Abstract
Rationale: Acute lung injury and acute respiratory distress syndrome are common clinical syndromes resulting largely from the accumulation of and inability to clear pulmonary edema, due to injury to the alveolar epithelium. Gene therapy may represent an important alternative for the treatment and prevention of these diseases by restoring alveolar epithelial function. We have recently developed an electroporation strategy to transfer genes to the lungs of mice, with high efficiency and low inflammation.
Objectives: We asked whether electroporation-mediated transfer of genes encoding subunits of the Na+,K+-ATPase could protect from LPS-induced lung injury or be used to treat already injured lungs by up-regulating mechanisms of pulmonary edema clearance.
Methods: Plasmids were delivered to the lungs of mice using transthoracic electroporation. Lung injury was induced by intratracheal administration of LPS (4 mg/kg body weight). Biochemical, cellular, and physiologic measurements were taken to assess gene transfer and lung injury.
Measurements and Main Results: Improvements in wet-to-dry ratios, pulmonary effusions, bronchoalveolar lavage protein levels and cellularity, alveolar fluid clearance, and respiratory mechanics were seen after delivery of plasmids expressing Na+,K+-ATPase subunits, but not control plasmids, in LPS-injured lungs. Delivery of plasmids expressing Na+,K+-ATPase subunits both protected from subsequent lung injury and partially reversed existing lung injury by these measures.
Conclusions: These results demonstrate that electroporation can be used effectively in healthy and injured lungs to facilitate gene delivery and expression. To our knowledge, this is the first successful use of gene delivery to treat existing lung injury, and may have future clinical potential.
Keywords: acute lung injury, alveolar fluid clearance, electroporation, gene therapy, pulmonary edema
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Gene transfer of the Na+,K+-ATPase has been shown to increase alveolar fluid clearance and protect from lung injury in animals, but not to reverse established injury. We have shown previously that electric fields can deliver genes to rodent lungs.
What This Study Adds to the Field
Gene delivery of plasmids expressing Na+,K+-ATPase subunits by electroporation to LPS-injured lungs both protected from subsequent injury and partially reversed existing lung injury.
Acute lung injury (ALI) and acute respiratory distress syndrome are common, devastating clinical syndromes that affect large numbers of patients and have up to 40% mortality with the current standard of care (1). It is estimated that there are almost 200,000 cases of ALI in the United States every year, leading to 74,500 deaths and 3.6 million hospital days (2). Despite 30 years of research, only moderate advances have been made in the treatment, and the mortality remains unacceptably high.
Gene therapy is a potentially powerful approach to treating a variety of disease states, including ALI. Unfortunately, to date, only limited successes have been made in clinical trials. Perhaps the major reason for this is that the majority of vectors, either viral or nonviral, have serious drawbacks that limit efficacy, including inefficiency of gene transfer and immunologic and inflammatory responses. Although viral vectors typically elicit greater inflammatory responses, advances in viral vector development have lessened these effects. Furthermore, it has become clear that certain nonviral lipid or polymer formulations can also induce these responses, albeit at lower levels. However, although nonviral vectors may be safer than their viral counterparts, their efficiency of gene transfer remains low.
Recent research from our laboratory and others has demonstrated that electroporation can be used to efficiently deliver genes to various tissues, including the lung, in vivo with no apparent damage and yields high-level gene expression (3, 4). The method is simple, fast, and safe: purified plasmid DNA is administered to the lungs of anesthetized animals via an endotracheal tube and a series of eight consecutive, square-wave electric pulses is applied to the lungs using electrodes placed on either side of the chest over a 10-second period. In recent studies in mice and rats, we have demonstrated that levels of transfer and expression of reporter and therapeutic genes approaching that obtained with recombinant adenoviral vectors can be achieved using this approach (3, 4). Indeed, when subunits for the Na+,K+-ATPase were transferred to the lungs of healthy rats using transthoracic electroporation, rates of alveolar fluid clearance (AFC) increased almost twofold, indistinguishable from those seen after gene transfer using a recombinant adenovirus expressing the same gene (4). Although these results are encouraging, they do not address the application of this technique to injured lungs.
In the current study, we have asked whether electroporation-mediated transfer of genes encoding subunits of the Na+,K+-ATPase could protect from lung injury or be used to treat already injured lungs by up-regulating mechanisms of pulmonary edema clearance. We have employed a well established model of lung injury using LPS, and transfer the genes either before or after injury to determine the efficacy of the treatment (5). We found that, even in the injured lung with increased pulmonary edema and increased numbers of inflammatory cells, electroporation can be used effectively to transfer genes to alveolar epithelial cells. Moreover, transfer of the α or β subunits of the Na+,K+-ATPase, or a combination of the two can both protect from LPS-induced lung injury and reduce injury in an already injured lung. To our knowledge, this is the first time that gene transfer has been used as a therapy for already established lung injury as opposed to being used as a preventive measure that can protect from future injury, suggesting that this approach may have clinical therapeutic potential. Some of the results of these studies have been previously reported in the form of an abstract (6).
METHODS
Plasmids
pCMV-lux-DTS and pCMV-lacZ-DTS contain an SV40 DNA nuclear targeting sequence (DTS) and express firefly luciferase and β-galactosidase, respectively, from the cytomegalovirus (CMV) immediate early promoter/enhancer (3, 7). pCMV-α1 and pCMV-β1 express the α1 and β1 subunits of Na+,K+-ATPase, respectively, from the CMV promoter. Plasmids were purified using Qiagen Giga-prep kits (Qiagen, Chatsworth, CA) and suspended in 10 mM Tris, pH 8.0, 1 mM ethylenediaminetetraacetic acid, and 140 mM NaCl.
In Vivo Gene Transfer to the Lung
Female Balb/c mice (15–18 g) were anesthetized with sodium pentobarbital (50 mg/kg body weight) and pediatric cutaneous pacemaker electrodes (Quik-Combo RTS; Medtronic Physio-Control Corp., Redmond, WA) were cut to 1 × 2 cm and placed on either side of the chest under the forelimbs using a small amount of KY Jelly (Johnson & Johnson, New Brunswick, NJ) to aid conductance. A 20-gauge Angiocath catheter used as an endotracheal tube was inserted into the airway, and 50 μl of plasmid was administered between breaths over a 2-second period. The animal was allowed to recover breathing for 20 seconds, and a series of 8 consecutive square-wave electric pulses (200 V/cm for 10 ms each) were administered using an ECM830 electroporator (Gentronics, San Diego, CA). Animals were allowed to recover from anesthesia and were returned to the vivarium. At later times, the lungs were removed and analyzed. All experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the Guide for Care and Use of Laboratory Animals.
Induction of Lung Injury
Female Balb/c mice (15–18 g) were anesthetized with sodium pentobarbital (50 mg/kg body weight), and 4 mg/kg of LPS (Escherichia coli O55:B5, 15,000,000 endotoxin units/mg protein), unless otherwise indicated, was delivered in 50 μl of phosphate-buffered saline (PBS) to the lungs via a 20-gauge angiocath catheter used as an endotracheal tube. The solution was delivered in two doses of 25 μl each separated by 30 seconds, and the animals were rolled from side to side after each administration to ensure even distribution.
Histologic Analysis
Paraffin-embedded thin sections were cut from lungs inflated to total lung capacity with 3% paraformaldehyde immediately after mice were killed. Lungs were X-gal stained as previously described (3). Immunohistochemistry was performed using the Vectastain ABC-AP system (Vector Laboratories, Foster City, CA). Hematoxylin and eosin–stained sections (three sections each from three animals per condition) were blinded and reviewed by a pathologist for lung injury. Lung injury was scored using five criteria (perivascular and peribronchial inflammation, hyaline membranes, alveolar infiltrates, interstitial infiltrates, and alveolar hemorrhage) on a 5-point scale, as previously described (4).
Bronchoalveolar Lavage Analysis
Bronchoalveolar lavage (BAL) was performed using a 20-gauge angiocath, as described previously here. Briefly, 1 ml of sterile saline at 4°C was instilled into mouse lungs and lavaged three times before it was aspirated for analysis.
Measurement of Luciferase Expression
Lungs were frozen in liquid nitrogen immediately after removal and extracts were prepared as previously described (3). Luciferase activity was measured in duplicate using the Luciferase Assay System (Promega, Madison, WI) in a Turner luminometer (Turner Biosystems, Sunnyvale, CA). Purified recombinant luciferase (Promega) was used to produce a standard curve for each experiment. The level of sensitivity of this assay is between 0.01 and 0.05 pg/lung for animals receiving no plasmid (i.e., “background”).
Measurement of Lung Mechanics
Mice were sedated with pentobarbital, tracheostomized with an 18-guage, 1-inch-long cannula, and paralyzed with pancuronium. Mice were connected to the Flexivent ventilator (Scireq, Montreal, PQ, Canada), and ventilated for 10 minutes. After this initial ventilation, the input impedance of the lung was measured between 0.25 and 19.625 Hz by applying a composite signal containing 19 mutually prime sinusoidal waves. The model used is described by the equation
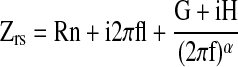 |
where i is a positive square root of −1, α = 2/π arctan H/G, Rn is a Newtonian, frequency-independent component of respiratory resistance and reflects airway resistance, I is inertance due to the gas in central airways, G is a parameter reflecting viscous energy dissipation in the tissue (cm H2O · s/ml, related to tissue resistance), and H is a parameter reflecting energy storage in the tissues (related to tissue elastance) (8, 9). Tissue hysteresivity (η) was calculated as the ratio of G to H. Data are presented as means and SEM. Groups were compared using a Student's t test.
Measurement of Lung Water Content—Wet-to-Dry Ratio
The effect of LPS on total lung water content was assessed at 24 and 72 hours after intratracheal administration of LPS. Mice were exsanguinated via laceration of left renal artery and vein. Lungs were then excised, surface liquid was blotted away, and wet lung weight determined. Lungs were placed in a heated vacuum centrifuge and weighed every 24 hours until a stable dry weight was obtained (usually at 72 h).
Pleural Effusion Measurements
After thoracotomy and before lungs and heart were removed en bloc, chest cavity was inspected for the presence of pleural changes and effusion. When pleural effusion was observed, volume of effusion was measured after it was carefully aspirated out of the chest cavity.
Alveolar Fluid Clearance Measurements in Live Mice
Alveolar fluid clearance was measured in living mice as previously described (10). Briefly, mice maintained at a body temperature of 37°C were anesthetized, the trachea was cannulated with a 5-mm, 20-gauge angiocath, and the catheter was connected to a small animal ventilator (Harvard Apparatus, Holliston, MA) before paralysis with pancuronium bromide (2.0 mg/kg, intraperitoneally). The animals were ventilated with a tidal volume of 10 ml/kg at a frequency of 160 breaths per minute and 100% oxygen. A total of 300 μl of an iso-osmolar NaCl solution containing 5% acid-free Evans Blue–labeled (0.15 mg/ml Evans Blue) bovine serum albumin was instilled into the endotracheal catheter over 10 seconds followed by 200 μl of air. The animals were kept supine, inclined to 30°, and ventilated for 30 minutes, after which the chest was opened to allow aspiration of fluid from the tracheal catheter. Protein concentration in the aspirate was measured and AFC was calculated using the following equation: AFC = (1 − C0/C30), where C0, is the protein concentration of the instillate before instillation, and C30 is the protein concentration of the sample obtained at the end of 30 minutes of mechanical ventilation. Clearance is expressed as a percentage of total instilled volume cleared/30 minutes.
Statistical Analysis
Nonparametric Mann-Whitney U tests were performed to determine statistical significance between individual samples, and one-way analysis of variance tests were performed for analysis of multiple samples followed post hoc by Tukey-Kramer multiple comparisons tests when differences were noted using Prism 4.0 software (GraphPad Software, San Diego, CA).
RESULTS
Establishment of LPS-induced Lung Injury Model
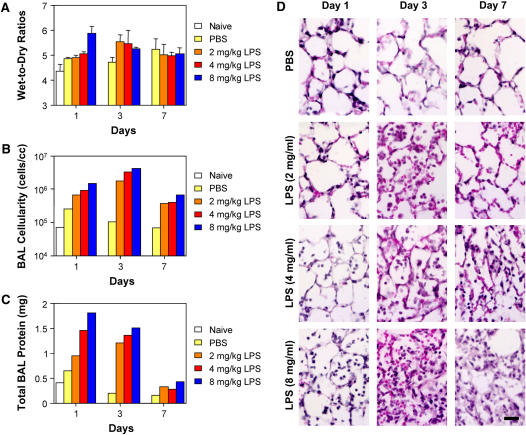
To determine whether electroporation could be used as a suitable method for gene delivery to the injured lung, we first established the lung injury model. Increasing doses of LPS were delivered to mice by intratracheal instillation and the degree of lung injury was analyzed 1, 3, and 7 days later (Figure 1). As measured by wet-to-dry ratios of the lungs (n = 4; Figure 1A), cellularity (n = 1; Figure 1B) and protein content (n = 1; Figure 1C) of BAL fluid, and histology (n = 3; Figure 1D and Table 1), all doses of LPS induced lung injury by 1 day after delivery, showed maximal injury at 3 days, and resolved by 7 days after delivery. Although delivery of saline alone caused a minor increase in wet-to-dry ratios and BAL cellularity and protein content compared to naive animals, significant differences could be easily detected between all doses of LPS and either saline-treated or naive animals. As relatively high numbers of red blood cells were seen in the lungs of mice that received 8 mg/kg of LPS, suggesting alveolar hemorrhage and severe disruption of alveolocapillary permeability, we chose for our studies a dose of 4 mg/kg LPS to induce lung injury. At this dose, even at Day 1, significant numbers of inflammatory cells (primarily neutrophils) could be seen in the lungs, and by Day 3 the infiltrates (neutrophils, lymphocytes, and plasma cells) were pronounced, as was the change in wet-to-dry ratio (increased by 26% vs. naive and 17% vs. saline).
Figure 1.
LPS-induced lung injury. LPS (0, 2, 4, and 8 mg/kg body weight in phosphate-buffered saline [PBS]) was administered intratracheally to Balb/c mice. At 1, 3, and 7 days after administration, lung injury was assessed (A) gravimetrically, (B) by cellularity of bronchoalveolar lavage (BAL) fluid, (C) by total BAL protein content, and (D) by histology in hematoxylin and eosin–stained sections. Bar = 20 μm.
TABLE 1.
LUNG INJURY IN LPS-TREATED ANIMALS
| LPS Dose (mg/kg) | Day 1 | Day 3 | Day 7 |
|---|---|---|---|
| 0 | 0.38 ± 0.25 | 0.38 ± 0.25 | 0.38 ± 0.48 |
| 2 | 0.50 ± 0.58 | 2.00 ± 0.82 | 1.13 ± 0.25 |
| 4 | 0.75 ± 0.50 | 2.25 ± 0.87 | 1.25 ± 0.50 |
| 8 | 1.25 ± 0.50 | 2.50 ± 0.41 | 1.50 ± 0.41 |
LPS (0, 2, 4, and 8 mg/kg body weight in phosphate-buffered saline was administered intratracheally to Balb/c mice and, at 1, 3, and 7 days after administration, lung injury was scored using five criteria (perivascular and peribronchial inflammation, hyaline membranes, alveolar infiltrates, interstitial infiltrates, and alveolar hemorrhage) on a 5-point scale. Values shown are mean ± SEM.
Effects of Lung Injury on Electroporation-mediated Gene Delivery and Expression
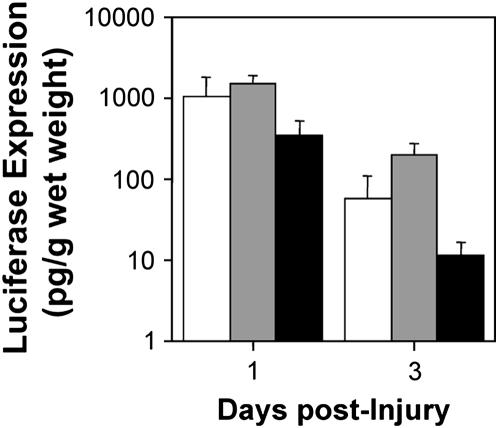
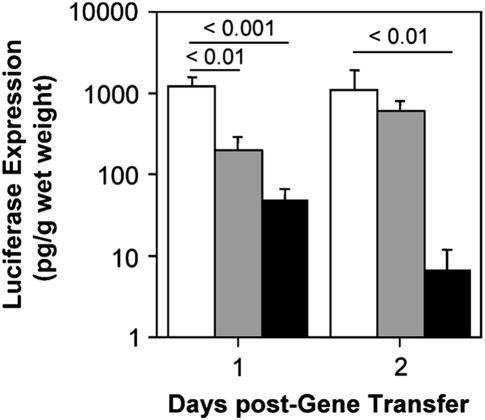
Although electroporation efficiently delivers genes to the lungs of healthy mice and rats, it remained to be seen whether it could also be used in the injured lung. Two different approaches were taken. First, we tested whether administration of LPS to mice would decrease gene expression from previously delivered reporter genes. Plasmids expressing the luciferase gene from a CMV promoter were transferred to the lungs of mice by electroporation and, 1 day later, LPS (4 mg) was administered to induce lung injury. Under this protocol, we observed less than a 10% mortality rate after DNA delivery and electroporation, as previously reported in mice and rats (3, 4). In noninjured lungs, maximal gene expression using this promoter occurs at 1 day after electroporation, plateaus for 4 days, and, by 7 days, expression ceases (3, 4). Thus, by assaying gene expression 1 and 3 days after injury (2 and 4 d after electroporation), maximal gene expression should be detectable. Although administration of saline slightly increased the levels of reporter gene expression, LPS decreased gene expression by 67–80%, but did not abolish it (Figure 2). Indeed, levels of gene expression at 3 days after LPS delivery were still 100- to 2,000-fold over background. Because all gene transfer to cells is accomplished within the first minute of electroporation, this decrease in reporter gene expression by LPS is indeed at the transcriptional and post-transcriptional level. To determine what effect lung injury has on gene delivery, as opposed to gene expression, lungs of mice were injured with LPS and, 1 day later, luciferase-expressing plasmids were electroporated into the lungs and assayed for gene delivery and expression 1 and 2 days after electroporation (Figure 3). Mortality in these experiments after DNA delivery and electroporation 1 day after treatment with PBS or LPS was higher than in untreated animals (15 and 22%, respectively), presumably due to the physiological state of the lungs after induction of injury. Electroporation of mice 1 day after treatment with PBS or LPS resulted in 9.7- and 24-fold-lower levels of gene expression at Day 1 after electroporation and 2.2- and 97-fold-lower levels at Day 2 after electroporation, respectively. Taken together, these results suggest that LPS has effects both on gene expression (i.e., transcription and translation) and gene delivery.
Figure 2.
LPS-induced lung injury modulates transgene expression. A total of 100 μg of pCMV-Lux-DTS in 50 μl were administered intratracheally to mice by electroportation (8 pulses of 10-ms duration and a field strength of 200 V/cm). After 24 hours, phosphate-buffered saline (gray bars) or LPS (black bars; 4 mg/kg) was administered to the lungs, or the animals were not treated with anything (naive, white bars). At 1 or 3 days later, lungs were removed and luciferase activity was measured in tissue extracts. Mean luciferase activities (±SEM; n = 5) are shown.
Figure 3.
Electroporation can be used successfully for gene transfer in LPS-injured lungs. Phosphate-buffered saline (gray bars) or LPS (black bars; 4 mg/kg) was administered intratracheally to mice and, 1 day later, 100 μg of pCMV-Lux-DTS in 50 μl were administered by electroportation. The lungs of untreated mice (naive, white bars) were also electroporated with plasmid. At 1 or 2 days after electroporation, luciferase activity was measured in lung extracts (mean ± SEM; n = 5).
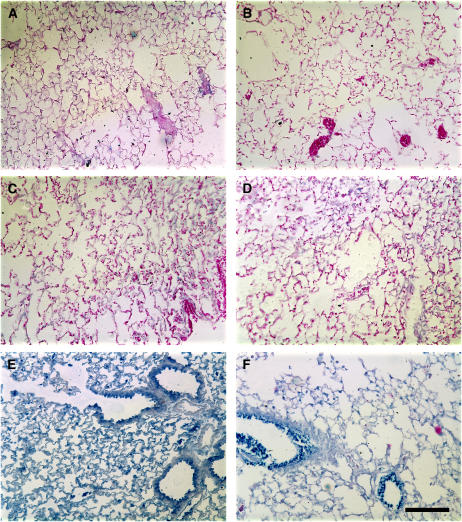
To determine whether the reduced gene expression seen in LPS-injured lungs was the result of reduced or altered distribution of delivered genes, a β-galactosidase–expressing plasmid was electroporated into the lungs of mice 1 day after administration of LPS, and the distribution and extent of transgene expression was measured by immunohistochemistry 1 day later (Figure 4). When plasmids were delivered to the lungs and electroporated, high levels of expression in multiple cell types, including airway and alveolar epithelial cells, were seen in saline-treated animals (Figure 4E), as previously reported (3,4). Although the levels of β-galactosidase staining were decreased in LPS-treated animals (Figure 4F), a similar distribution pattern of expression was seen. By contrast, neither mice receiving saline (Figure 4A) nor LPS (Figure 4B) without plasmid or electroporation showed any expression of β-galactosidase, nor did saline-treated (Figure 4C) or LPS-treated (Figure 4D) mice electroporated without addition of plasmid, as expected. Collectively, these results suggest that, although gene transfer and expression is reduced in LPS-injured lungs, electroporation can be used to deliver genes effectively to these lungs.
Figure 4.
Distribution of gene transfer and expression in LPS-injured lungs. Saline (A, C, and E) or LPS (4 mg; B, D, and F) was administered intratracheally to mice. Twenty-four hours later, mice were electroporated in the absence of plasmid (C and D) or after intratracheal delivery of 50 μg pCMV-LacZ-DTS (E and F) and their lungs were removed and processed 24 hours later. The lungs shown in (A) and (B) received neither plasmid or electroporation. Immunohistochemistry for LacZ (blue) was performed on all sections and samples were counterstained with eosin. Bar = 100 μm.
Transfer of Na+,K+-ATPase Subunit Genes Can Protect from LPS-induced Lung Injury
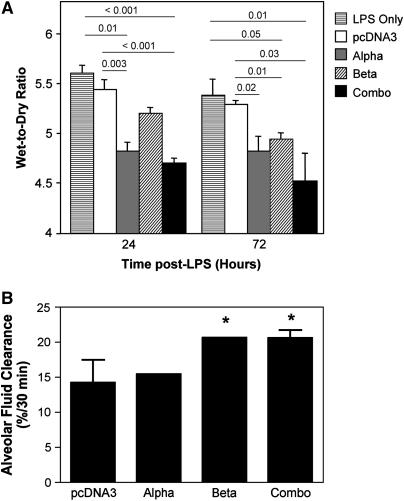
We have previously demonstrated that gene transfer of the β1 subunit of the Na+,K+-ATPase using electroporation can increase AFC in healthy rats (4). To determine whether electroporation-mediated gene transfer of subunits of the Na+,K+-ATPase can protect from subsequent LPS-induced lung injury, individual α1 or β1 subunits, or a combination of the two, were transferred to the lungs of mice. After 24 hours, at the height of transgene expression (4), LPS (4 mg/kg) was delivered intratracheally to induce lung injury, and the extent of lung injury, or protection thereof, was measured by wet-to-dry ratio 1 or 3 days later (Figure 5A). The wet-to-dry ratio of naive lungs was 4.28 ± 0.06 (±SEM; n = 6), whereas administration of LPS caused this to increase to 5.44 ± 0.1 (n = 6) at 24 hours, 5.29 ± 0.13 (n = 5), and 5.27 ± 0.06 (n = 8), at 24, 48, and 72 hours, respectively. Compared with lungs that received a plasmid expressing no transgene, those electroporated with plasmids expressing either the α subunit or the combination of subunits showed significantly less pulmonary edema at both 24 and 48 hours after injury. Indeed, the protection provided by coadministration of the α1 and β1 subunits decreased the difference in wet-to-dry ratios between naive and LPS-injured lungs by 64 and 79% at 24 and 48 hours, respectively. Although administration of the β1 subunit provided statistically significant protection over control treatment at 48 hours (5.01 ± 0.06 vs. 5.29 ± 0.05; P < 0.001), it only trended toward significance at 24 hours (5.20 ± 0.06 vs. 5.44 ± 0.10; P ≤ 0.07). To determine whether the protection seen after transfer of genes for subunits of the Na+,K+-ATPase was due to increased pump activity, AFC was measured in a group of DNA-electroporated, LPS-exposed mice at 48 hours after LPS administration (Figure 5B). As can be seen, transfer of the control pcDNA3 plasmid before LPS administration resulted in no change in AFC compared to LPS-treated animals. By contrast, transfer of the β1 subunit alone, or both α1 and β1 plasmids together, resulted in significantly increased fluid clearance in these animals. Taken together, these results demonstrate that electroporation-mediated gene transfer of individual or combinations of Na+,K+-ATPase subunits can protect from LPS-induced lung injury.
Figure 5.
Electroporation-mediated gene transfer of subunits of the Na+,K+-ATPase increases alveolar fluid clearance (AFC) and can protect from subsequent LPS-induced lung injury. (A) A total of 100 μg of plasmid in 50 μl were administered intratracheally to mice by electroportation. After 1 day, LPS (4 mg/kg) was administered to the lungs and 24 or 72 hours after this, lungs were removed for gravimetric analysis. No DNA (i.e., LPS-treated only; horizontal lined bars), or plasmids expressing no transgene (pcDNA3, open bars), the α subunit (gray bars), the β subunit (hatched bars), or a mixture of α and β (50 μg of each; “combo,” closed bars) were used. Wet-to-dry ratios are shown as mean ± SEM (n = 5). (B) Mice were electroporated and treated with LPS as in (A), and AFC was measured in living animals 72 hours after LPS administration. AFC is shown as percentage of total instilled volume cleared in 30 minutes (mean ± SEM; n = 4). Statistical analysis was by one-way analysis of variance followed by Tukey-Kramer post hoc testing. *P ⩽ 0.05 compared with pcDNA3.
Transfer of Na+,K+-ATPase Subunit Genes Can Reverse LPS-induced Lung Injury
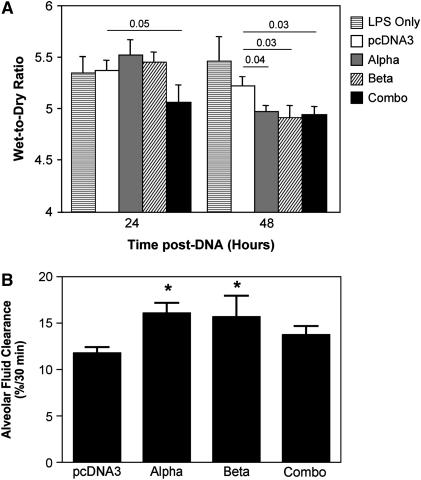
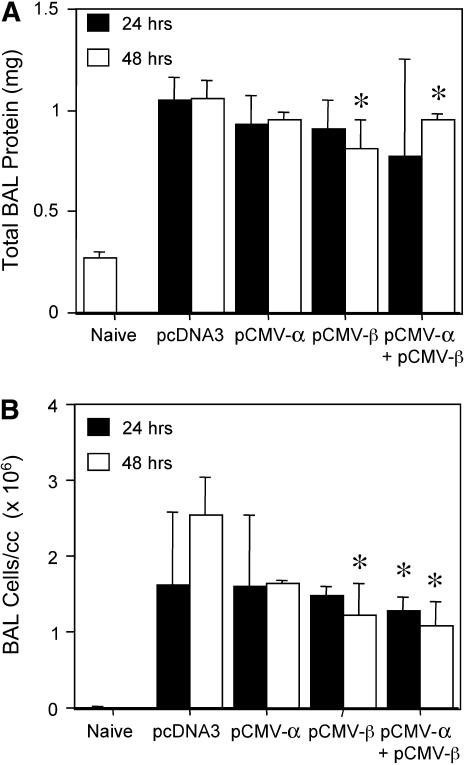
We next determined whether the same approach could be used to reverse previously induced lung injury by electroporating the Na+,K+-ATPase subunit genes into LPS-injured lungs. Lungs were injured by administration of LPS (4 mg/kg) and, 24 hours later, plasmids expressing the α1 or β1 subunit of the Na+,K+-ATPase were electroporated into the lungs either individually or in combination. At 24 or 48 hours after gene delivery (corresponding to 2 and 3 days after LPS injury, respectively), lungs were removed and injury was assessed by measurement of wet-to-dry ratios. Although little difference in the degree of lung injury was seen 1 day after administration of either the α1 or β1 subunit plasmid alone, coadministration of both plasmids yielded a statistically significant decrease in the wet-to-dry ratios compared with mice receiving the empty vector pcDNA3 (5.37 ± 0.1 vs. 5.06 ± 0.17; Figure 6A). Coadministration decreased the difference in wet-to-dry ratios between LPS-injured lungs and uninjured lungs by 30%. By contrast, transfer of either plasmid individually or coadministration of both plasmids reduced lung injury at 48 hours after electroporation. At this time point, transfer of α1 and/or β1 subunits resulted in significant reductions in lung injury, as measured by wet-to-dry ratio. Transfer of the α1, β1, or the combination reduced the ratios from 5.22 ± 0.09 for pcDNA3 alone to 4.97 ± 0.06, 4.91 ± 0.12, and 4.94 ± 0.08, respectively. When AFC was measured directly in LPS-injured mice that subsequently received α1 and/or β1 subunit plasmids by electroporation, transfer of either α1 or β1 subunit genes alone resulted in increased AFC compared with LPS-treated animals alone or LPS-treated animals that received an empty plasmid (Figure 6B). Transfer of α1 and/or β1 subunits to similarly injured mice also reduced total protein levels in BAL fluids by between 11 and 27% at 1 and 2 days after electroporation compared with LPS-injured mice receiving empty plasmid (Figure 7). Similarly, total cells in BAL fluids from these mice were reduced after electroporation of α1 and/or β1 subunits (Figure 7). Although these reductions were not large, they were statistically significant. Finally, cotransfer of both the α1 and β1 subunits, which gave the highest degree of reversal of injury as measured above, also significantly improved lung mechanics in LPS-injured mice at 2 days after transfer. Tissue resistance of the peripheral lung was measured as G, a parameter reflecting viscous energy dissipation in the tissue, using the Flexivent system (8, 9). LPS-injured animals that received pcDNA3 had a mean resistance of 9.31 ± 0.49 cm H2O · s/ml, whereas transfer of both subunits significantly reduced peripheral resistance to 6.02 ± 0.18 cm H2O · s/ml (P ⩽ 0.03). Taken together, these results demonstrate that electroporation-mediated transfer of Na+,K+-ATPase subunit genes can reduce previously established lung injury.
Figure 6.
Electroporation-mediated gene transfer of the Na+,K+-ATPase can reverse LPS-induced lung injury. (A) LPS (4 mg/kg) was administered intratracheally to Balb/c mice and, 1 day later, 100 μg of plasmid was delivered to the lungs by electroporation. At 24 and 48 hours later, lungs were removed for gravimetric analysis. No DNA (LPS-treated only, horizontal lined bars) or plasmids expressing no transgene (pcDNA3, open bars), the α subunit (gray bars), the β subunit (hatched bars), or a mixture of α and β (50 μg of each; “combo,” closed bars) were used. Wet-to-dry ratios are shown as mean ± SEM (n = 5). (B) Mice were treated with LPS and electroporated as in (A), and alveolar fluid clearance (AFC) was measured in living animals 48 hours after electroporation. AFC is shown as percentage of total instilled volume cleared in 30 minutes (mean ± SEM; n = 4). Statistical analysis was by one-way analyis of variance followed by Tukey-Kramer post hoc testing. *P ⩽ 0.05 compared with pcDNA3.
Figure 7.
Electroporation-mediated gene transfer of the Na+,K+-ATPase reduces bronchoalveolar lavage (BAL) cellularity and protein levels. LPS (4 mg/kg) was administered intratracheally to Balb/c mice and, 1 day later, 100 μg of plasmid was delivered to the lungs by electroporation. At 24 or 48 hours later, BAL fluid was collected from lungs and analyzed for protein levels (A) and total cell counts (B) (mean ± SEM; n = 4). Statistical analysis was by one-way analysis of variance followed by Tukey-Kramer post hoc testing. *P < 0.05 compared with levels seen at 48 hours in LPS-injured mice receiving pcDNA3.
Transfer of Na+,K+-ATPase Subunit Genes Modulates Pleural Effusion
During the course of these experiments, we noticed that, upon injury with LPS, the incidence of pleural effusions increased, as has been previously noted (11). At 2 and 3 days after LPS treatment, 3.4 ± 2.1 and 3.3 ± 1.2 μl of pleural effusion was collected from the mice, respectively. When either the α or β subunit of the Na+,K+-ATPase were transferred to the LPS-injured mice, pleural effusion volume increased by 2- to 3.4-fold (7.9 ± 5.5 and 11.7 ± 3.8 μl, respectively), but was not statistically significant. Transfer of both subunits caused a slight decrease (1.6 ± 1.0 μl), but again, statistical significance was not reached. However, 2 days after gene transfer of α1 and/or β1 subunits, volumes collected from the pleura decreased significantly (P < 0.05), by between 5- and 48-fold, to 0.2 ± 0.2, 0.06 ± 0.04, and 0.6 ± 0.3 μl for the α1, β1, and α + β genes, respectively. Improvement in the size of pleural effusions provides further support for the improvement in lung edema as a result of electroporation-mediated gene transfer. Although the mechanism responsible for reduction in the size of LPS-induced pleural effusion was not studied, it is likely due to electroporation-mediated transfer of the Na+,K+-ATPase genes and up-regulation of active Na+ transport, which has been previously shown to be associated with improvement in pleural effusion in other models of ALI (12).
DISCUSSION
Gene therapy for ALI is an attractive approach for treatment. However, most studies to date have demonstrated either that gene transfer of various genes can increase fluid clearance in the lungs of healthy animals or that transfer of these genes can provide protection from subsequent injury. In this report, we demonstrate that, not only can electroporation of subunit genes for the Na+,K+-ATPase result in protection from subsequent LPS-induced lung injury, but also that this approach can improve and reduce lung injury in previously injured animals. To our knowledge, this is the first time that gene transfer of any gene product has been successfully used to treat preexisting lung injury. Improvements in wet-to-dry ratios, pleural effusions, and respiratory mechanics, as well as limited improvements in BAL protein levels and cellularity, were seen after delivery of plasmids expressing Na+,K+-ATPase, but not empty control plasmids, in LPS-injured lungs. These results demonstrate that electroporation can be used effectively in healthy and edematous lungs to facilitate gene delivery and expression.
Electroporation is a potentially powerful approach that has several advantages, including the use of easily and cheaply produced plasmids, the relative absence of inflammation and trauma to the lungs of animals, and the ease and simplicity of administration (3, 4, 13). Perhaps the greatest advantage is that electroporation facilitates gene delivery to multiple cell layers, unlike gene delivery methods using liposomes, polymers, and viruses (14, 15). In the case of the lung, electroporation of DNA administered to the airways causes DNA delivery and expression, not only in the airway and alveolar epithelium, but also in subepithelial cells, including fibroblasts, endothelial cells, and vascular and airway smooth muscle cells. In the vasculature, electroporation causes DNA to move through multiple smooth muscle cell layers and even through the elastic lamina, after adventitial delivery (15, 16). This suggests that electroporation facilitates movement through cell junctions and interstitial and extracellular matrix. In this study, we have shown that electroporation can also be used to deliver genes in edematous lungs, allowing DNA to move through the protein and cell-rich edema fluid. Although expression levels were lower after delivery in previously injured lungs than in healthy lungs, the distribution of gene expression throughout the lungs and the cell types that received and expressed the transgenes were the same. Similar findings concerning delivery distribution have been made after delivery of adenoviral vectors in edematous hyperoxia-injured lungs (17). However, delivery by other methods, including liposomes and polymers, appears greatly inhibited in the injured lung (18).
Transfer of subunits of the Na+,K+-ATPase decreased severity of LPS-induced lung injury as measured by a number of criteria, including wet-to-dry ratios, BAL cellularity and protein content, and lung resistance (19). Athough we saw increased AFC in animals receiving subunits of the Na+,K+-ATPase, one of the limitations of our AFC calculation in the experiments where we tested the effect of gene therapy on an already established ALI is the accuracy of baseline albumin concentration used to calculate AFC. Pulmonary edema fluid may dilute the instilled albumin and, therefore, use of albumin concentration of 5 g/dl as the baseline may lead to underestimation of AFC in these animals. Although we acknowledge the limitation of our AFC calculations, the impact of pulmonary edema fluid on baseline albumin concentration is likely to be small (2.5 ± 1.7% reduction), as reported by Davis and colleagues in another model of ALI (20). When the subunits were transferred before induction of lung injury, transfer of the β1 subunit alone gave the least amount of protection from injury, whereas transfer of α1 alone or the combination of α1 and β1 subunits gave significant protection based on wet-to-dry ratios. This is contrary to previous reports in rats, showing that transfer of the β1 subunit produces greater increases in AFC in healthy lungs and provides greater protection from lung injury induced by hyperoxia, ventilation, or elevated left atrial pressure (7, 21–24). Indeed, after adenovirus-mediated transfer of the α1 subunit to rats, no increase is AFC was detected over naive lungs (21, 24). However, transfer of the α2 subunit to A549 and isolated rat type II cells increased Na+,K+-ATPase activity and AFC in rat lungs (25). Results from Alton's group showed that liposome-mediated gene transfer of an α–β fusion protein gave protection from subsequent thiourea-induced lung injury in mice, although these authors did not test the individual subunits for activity (26). Whether mice are somehow different than rats in their response to overexpression of Na+,K+-ATPase subunits is unknown. Previous explanations for the differential effects of transfer of Na+,K+-ATPase subunits have suggested that, although the catalytic α subunit of the pump is relatively abundant, the regulatory β subunit may be limiting, thus accounting for the greater increases seen after β transfer. However, this has not been evaluated in mice.
When subunits were transferred to LPS-injured lungs, the only protection seen 1 day later was after transfer of both subunits. By 2 days after gene transfer, significant improvements in lung injury were seen with transfer of either individual subunit or the combination of the two, and there was no statistical difference between any of these conditions. Although numerous studies have demonstrated that gene transfer of a number of genes, including Na+,K+-ATPase subunits, hemoxygenase-1, heat shock protein-70, and the β2-adrenergic receptor, by either recombinant adenoviruses or liposomes, can protect from subsequent lung injury (22, 23, 26–31), these are the first results demonstrating that gene transfer can be used to treat previously injured lungs. Although Factor and colleagues have shown that adenoviruses can transfer genes to the pulmonary epithelium after hyperoxic lung injury as efficiently as they can in healthy rat lungs, they have only shown this using reporter genes (17). Thus, these results are encouraging for future clinical application towards ALI, where the ideal therapy would be administered after lung injury and edema have developed. We also performed a set of experiments in mice with severely injured lungs by administering LPS at a dose of 8 mg/kg before electroporation of Na+,K+-ATPase subunit plasmids and found no improvement in lung injury as measured by wet-to-dry ratios (data not shown). Indeed, we found that fluid delivery with or without electroporation 1 day after LPS administration resulted in increased mortality and, of the animals that survived all procedures, immense variation in the wet-to-dry ratios were obtained, without respect to treatment group. Thus, we concluded that these animals were likely too sick to receive benefit from any treatment and that the treatment used that provides the greatest degree of gene delivery and expression in itself was harmful to the animals. This is potentially due to the model of injury or may be secondary to the fact that the treatment was applied to animals that were not ventilated. Overall, this suggests that, although this therapeutic avenue might be effective and a logical approach to treat lung injury, its application in severe lung injury still needs refinement.
We and others have previously demonstrated that gene transfer of Na+,K+-ATPase subunits results in increased pump activity, as measured by either ATP hydrolysis or 86Rb+ uptake by cells (7, 21). Thus, the assumption is that transfer of the pump results in increased alveolar Na+ transport and edema clearance from the lungs. In support of this, similar increases in fluid clearance from the lung can be achieved after β2-adrenergic receptor overexpression and cystic fibrosis transmembrane regulator (32, 33). Although it is clear that increased Na+ absorption from the airspaces results in fluid clearance from the lung, this may not be the only mechanism by which overexpression of the Na+,K+-ATPase can lessen lung injury. Several reports have suggested that the Na+,K+-ATPase may be involved in the formation and maintenance of tight junctions in the alveolar epithelium (34–36). Others have suggested that the β1 subunit may increase adhesiveness between cells, independent of tight junction formation (37). These additional activities of the Na+,K+-ATPase resulting in increased clearance and maintenance of barrier integrity may be responsible for our findings of reduced inflammation after gene transfer of the α1 and/or β1 subunits in previously injured lungs. Because ALI results from barrier dysfunction and the ensuing pulmonary edema, its resolution requires both repair of the epithelial barrier function and edema clearance. However, the experiments presented here did not have the power necessary to clearly demonstrate this. Regardless of which of these activities in which the Na+,K+-ATPase carries out a major function, its gene transfer represents a logical approach to treat the injured lung.
Acknowledgments
The authors thank Drs. Tom Kuzniar, J. Iasha Sznajder, Anjana Yeldandi, G. R. Scott Budinger, Jennifer Young, and Phil Factor for helpful discussions.
Supported by the Sandler Program for Asthma Research (D.A.D.), National Institutes of Health grants HL71643 (D.A.D.), HL81148 (D.A.D.), and ES015024 (G.M.M.), and a research grant from the American Lung Association (G.M.M.).
Originally Published in Press as DOI: 10.1164/rccm.200608-1246OC on June 7, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–1693. [DOI] [PubMed] [Google Scholar]
- 3.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther 2003;10:1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GRS, Yeldandi A, Sznajder JI, Dean DA. Gene transfer of the Na+,K+-ATPase b1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med 2005;171:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, Nakajima H. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med 2001;163:762–769. [DOI] [PubMed] [Google Scholar]
- 6.Machado-Aranda D, Kuzniar T, Mutlu GM, Dean DA. Electroporation-mediated gene transfer in lipopolysaccaride (LPS) injured murine lung [abstract]. Proc Am Thorac Soc 2005;2:A739. [Google Scholar]
- 7.Factor P, Senne C, Dumasius V, Ridge K, Jaffe HA, Uhal B, Gao Z, Sznajder JI. Overexpression of the Na+,K+-ATPase α1 subunit increases Na+,K+-ATPase function in A549 cells. Am J Respir Cell Mol Biol 1998;18:741–749. [DOI] [PubMed] [Google Scholar]
- 8.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178. [DOI] [PubMed] [Google Scholar]
- 9.Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 1989;67:2408–2419. [DOI] [PubMed] [Google Scholar]
- 10.Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med 2007;175:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintana HK, Cannet C, Schaeublin E, Zurbruegg S, Sugar R, Mazzoni L, Page CP, Fozard JR, Beckmann N. Identification with MRI of the pleura as a major site of the acute inflammatory effects induced by ovalbumin and endotoxin challenge in the airways of the rat. Am J Physiol Lung Cell Mol Physiol 2006;291:L651–L657. [DOI] [PubMed] [Google Scholar]
- 12.Factor P, Ridge K, Alverdy J, Sznajder JI. Continuous enteral nutrition attenuates pulmonary edema in rats exposed to 100% oxygen. J Appl Physiol 2000;89:1759–1765. [DOI] [PubMed] [Google Scholar]
- 13.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-κB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol 2005;175:7530–7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean DA. Electroporation of the vasculature and the lung. DNA Cell Biol 2003;22:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean DA. Nonviral gene transfer to skeletal, smooth, and cardiac muscle in living animals. Am J Physiol Cell Physiol 2005;289:C233–C245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin JB, Young JL, Benoit JN, Dean DA. Gene transfer to intact mesenteric arteries by electroporation. J Vasc Res 2000;37:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Factor P, Mendez M, Mutlu GM, Dumasius V. Acute hyperoxic lung injury does not impede adenoviral-mediated alveolar gene transfer. Am J Respir Crit Care Med 2002;165:521–526. [DOI] [PubMed] [Google Scholar]
- 18.Weiss D. Delivery of gene transfer vectors to lung: obstacles and the role of adjunct techniques for airway administration. Mol Ther 2002;6:148–152. [DOI] [PubMed] [Google Scholar]
- 19.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol 2004;286:L231–L246. [DOI] [PubMed] [Google Scholar]
- 20.Davis IC, Sullender WM, Hickman-Davis JM, Lindsey JR, Matalon S. Nucleotide-mediated inhibition of alveolar fluid clearance in BALB/c mice after respiratory syncytial virus infection. Am J Physiol Lung Cell Mol Physiol 2004;286:L112–L120. [DOI] [PubMed] [Google Scholar]
- 21.Factor P, Dumasius V, Saldias F, Brown LA, Sznajder JI. Adenovirus-mediated transfer of an Na+/K+-ATPase β1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum Gene Ther 2000;11:2231–2242. [DOI] [PubMed] [Google Scholar]
- 22.Azzam ZS, Dumasius V, Saldias FJ, Adir Y, Sznajder JI, Factor P. Na,K-ATPase overexpression improves alveolar fluid clearance in a rat model of elevated left atrial pressure. Circulation 2002;105:497–501. [DOI] [PubMed] [Google Scholar]
- 23.Adir Y, Factor P, Dumasius V, Ridge KM, Sznajder JI. Na,K-ATPase gene transfer increases liquid clearance during ventilation-induced lung injury. Am J Respir Crit Care Med 2003;168:1445–1448. [DOI] [PubMed] [Google Scholar]
- 24.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe HA, Blanco G, Barnard M, Mercer R, Perrin R, et al. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na,K-ATPase β1 subunit gene. J Clin Invest 1998;102:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the α2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res 2003;92:453–460. [DOI] [PubMed] [Google Scholar]
- 26.Stern M, Ulrich K, Robinson C, Copeland J, Griesenbach U, Masse C, Cheng S, Munkonge F, Geddes D, Berthiaume Y, et al. Pretreatment with cationic lipid-mediated transfer of the Na+ K+-ATPase pump in a mouse model in vivo augments resolution of high permeability pulmonary oedema. Gene Ther 2001;7:960–966. [DOI] [PubMed] [Google Scholar]
- 27.Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, et al. Upregulation of alveolar epithelial active Na+ transport is dependent on β2-adrenergic receptor signaling. Circ Res 2004;94:1091–1100. [DOI] [PubMed] [Google Scholar]
- 28.Zhou MY, Lo SK, Bergenfeldt M, Tiruppathi C, Jaffe A, Xu N, Malik AB. In vivo expression of neutrophil inhibitory factor via gene transfer prevents lipopolysaccharide-induced lung neutrophil infiltration and injury by a β2 integrin–dependent mechanism. J Clin Invest 1998;101:2427–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashiba T, Suzuki M, Nagashima Y, Suzuki S, Inoue S, Tsuburai T, Matsuse T, Ishigatubo Y. Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther 2001;8:1499–1507. [DOI] [PubMed] [Google Scholar]
- 30.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest 2002;110:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulrich K, Stern M, Goddard ME, Williams J, Zhu J, Dewar A, Painter HA, Jeffery PK, Gill DR, Hyde SC, et al. Keratinocyte growth factor therapy in murine oleic acid–induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2005;288:L1179–L1192. [DOI] [PubMed] [Google Scholar]
- 32.Dumasius V, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, Factor P. β2-adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res 2001;89:907–914. [DOI] [PubMed] [Google Scholar]
- 33.Mutlu GM, Adir Y, Jameel M, Akhmedov AT, Welch L, Dumasius V, Meng FJ, Zabner J, Koenig C, Lewis ER, et al. Interdependency of β-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res 2005;96:999–1005. [DOI] [PubMed] [Google Scholar]
- 34.Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell 2001;12:3717–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajasekaran SA, Hu J, Gopal J, Gallemore R, Ryazantsev S, Bok D, Rajasekaran AK. Na,K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol 2003;284:C1497–C1507. [DOI] [PubMed] [Google Scholar]
- 36.Rajasekaran AK, Rajasekaran SA. Role of Na-K-ATPase in the assembly of tight junctions. Am J Physiol Renal Physiol 2003;285:F388–F396. [DOI] [PubMed] [Google Scholar]
- 37.Shoshani L, Contreras RG, Roldan ML, Moreno J, Lazaro A, Balda MS, Matter K, Cereijido M. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between β-subunits located in neighboring cells. Mol Biol Cell 2005;16:1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]