidly than NO.
We therefore hypothesized that ENO might be superior to NO in its ability to inactivate NF-κB, an important regulator of lung inflammation via induction of inflammatory cytokine transcription factors (30). SNO modifications inactivate I-κ kinase-kinase β in vitro, which derepresses NF-κB activation (31). S-nitrosylation inactivates the p50 subunit of NF-κB, preventing transcriptional activation (32). Preliminary studies show that ENO can block superoxide-induced NF-κB activation in vitro (15). To determine if hyperoxia induced NF-κB activation in newborn rat lung, or if inhaled ENO or NO blocked hyperoxia-induced pulmonary NF-κB activation in vivo, we measured NF-κB activation in nuclear protein extracts from whole lung in each treatment group at Day 8. We found that the overall magnitude of NF-κB activation in whole-lung nuclear extracts was small compared with the more robust stimulus of LPS injection in adult rat lung. We found that hyperoxia exposure increased activation in a subset of cells identified by immunohistochemistry—most prominently, the bronchiolar epithelium—which was prevented in ENO-treated pus, but not in NO-treated pups. In contrast with the LPS-treated adult rats, alveolar epithelial NF-κB activation was qualitatively decreased and heterogeneously expressed.
NF-κB abundance is reportedly induced by hyperoxia in neonatal rats, but activation and nuclear localization were not directly examined in the previous report (33). Hyperoxia-exposed transgenic newborn mice expressing NF-κB–driven luciferase demonstrate NF-κB activation, which persisted until 72 hours of hyperoxia exposure (later time points were not examined), but not in all cells (34), in agreement with our findings in newborn rats. NF-κB activation may protect some cells against oxidative stress–induced apoptosis (35), and may serve as an adaptive response under certain circumstances. Conducting similar studies using the NF-κB luciferase transgenic mouse could help to clarify timing and location of inhaled ENO or NO effects on pulmonary NF-κB activation.
Our main finding was that ENO significantly prevented hyperoxia-impaired lung compliance and alveolar development in animals recovered in air at Postnatal Day 14. This was not observed in NO-treated pups. The physiologic impact of this benefit is reflected by the superior protection against hyperoxia-impaired body growth in ENO- versus NO-treated pups at Days 8 and 14. The effects on static compliance could have been due, in part, to differences in inflammation, with most, but not all, parameters superior in ENO- versus NO-treated animals. Other possible factors include effects on surfactant that could have been a significant contributor to observed compliance differences. The effect of NO on the surfactant system is variable, with inhibition of surfactant function at high NO doses, (36) but apparent augmentation at low doses (1). Improvement or impairment of surfactant function in vitro does not necessarily correlate with in vivo effects on lung compliance (1). Interstitial edema could have contributed to the disparate effects on lung compliance, but this was not examined directly.
The beneficial effects of ENO on hyperoxia-impaired alveolar development were significant. Alveolar volume density, which estimates alveolar number, was significantly protected in ENO-treated pups, but not in NO-treated pups. ENO treatment also preserved alveolar surface area, estimated by surface density. Because interpretation of volume or surface density could have been confounded by differences in lung inflation, we measured secondary crest abundance normalized to septal tissue, which demonstrated even more striking differences between ENO and NO effects on alveolar development in hyperoxia-exposed rat pups. We did not observe obvious differences in elastin deposition in alveolar walls or in septal tips between the air-, O2-, O2 + ENO–, and O2 + NO–exposed animals at 14 days (data not shown). This contrasts with the finding of preserved elastin morphology in the NO-treated baboon model of BPD (37). This may be attributable to species differences, or the other important differences between the models, including the degree of immaturity, and the lack of mechanical ventilation as a contributor in our studies.
Because NO and ENO effects are likely to be mediated by a number of pathways, we measured effects of ENO and NO on antioxidants, as differences in ENO and NO effects on antioxidant defenses might have contributed to the disparity in effects on lung compliance and development. Low levels of NO induce catalase in vitro (38), and S-nitrosylation has been implicated as potentially regulating catalase expression (39). Sp1 transcription factor, which regulates extracellular SOD3 (40), appears to be affected by S-nitrosylation (41), although the specific thiol targets have not been identified. We found no large effects of either ENO or NO treatment on SOD or catalase activity. Total lung SOD activity was statistically greater in the NO-treated group, but the increment was modest. Increases in SOD activity unaccompanied by changes in catalase could potentially increase hydrogen peroxide accumulation, depending on the local conditions. Whole-lung expression of SOD1, −2, or −3, or catalase, were unaffected by either ENO or NO treatment in hyperoxia-exposed pups. Hyperoxia increased SOD3 expression, as expected (42). We cannot exclude disparities in local effects on antioxidant enzyme regulation, as these enzymes (43), particularly SOD3 (17, 42), are not expressed ubiquitously in the lung.
We examined the effects of NO and ENO inhalation on hyperoxia-induced 3-nitrotyrosine formation, as ENO should, theoretically, be less able to form peroxynitrite, the precursor of 3-nitrotyrosine, than is NO, assuming conditions of excess superoxide, as the reaction proceeds at essentially diffusion-limited rates (44). Peroxynitrite formation is associated with disrupted protein function and cellular metabolism (see Reference 45 for review). Both ENO- and NO-exposed animals had reduced 3-nitrotyrosine in whole lung compared with hyperoxia-exposed control animals. ENO and NO suppression of neutrophil accumulation and, therefore, neutrophil-generated superoxide may have been sufficient to prevent peroxynitrite formation and 3-nitrotyrosine accumulation.
There are a large number of pathways important to postnatal lung development that could be differentially affected by ENO and NO. Cui and colleagues demonstrated many cell-cycle, inflammation, and apoptosis-related genes that were induced in vitro by NO, independent of its effects on guanylyl cyclase, and, therefore, most likely affected via S-nitrosylation (9). We speculate that the improvements in lung development that we observed are partly attributable to preservation of lung cell proliferation and differentiation, which likely depend on a number of these S-nitrosylation–modified pathways. Disordered epithelial proliferation has been linked to the development of BPD in a number of animal models (46, 47).
The lack of beneficial effect of inhaled NO in our model system contrasts with beneficial effects in other systems designed to model BPD. This likely depends on a number of interacting factors, including, but not limited to, the overall burden of oxidative stress, the dose and timing of NO therapy, and the magnitude of endogenous NO production. For example, inhaled NO after hyperoxia exposure in newborn rats rescued impaired alveolar development (6), but treatment with an NO donor during hyperoxia exposure did not (48). In model systems with less oxidative stress (e.g., prematurely delivered lambs [49] and baboons [37]), inhaled NO, generally at doses lower than 10 ppm, improved lung development. It would be important to determine whether or not similar protective effects are conferred with less stringent oxidative stress (e.g., lower, more chronic oxidative stress [21], and in models that include mechanical ventilation, to more closely mimic clinical BPD).
In summary, we found that inhaled ENO and NO both inhibited hyperoxia-induced lung inflammation, ENO more so than NO, and prevented protein nitration in postnatal rats, but that ENO alone prevented hyperoxia-impaired weight gain, lung compliance, and alveolar development. The magnitude of differences in effects of ENO and NO on hyperoxia-induced inflammation can only partly account for the differences in lung development. Because the dosing regimens of ENO and NO were the same, we speculate that S-nitrosylation–dependent regulatory pathways account for the beneficial effects of ENO on lung development. Under conditions of oxidative stress, inhaled ENO may restore or augment airway lining fluid S-nitrosothiols that may be important to processes, yet undetermined, that govern postnatal alveolar development.
Supplementary Material
Acknowledgments
The authors are grateful for the careful reading of the manuscript by Drs. James A. Reynolds and Harvey Marshall, and for Dr. Marshall's help with the NF-κB assays, for the technical assistance of Erin N. Potts, and for the gift of nitric oxide gas by INO Therapeutics.
Supported by Nitrox, LLC, National Institutes of Health grants HL 067211 (R.L.A.) and ES 011961 (W.M.F.), and by Duke Translational Research Award (R.N.G. and J.S.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200605-662OC on May 3, 3007
Conflict of Interest Statement: R.L.A. received a grant of $121,580 in 2004/2005 from Nitrox, LLC. S.N.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.R.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.M.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.N.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S.S. received $10,000 in yearly consultancy fees from 2003 to 2006 and holds equity in Nitrox LLC. K.M.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ballard PL, Gonzales LW, Godinez RI, Godinez MH, Savani RC, McCurnin DC, Gibson LL, Yoder BA, Kerecman JD, Grubb PH, et al. Surfactant composition and function in a primate model of infant chronic lung disease: effects of inhaled nitric oxide. Pediatr Res 2006;59:157–162. [DOI] [PubMed] [Google Scholar]
- 2.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med 2006;355:354–364. [DOI] [PubMed] [Google Scholar]
- 3.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med 2005;353:13–22. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 2003;349:2099–2107. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JD Jr, Chiche JD, Weimann J, Steudel W, Zapol WM, Bloch KD. Nitric oxide inhalation decreases pulmonary artery remodeling in the injured lungs of rat pups. Circ Res 2000;87:140–145. [DOI] [PubMed] [Google Scholar]
- 6.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 2005;58:22–29. [DOI] [PubMed] [Google Scholar]
- 7.Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH. Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 1997;41:457–463. [DOI] [PubMed] [Google Scholar]
- 8.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med 2006;173:1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui X, Zhang J, Ma P, Myers DE, Goldberg IG, Sittler KJ, Barb JJ, Munson PJ, Cintron Adel P, McCoy JP, et al. cGMP-independent nitric oxide signaling and regulation of the cell cycle. BMC Genomics 2005;6:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon TJ, Ahearn GS, Moya MP, Gow AJ, Huang YC, Luchsinger BP, Nudelman R, Yan Y, Krichman AD, Bashore TM, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci USA 2005;102:14801–14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moya MP, Gow AJ, Califf RM, Goldberg RN, Stamler JS. Inhaled ethyl nitrite gas for persistent pulmonary hypertension of the newborn. Lancet 2002;360:141–143. [DOI] [PubMed] [Google Scholar]
- 12.Moya MP, Gow AJ, McMahon TJ, Toone EJ, Cheifetz IM, Goldberg RN, Stamler JS. S-nitrosothiol repletion by an inhaled gas regulates pulmonary function. Proc Natl Acad Sci USA 2001;98:5792–5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auten RL Jr, Mason SN, Tanaka DT, Welty-Wolf K, Whorton MH. Anti-neutrophil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. Am J Physiol Lung Cell Mol Physiol 2001;281:L336–L344. [DOI] [PubMed] [Google Scholar]
- 14.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 2006;355:343–353. [DOI] [PubMed] [Google Scholar]
- 15.Wratney AT, Whorton MH, Mason SN, Auten RL. Blockade of alveolar epithelial inflammatory cytokine transcription with nitrosoethanol [abstract]. Pediatr Res 2004;55:510A. [Google Scholar]
- 16.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA 1992;89:7674–7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auten RL, O'Reilly MA, Oury TD, Nozik-Grayck E, Whorton MH. Transgenic extracellular superoxide dismutase protects postnatal alveolar epithelial proliferation and development during hyperoxia. Am J Physiol Lung Cell Mol Physiol 2006;290:L32–L40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwell TS, Blackwell TR, Holden EP, Christman BW, Christman JW. In vivo antioxidant treatment suppresses nuclear factor-κ B activation and neutrophilic lung inflammation. J Immunol 1996;157:1630–1637. [PubMed] [Google Scholar]
- 19.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFκB. Nucleic Acids Res 2001;29:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS. Protection from experimental asthma by an endogenous bronchodilator. Science 2005;308:1618–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, et al. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med 2004;170:1188–1196. [DOI] [PubMed] [Google Scholar]
- 22.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol 1987;63:152–157. [DOI] [PubMed] [Google Scholar]
- 23.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc 2006;3:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su WY, Day BJ, Kang BH, Crapo JD, Huang YC, Chang LY. Lung epithelial cell–released nitric oxide protects against PMN-mediated cell injury. Am J Physiol 1996;271:L581–L586. [DOI] [PubMed] [Google Scholar]
- 25.Fortenberry JD, Owens ML, Brown MR, Atkinson D, Brown LA. Exogenous nitric oxide enhances neutrophil cell death and DNA fragmentation. Am J Respir Cell Mol Biol 1998;18:421–428. [DOI] [PubMed] [Google Scholar]
- 26.Prasad R, Giri S, Nath N, Singh I, Singh AK. GSNO attenuates EAE disease by S-nitrosylation–mediated modulation of endothelial-monocyte interactions. Glia 2007;55:65–77. [DOI] [PubMed] [Google Scholar]
- 27.Rios-Santos F, Alves-Filho JC, Souto FO, Spiller F, Freitas A, Lotufo CM, Soares MB, Dos Santos RR, Teixeira MM, de Queiroz Cunha F. Down-regulation of CXCR2 on neutrophils in severe sepsis is mediated by inducible nitric oxide synthase–derived nitric oxide. Am J Respir Crit Care Med 2007;175:490–497. [DOI] [PubMed] [Google Scholar]
- 28.Auten RL, Richardson RM, White JR, Mason SN, Vozzelli MA, Whorton MH. Nonpeptide CXCR2 antagonist prevents neutrophil accumulation in hyperoxia-exposed newborn rats. J Pharmacol Exp Ther 2001;299:90–95. [PubMed] [Google Scholar]
- 29.Li S, Whorton AR. Identification of stereoselective transporters for S-nitroso-L-cysteine: role of LAT1 and LAT2 in biological activity of S-nitrosothiols. J Biol Chem 2005;280:20102–20110. [DOI] [PubMed] [Google Scholar]
- 30.Blackwell TS, Holden EP, Blackwell TR, DeLarco JE, Christman JW. Cytokine-induced neutrophil chemoattractant mediates neutrophilic alveolitis in rats: association with nuclear factor κ B activation. Am J Respir Cell Mol Biol 1994;11:464–472. [DOI] [PubMed] [Google Scholar]
- 31.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory κB kinase through S-nitrosylation. Proc Natl Acad Sci USA 2004;101:8945–8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall HE, Stamler JS. Inhibition of NF-κ B by S-nitrosylation. Biochemistry 2001;40:1688–1693. [DOI] [PubMed] [Google Scholar]
- 33.Tang F, Yue S, Luo Z, Feng D, Wang M, Qian C, Zhen X, Duan Y. Role of N-methyl-D-aspartate receptor in hyperoxia-induced lung injury. Pediatr Pulmonol 2005;40:437–444. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-κB activation and their role in tolerance to hyperoxia. J Clin Invest 2004;114:669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Franek WR, Horowitz S, Stansberry L, Kazzaz JA, Koo HC, Li Y, Arita Y, Davis JM, Mantell AS, Scott W, et al. Hyperoxia inhibits oxidant-induced apoptosis in lung epithelial cells. J Biol Chem 2001;276:569–575. [DOI] [PubMed] [Google Scholar]
- 36.Robbins CG, Davis JM, Merritt TA, Amirkhanian JD, Sahgal N, Morin FC III, Horowitz S. Combined effects of nitric oxide and hyperoxia on surfactant function and pulmonary inflammation. Am J Physiol 1995;269:L545–L550. [DOI] [PubMed] [Google Scholar]
- 37.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2005;288:L450–L459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshioka Y, Kitao T, Kishino T, Yamamuro A, Maeda S. Nitric oxide protects macrophages from hydrogen peroxide–induced apoptosis by inducing the formation of catalase. J Immunol 2006;176:4675–4681. [DOI] [PubMed] [Google Scholar]
- 39.Foster MW, Stamler JS. New insights into protein S-nitrosylation: mitochondria as a model system. J Biol Chem 2004;279:25891–25897. [DOI] [PubMed] [Google Scholar]
- 40.Zelko IN, Folz RJ. SP1 and SP3 transcription factors mediate trichostatin A–induced and basal expression of extracellular superoxide dismutase. Free Radic Biol Med 2004;37:1256–1271. [DOI] [PubMed] [Google Scholar]
- 41.Zaman K, Vaughan J, Hunt JF, Palmer L, Gaston B. Transcription factor SP1 is involved in the regulation of cystic fibrosis transmembrane conductance regulator by endogenous S-nitrosoglutathione. Am J Respir Crit Care Med 2002;165:A279. [Google Scholar]
- 42.Mamo LB, Suliman HB, Giles BL, Auten RL, Piantadosi CA, Nozik-Grayck E. Discordant extracellular superoxide dismutase expression and activity in neonatal hyperoxic lung. Am J Respir Crit Care Med 2004;170:313–318. [DOI] [PubMed] [Google Scholar]
- 43.Kaarteenaho-Wiik R, Kinnula VL. Distribution of antioxidant enzymes in developing human lung, respiratory distress syndrome, and bronchopulmonary dysplasia. J Histochem Cytochem 2004;52:1231–1240. [DOI] [PubMed] [Google Scholar]
- 44.Ho YS. Transgenic and knockout models for studying the role of lung antioxidant enzymes in defense against hyperoxia. Am J Respir Crit Care Med 2002;166:S51–S56. [DOI] [PubMed] [Google Scholar]
- 45.van der Vliet A, Eiserich JP, Cross CE. Nitric oxide: a pro-inflammatory mediator in lung disease? Respir Res 2000;1:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 2006;291:L1101–L1111. [DOI] [PubMed] [Google Scholar]
- 47.O'Reilly MA, Watkins RH, Staversky RJ, Maniscalco WM. Induced p21Cip1 in premature baboons with CLD: implications for alveolar hypoplasia. Am J Physiol Lung Cell Mol Physiol 2003;285:L964–L971. [DOI] [PubMed] [Google Scholar]
- 48.Lopez E, Boucherat O, Franco-Montoya ML, Bourbon JR, Delacourt C, Jarreau PH. Nitric oxide donor restores lung growth factor and receptor expression in hyperoxia-exposed rat pups. Am J Respir Cell Mol Biol 2006;34:738–745. [DOI] [PubMed] [Google Scholar]
- 49.Kinsella JP, Parker TA, Galan H, Sheridan BC, Abman SH. Independent and combined effects of inhaled nitric oxide, liquid perfluorochemical, and high-frequency oscillatory ventilation in premature lambs with respiratory distress syndrome. Chest 1999;116:15S–16S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.