Abstract
Stromal cell–derived factor-1 (SDF-1) participates in mobilizing bone marrow–derived stem cells, via its receptor CXCR4. We studied the role of the SDF-1/CXCR4 axis in a rodent model of bleomycin-induced lung injury in C57BL/6 wild-type and matrix metalloproteinase (MMP)-9 knockout mice. After intratracheal instillation of bleomycin, SDF-1 levels in serum and bronchial alveolar lavage fluid increased. These changes were accompanied by increased numbers of CXCR4+ cells in the lung and a decrease in a population of CXCR4+ cells in the bone marrow that did not occur in MMP-9−/− mice. Both SDF-1 and lung lysates from bleomycin-treated mice induced migration of bone marrow–derived stem cells in vitro that was blocked by a CXCR4 antagonist, TN14003. Treatment of mice with TN14003 with bleomycin-induced lung injury significantly attenuated lung fibrosis. Lung tissue from patients with idiopathic pulmonary fibrosis had higher numbers of cells expressing both SDF-1 and CXCR4 than did normal lungs. Our data suggest that the SDF-1/CXCR4 axis is important in the complex sequence of events triggered by bleomycin exposure that eventuates in lung repair. SDF-1 participates in mobilizing bone marrow–derived stem cells, via its receptor CXCR4.
Keywords: bone marrow–derived stem cells, pulmonary fibrosis, SDF-1, CXCR4
CLINICAL RELEVANCE
In patients with idiopathic pulmonary fibrosis, there is an increase of stromal cell–derived factor-1 expression and an elevated number of CXCR4 cells, suggesting that chronic injury induces the recruitment of CXCR4+ cells that may be a perpetual source for new fibroblasts.
The chemokine, stromal cell–derived factor-1 α (SDF-1, also called CXCL-12) is critical to bone marrow (BM) stem cell development. Murine embryos lacking SDF-1 or its receptor CXCR4 show multiple lethal defects, including impaired BM lymphoid and myeloid hematopoiesis (1, 2). SDF-1, acting via its receptor, CXCR4, is a chemoattractant for a broad range of cell types (3–7), including BM-derived stem cells. Mobilization of stem cells from BM to peripheral blood, and thence to injured tissues, may be down an SDF-1 concentration gradient (7, 8). Proteolytic enzymes such as cathepsin G, elastase, and matrix metalloproteinase (MMP)-9 also play a role in the mobilization process by degrading SDF-1 in BM, increasing expression of CXCR4 and mobilizing maturing leukocytes, progenitors, and stem cells (9).
Idiopathic pulmonary fibrosis (IPF) is a fatal disease characterized by inexorably progressive respiratory failure (10, 11). The hallmark histologic lesions are focal areas of active fibrogenesis called fibroblastic foci that progress to obliteration of the distal airspaces (12). Conventional therapy for IPF with corticosteroids and immunosuppressive agents has limited success. The fibrosis has been usually attributed to activation of lung tissue fibroblasts, but recent studies from animal models suggest that fibrosis is the result of dysrepair in which recruitment of a subpopulation of BM-derived cells called “fibrocytes” are sources of active fibroblasts (13, 14).
In the present study, we report an important role for the SDF-1/CXCR4 axis in recruitment of BMDMSC and lung tissue responses in an established animal model of lung injury, fibrosis, and repair. Studies of lung tissue from humans with IPF are consistent with a similar role in IPF in humans.
MATERIALS AND METHODS
Animal Maintenance
Six- to eight-week-old wide-type and MMP-9 knockout C57BL/6 female mice were used in all experiments. They were randomized into various groups before initiating experimental protocols. Animals were maintained in the animal care facility at Emory University, which is a fully accredited facility. Approval of the experimental protocol by the Emory University Institutional Animal Care and Use Committee (IACUC) was obtained before conducting the experiments.
Bleomycin Administration
Mice were anesthetized by isofluorane inhalation, the trachea exposed using sterile technique and 4 U/kg bleomycin (Sigma, St. Louis, MO) in 100 μl of PBS or PBS vehicle injected into the tracheal lumen. After inoculation, the incision was closed and the animals were allowed to recover.
Enzyme-Linked Immunosorbent Assay for Serum and BAL SDF-1
BAL was collected through a tracheal cannula with two instillations of 0.6 ml of PBS. Peripheral blood was collected from mouse orbital sinus and serum collected after clotting. Quantitative immunoassay was used for SDF-1 measurement, using a commercially available kit according to the manufacturer's protocol (R&D Systems, Minneapolis, MN).
Western Blot for Detecting CXCR4
Samples (20 μg protein per lane) were run on 4–20% SDS-PAGE gels (Invitrogen, Carlsbad, CA) for 1 h at 150 V and then transferred to nitrocellulose membranes. The blots were blocked in “blot buffer” (2% non-fat dry milk, 0.1% Tween 20, 50 mM NaCl, 10 mM Hepes, pH 7.4) for at least 30 min. Blots were then incubated with a goat anti-mouse CXCR4 antibody (Abcam, Cambridge, MA) or a mouse anti-β-actin antibody (Sigma). The blots were then washed three times with 10 ml of blot buffer and incubated for 1 h at room temperature with a horseradish peroxidase-conjugated anti-goat secondary antibody (Amersham Biosciences, Piscataway, NJ) in blot buffer. Finally, the blots were washed three more times with 10 ml of blot buffer and visualized via enzyme-linked chemiluminescence using the SuperSignal West Pico kit (Pierce, Holmdel, NJ).
Gelatin Zymography
Mouse femurs were flushed with 1 ml of PBS. Supernatant of the flush was used for zymography analysis. Gelatin zymography was performed by using a 9% SDS-PAGE gel saturated with 1 mg/ml gelatin (300 bloom; Sigma). Samples with equal protein amount (10 μg) were loaded onto the gel and electrophoresed at 150 V for 3 h. The gels were incubated for 1 h at room temperature in 2.5% Triton X-100, followed by an overnight incubation at 37°C in gelatinase substrate buffer (50 mM Tris, 10 mM CaCl2, and 0.02% NaN2, pH 8.0). The gels were stained with 0.5% Coomassie blue followed by destaining with 50% methanol. The gels were dried onto cellophane and scanned under a densitometer for determination of gelatinolytic activity.
Fluorescence-Activated Cell Sorter
BM cells were isolated by flushing PBS through mouse femurs. For flow cytometry, cells were treated with H2O briefly to lyse red blood cells. Cells were pelleted and resuspended with fluorescence-activated cell sorter (FACS) buffer (3% BSA and 0.1% sodium azide in PBS) to a concentration of 5 × 106 cells/ml. CD45- fractions were separated by MACS system as described by the manufacturer (Miltenyi Biotec, Auburn, CA). The following conjugated antibodies specific for surface antigens were used for total BM samples: PE anti-mouse CD45, FITC anti-mouse CD11b, and streptavidin-PerCP-Cy5.5 plus biotinylated anti-mouse CXCR4 (BD Pharmingen, San Jose, CA). For samples after MACS separation, the conjugated antibodies were PE anti-mouse CXCR4, FITC anti-mouse CD45, APC anti-mouse CD44, and streptavidin-PerCP-Cy5.5 plus biotinylated anti-mouse CD105 (eBiosciences, San Diego, CA). Cells were stained with saturating antibody concentrations for 30 min at 4°C, and washed 2 times. Both labeled and unlabeled samples were then analyzed on a FACSCalibur (Becton Dickinson, Mountain View, CA). Flow cytometry data were analyzed by using the FlowJo 6.1.1 software (Tree Star, Inc., San Carlos, CA).
CXCR4 Antagonist
The CXCR4 antagonist TN14003 was synthesized by the Microchemical Core Facility at Emory University and has been characterized in the literature before (15). TN14003 was designed based on a specific CXCR4 inhibitor T140, a 14-residue peptide that possessed a high level of anti-HIV activity and antagonism of T cell line–tropic HIV-1 entry among all antagonists of CXCR4 (16). TN14003 was generated by amidating the COOH-terminal of T140 and by substituting basic residues with nonbasic polar amino acids to reduce the total-positive charges of the molecule (17). TN14003 is far less cytotoxic and more stable in serum compared with T140. The concentrations of T140 and TN14003 required for 50% protection of HIV-induced cytopathogenicity in MT-4 cells (EC50) are 3.3 and 0.6 nM, respectively. The concentrations of T140 and TN14003 that induce a 50% reduction of the viability of MT-4 cells (50% cytotoxic concentration [CC50]) are 59 and 410 μM, respectively. These results reflect the improved therapeutic index for TN14003 over T140 (SITN14003 = 680,000; SIT140 = 17,879; selective index [SI] = CC50/EC50).
Migration Assay
Fresh BM cells were isolated by flushing with PBS through the end of both femurs and washed once with Dulbecco's modified Eagle's medium containing penicillin-streptomycin. Cells were plated at 106 cells per 100-mm cell culture dish in a medium containing 20% fetal calf serum, nonessential amino acids, pyruvate, the antibiotics penicillin at 200 IU/ml, and streptomycin at 250 μm/ml, and cultured at 5% CO2 atmosphere. After 48 h, nonadherent cells were removed and fresh media added to the culture. At Day 7, cells were harvested by treating the culture with 0.25% trypsin for 5 min followed by gentle scraping to remove cells. MSC were purified by passing a MACS system column twice (Miltenyi Biotec) to eliminate CD45+ and CD11b+ cells. The resulting MSC were over 99% CD45−. Migration of MSC was determined by incubation in Transwells (Corning, Corning, NY) assay. Cells were resuspended in modified Dulbecco's medium and 1 × 105 cells were loaded into the upper chamber of 8-μm pore transwell inserts. Recombinant SDF-1 (200 ng/ml; R&D Systems), MIP-2 (100 ng/ml; R&D Systems) or lysates of lungs from mice 3 d after treatment with bleomycin or PBS were added to the lower chamber. To produce lung lysates, at Day 3 after bleomycin or PBS treatment, lungs were harvested and homogenized with 1 ml of PBS plus proteinase inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). The homogenates were centrifuged with 13,000 rpm and supernatants were used for migration assay. Protein concentrations in the supernatants were determined by Coomassie assay. A total of 100 μg of protein was used for each migration. To determine the effect of CXCR4 antagonist on stem cell migration, cells were incubated with 1 μM of TN14003 for 30 min at 37°C before performing the migration assay. The assays were performed at 37°C for 2 h. Transmigrated cells were collected by centrifuging the liquid from the lower well and quantified by a hemocytometer.
Hydroxyproline Assay
Hydroxyproline content in whole mouse lungs was used to quantify lung collagen content and was measured colorimetrically by a method described previously, with modifications (18). At the time of killing, all lobes of lung were removed and the extrapulmonary airways and blood vessels excised and discarded. The lung parenchyma was homogenized in 1.0 ml of PBS, after which 1.0 ml of 12 N HCl was added, and the samples were hydrolyzed at 110°C for 24 h. Five microliters of each sample was mixed with 5 μl of citrate-acetate buffer (5% citric acid, 1.2% glacial acetic acid, 7.25% sodium acetate, and 3.4% sodium hydroxide). One hundred microliters of chloramine-T solution (1.4% chloramine-T, 10% N-propanol, and 80% citrate-acetate buffer) was added, and the mixture was incubated for 20 min at room temperature. Ehrlich's solution was added and the samples were incubated at 65°C for 18 min. Absorbance was measured at 550 nm. A standard curve was generated for each experiment using reagent hydroxyproline as a standard. Results were expressed as micrograms of hydroxyproline per lung.
Histology, Immunohistochemistry, and Immunofluorescence
Five mice per group at each experimental time point were used for immunohistochemistry and immunofluorescence analysis. To study tissue expression of CXCR4, frozen sections were incubated with a rabbit anti-CXCR4 antibody (Abcam). Slides were then treated with fluorescence-conjugated secondary antibodies. Nuclei were detected by DAPI staining. For experiments to determine the effects of CXCR4 antagonist on lung fibrosis, after inflation and fixation with 4% paraformaldehyde for 24 h, lung tissue was paraffin embedded, sectioned, and stained with hematoxylin and eosin (H&E) for routine histologic examination and Masson's trichrome staining to delineate collagen. In human tissue, immunohistochemistry was performed with an antibody for human SDF-1 and an antibody for human CXCR4 (R&D Systems). DAB was used as the chromogen.
Patient Population
With Institutional Review Board (IRB) approval (Emory University), four pairs of archived lung samples with the diagnosis of IPF and normal control were enrolled into the study. The diagnosis was provided by pulmonary pathologist and meet the criteria for UIP as defined by Katzenstein and Myers (19).
Statistical Methods
For comparisons between groups, paired or unpaired t test one-way and two-way ANOVA tests were used (P values < 0.05 were considered significant). We used GraphPad Prism and GraphPad InStat to calculate the statistics.
RESULTS
Bronchoalveolar Lavage and Serum SDF-1 Concentrations Increase after Intratracheal Bleomycin
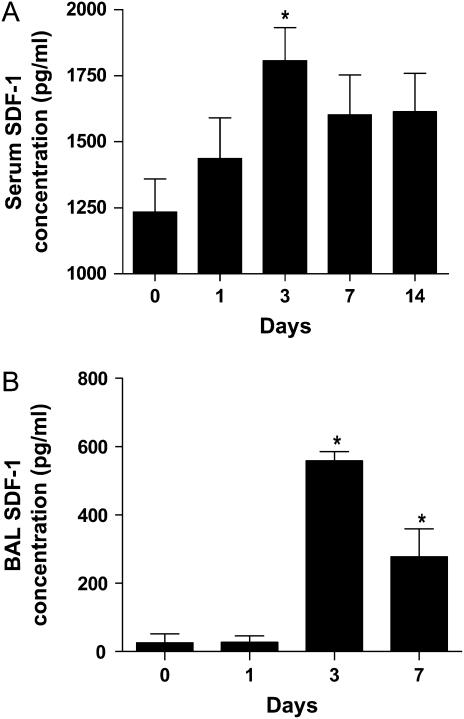
To determine the role of SDF-1 in lung injury, female C57BL/6 mice were treated with a single intratracheal instillation of 4 U/kg bleomycin or phosphate buffered saline (PBS). After 1, 3, 7, and 14 d, serum and BAL samples were collected for analysis. Figure 1A summarizes serum concentrations of SDF-1 determined by enzyme-linked immunosorbent assay (ELISA). Serum levels increased after bleomycin administration, reaching a peak at Day 3 and remained high through Day 14. Figure 1B shows bronchoalveolar lavage (BAL) SDF-1 levels and the expression patterns were very similar to that of serum. There were no significant changes in SDF-1 concentrations in serum and BAL in animals treated with PBS (data not shown). These data demonstrate a temporally coincident increase in expression of SDF-1 at the site of lung injury and in circulating SDF-1 concentrations.
Figure 1.
SDF-1 expression in lungs of mice after bleomycin treatment. After 0, 1, 3, 7, and 14 d of bleomycin treatment, mice were killed. Serum (A) and BAL (B) SDF-1 levels were determined by ELISA. Values represent mean ± SE (n = 6, *P < 0.05).
CXCR4 Levels Increase in the Lung after Intratracheal Bleomycin
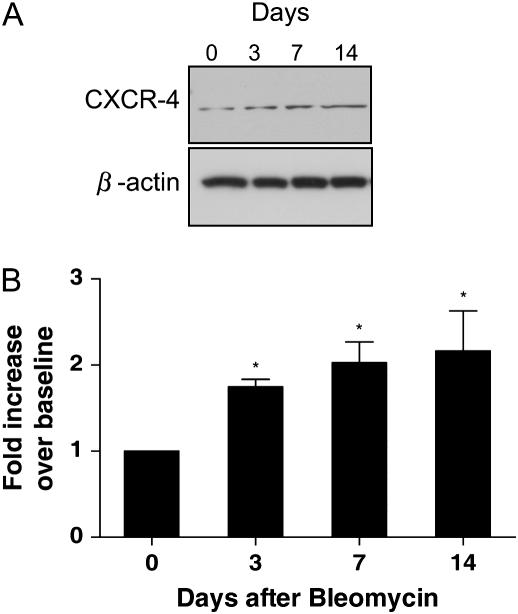
It has been shown that SDF-1 recruits CXCR4+ bone marrow–derived mesenchymal stem cells (BMDMSC) to ischemic tissues (20). To determine the effects of bleomycin treatment on CXCR4 levels, Western blotting was used to detect the CXCR4 expression in whole lung tissue at different time points. Figure 2A illustrates a blot, and Figure 2B summarizes quantitative data from the blots. CXCR4 levels in the lungs increased gradually after bleomycin, reaching a peak of 2.4-fold over baseline by Day 14. The time course of the increase in CXCR4 expression in the lungs was delayed relative to the time course of serum and BAL SDF-1 concentrations. That temporal relationship is consistent with the concept that SDF-1 recruits CXCR4-expressing cells from BM to the injured lung.
Figure 2.
CXCR-4 expression in mice after bleomycin treatment. Female C57BL/6 mice, 8 wk old, were given 4 U/kg bleomycin in 100 μl of PBS or 100 μl of PBS alone intratracheally. After 0, 3, 7, and 14 d, mice were killed. (A) CXCR4 protein expression as shown in Western blot. (B) Quantitation of CXCR4 levels from Western blot by densitometry. Basal level was designated as 1. Values represent mean ± SE (n = 4, *P < 0.05).
A Small Population of BMDMSC Expresses CD45−CD105+CD44+CXCR4+
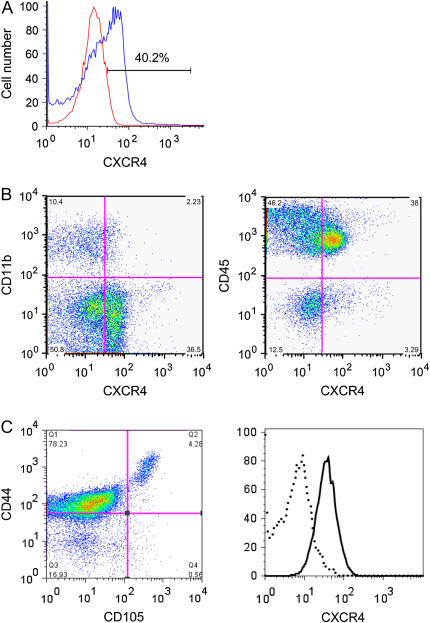
BM stem cells are classified as hematopoietic stem cells and mesenchymal stem cells. We determined by FACS analysis that 40.2% of mouse bone marrow cells express CXCR4 (Figure 3A). A fraction (38%), of bone marrow cells were double positive for CXCR4 and CD45, which include hematopoietic stem cells. On the other hand CXCR4+CD45− cells were 3.3% of the total cells (Figure 3B).
Figure 3.
FACS analysis of BM cells. (A) Total bone marrow cells were labeled with anti-mouse CXCR4. Both labeled (blue curve) and unlabeled (red curve) samples were subjected to FACS analysis. (B) Total bone marrow cells were labeled with anti-mouse CD45, CD11b, and CXCR4. Samples were subjected to FACS analysis. (C) CD45− fraction of BM cells was purified by MACS separation system. The resulting cells were labeled with anti-mouse CD45, CD105, CD44, and CXCR4 antibodies and gated for CD45− cells. Data shown in left panel were dual expression of CD105 and CD44. In the right panel, the CD45−CD105+CD44+ fractions were further examined for the expression of CXCR4 (solid line). Flow cytometry data were generated using the FlowJo 6.1.1 software.
Although there are no definitive markers for BMDMSC, several surface proteins have been associated with these cells. Based on such markers, we describe a BMDMSC population, with the phenotype CD45−CD105+CD44+CXCR4+, that comprise less than 0.1% of total bone marrow cells. To identify these cells, we first selected the CD45−CD11b− fraction by MACS system. For FACS analysis, cells were gated for CD45− cells and examined for dual expression of CD105 and CD44. A small population of cells in the fraction expressed CD45−CD105+CD44+ cells. The majority of CD45−CD105+CD44+ cells express CXCR4 (Figure 3C).
Mobilization of CD45−CD105+CD44+CXCR4+ Cells from BM in MMP-9−/− Compared with Wild-Type Mice
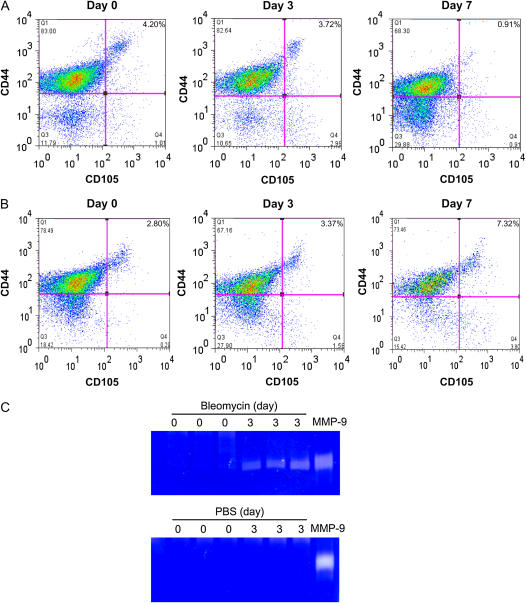
To assess mobilization of BMDMSC from marrow, we measured the subpopulation of CD45−CD105+CD44+CXCR4+ cells in BM over the course of the bleomycin response. Figures 4A and 4B showed representative flow cytometry scatter plots. At baseline, ∼ 4% of CD45− BM cells expressed CD105+CD44+CXCR4+, assuming that the large majority, more that 70%, of CD105+CD44+ cells were CXCR4+ as shown in Figure 3C. After bleomycin treatment in wild-type mice, the percentage of CD45−CD105+CD44+CXCR4+ cells was slightly reduced by Day 3 and significantly depleted by Day 7 (Figure 4A). There were no significant changes in percentage of CD45−CD105+CD44+CXCR4+ cells in PBS treated control group (data not shown). These data indicate that the migration from bone marrow of CD45−CD105+CD44+CXCR4+ BMDMSC occurred mainly between Days 3 and 7 after bleomycin injury.
Figure 4.
Effect of MMP-9 on recruitment of CD45−CD105+CD44+CXCR4+ cells from BM after bleomycin treatment. After 0, 3, and 7 d of bleomycin treatment, BM samples were harvested and CD45− fraction collected by MACS separation. The samples were stained with anti-mouse CD45, CD105, CD44, and CXCR4 antibodies and analyzed by FACS. (A) In wild-type C57BL/6 mice, FACS data showed changes of CD45−CD105+CD44+expression at different time points. (B) Results from MMP-9−/− C57BL/6 mice. (C) MMP-9 activity in BM stromal supernatant as determined by gelatin zymography. Clear bands revealed MMP gelatinase activity.
MMP-9 has been reported to participate in migration of maturing leukocytes, progenitors, and stem cells (9). To demonstrate the role of MMP-9 in the migration from bone marrow of BMDMSC in our system, we determined the kinetics of CD45−CD105+CD44+CXCR4+ cells in the BM of MMP-9 knockout mice treated with bleomycin. In stead of reducing the percentage of cells expressing CD45−CD105+CD44+CXCR4+ markers as shown in wild-type mice, bleomycin treatment increased the percentage of these cells in the BM (Figure 4B). These data suggest that there are signals produced by the lung that induce an increase in the number of BMDMSC in the bone marrow and that MMP-9 plays a role in the mobilization of CD45−CD105+CD44+CXCR4+ cells from the bone marrow into circulation.
We also determined MMP-9 activity in BM stromal supernatant in response to administration of bleomycin in wild-type mice. As shown by the zymogram in Figure 4C, MMP-9 activity increased significantly after bleomycin treatment, while no change was seen in PBS-treated group.
SDF-1 and CXCR4 Mediate Stem Cell Migration In Vitro
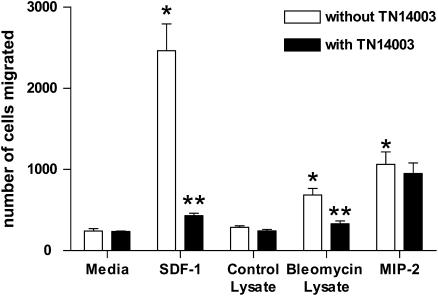
To explore the roles of SDF-1/CXCR4 axis in homing of BMDMSC to injured lungs, we performed an in vitro migration assay using cultured BMDMSC and lung extracts obtained from mice at Day 3 after bleomycin treatment. BMDMSC were cultured and selected CD45−CD11b− cells purified using a MACS system as described (21). As shown in Figure 5, SDF-1 induced marked chemotactic migration of stem cells (∼ 11-fold increase over control, *P < 0.01). Lung extracts from saline-treated mice did not affect stem cell migration. Extracts of lungs from mice after bleomycin treatment mimicked the effect of SDF-1 (∼ 3-fold increase over control, *P < 0.01). To demonstrate that this was an SDF-1–dependent effect, a synthetic specific CXCR4 antagonist, TN14003 (15) (see below for fuller description), completely blocked chemotaxis of the stem cells in response to either SDF-1 or lung extracts from bleomycin-treated animals (Figure 5, **P < 0.01). TN14003 did not affect MIP-2–induced cell migration, indicating specificity of the agent for SDF-1 (Figure 5). These results implicate SDF-1 as a chemoattractant produced in injured lung that recruits BMDMSC to the areas of injury via CXCR4 receptors.
Figure 5.
Effect of lung lysate on MSC migration. Cultured bone marrow cells were depleted with CD45 and CD11b antibodies to purify MSC (1 × 105) and plated in Matrigel-coated transwells. Minimal essential medium was added to both upper and lower chambers. Extracts of lungs harvested from mice 3 d after bleomycin or PBS treatment (200 μg of total protein), SDF-1 (200 ng/ml), MIP-2 (100 ng/ml) or PBS was added to the lower chamber. For effects of the CXCR4 antagonist on stem cell migration, cells were incubated with 1 μM of TN14003 for 30 min at 37°C before migration. Migration assays were performed at 37°C for 2 h. Transmigrated cells were collected and quantified by a hemocytometer. Values represent mean ± SE (*,**P < 0.01, n = 4).
A CXCR4 Antagonist Attenuates Bleomycin-Induced Lung Fibrosis in Mice
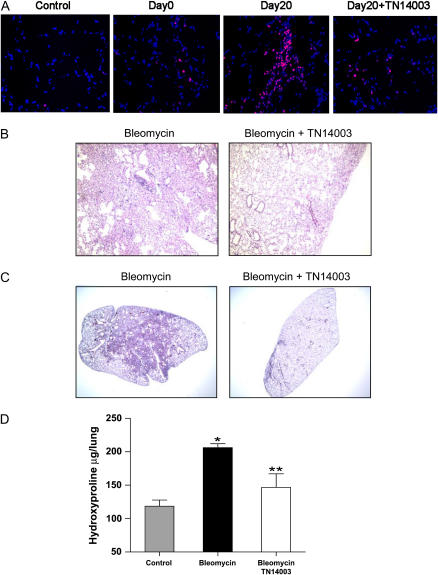
We determined the effects of a synthetic specific CXCR4 antagonist, TN14003, on the mobilization and recruitment of BMDMSC into the injured lung. TN14003 is a 14-residue peptide and its specificity for CXCR4 has been characterized and reported in the literature (15–17). Mice received intraperitoneally 160 ng/g of TN14003 or saline 1 d before bleomycin treatment and daily for 20 d. All mice tolerated the antagonist well. Lungs harvested 20 d after bleomycin treatment were analyzed histologically and assayed for collagen content. Results from immunofluorence staining showed that CXCR4+ cells were greatly increased with bleomycin treatment and were dramatically reduced with TN14003 treatment (Figure 6A). Figure 6B shows photomicrographs of H&E-stained sections. As reported in the literature, bleomycin caused marked alterations in lung architecture with increased interstitial wall thickness and mononuclear cell infiltrates (13). TN14003-treated mice receiving bleomycin showed a decrease in interstitial and alveolar structural distortion (Figure 6B). Figure 6C shows histologic sections of lung stained with Masson's trichrome to highlight collagen and Figure 6D summarizes biochemical measurements of collagen in the lungs as determined by hydroxyproline assay. Bleomycin caused marked increases in collagen deposition in the trichrome-stained sections, which was largely prevented by treatment with the CXCR4 antagonist. Biochemical measurements of lung collagen content were consistent with the histologic sections, showing significantly elevated hydroxyproline in bleomycin-treated animals that was significantly inhibited by treatment with CXCR4 antagonist.
Figure 6.
A CXCR4 antagonist inhibits bleomycin induced pulmonary fibrosis. Mice were treated with bleomycin in the presence of CXCR4 antagonist, TN14003 or PBS. (A) Frozen lung sections from mice treated with bleomycin on Days 0 and 20 were stained with a rabbit anti-CXCR4 antibody or a control rabbit IgG (control). (B) Representative H&E-stained histopathologic sections of saline-treated (left panels) or TN14003-treated (right panels) lung tissues on Day 20 after bleomycin treatment. (C) Masson's Trichrome staining of lung sections from the same experimental groups. (D) Collagen content of lung tissue as determined by hydroxyproline assay. *= significant increase as compared to control, ** = significant reduction as compared to bleomycin treatment. Values represent mean ± SE (*,**P < 0.05, n = 5).
SDF-1 and CXCR4 Are Increased in Lungs of Humans with IPF
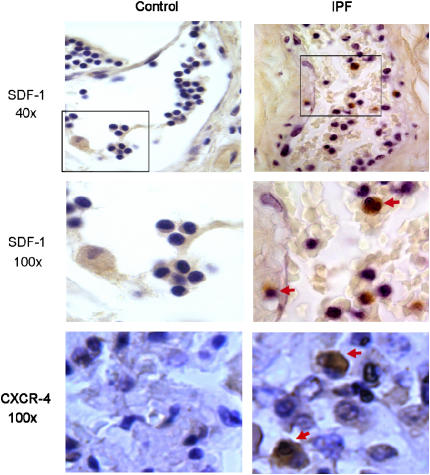
The above data implicate the SDF-1/CXCR4 axis in the pathogenesis of bleomycin-induced pulmonary fibrosis in mice, but whether similar pathogenetic processes are operative in pulmonary fibrosis in humans remains to be shown. To obtain data relevant to that question, we analyzed tissue samples of normal human lungs and samples of human lungs from patients with the clinical picture of IPF that showed the typical pathology (termed “usual interstitial pneumonia” [UIP]). Figure 7 shows photomicrographs of sections from normal and IPF lungs stained with antibodies specific for either SDF-1 or CXCR4. SDF-1– and CXCR4-positive cells were clearly identifiable in lungs from patients with IPF, but very few were seen in normal lungs. In a high-magnification field (×100), several cells within and around a small blood vessel stained positive for SDF-1. These results are similar to the data from the rodent bleomycin model and are consistent with the conclusion that chronic expression of SDF-1 mediates recruitment of CXCR4+ stem cells which play a role in perpetuating fibrosis.
Figure 7.
Increased SDF-1 and CXCR-4 in IPF lung samples compared with normal human lungs. Lung tissues from patients with IPF (right panels) and from normal control subjects (left panels) were paraffin embedded and sectioned. Immunohistochemistry was performed for SDF-1 and CXCR-4. Cells stained dark brown were SDF-1– (inside and around a vessel) or CXCX4 (interstitium)-expressing cells (see arrows).
DISCUSSION
Recent studies have shown that BMDMSC may be involved in the repair of bleomycin-induced lung injury (13). Phillips and associates described a population of bone marrow derived cells called “fibrocytes” that are implicated in the pathogenesis of lung fibrosis (14). In this study we sought to investigate the role of SDF-1/CXCR4 axis in the recruitment of bone marrow–derived stem cells into the injured lung and to determine the consequences of inhibiting the recruitment of CXCR4+ cells into the lungs in response to bleomycin-induced injury. In addition, a work recently published by Yang and collaborators demonstrated an increase of SDF-1 expression in the lungs of patients with idiopathic interstitial pneumonia, which emphasizes the importance of the axis SDF-1/CXCR4 in pulmonary fibrosis (22). Based on studies in the literature, we hypothesized that the SDF-1/CXCR4 axis is an important system for recruitment of profibrotic BMDMSC to the injured lung and therefore might play a critical role in perpetuating a fibrotic response.
We found that SDF-1 levels in BAL and circulating blood increased late after bleomycin injury, a finding that is consistent with other reports (13). This increase in SDF-1 was accompanied by an increase in CXCR4 expression in the lungs with a peak at the second week after injury. In the bone marrow, a subpopulation of BM cells expressing the marker profile CD45−CD105+CD44+CXCR4+ decreased in number simultaneously with an increase in the levels of SDF-1 after bleomycin, changes that were reversed in mice deficient on MMP-9. In an in vitro assay, extracts of lungs harvested from mice after administering bleomycin were, like SDF-1, chemotactic for BM derived stem cells and this effect was blocked by a CXCR4 antagonist. The same CXCR4 antagonist given to mice that received bleomycin largely prevented bleomycin lung fibrosis. In lungs from patients with idiopathic pulmonary fibrosis, we found increased positive staining cells for SDF-1 and CXCR4 compared with normal human lungs. These data are consistent with the hypothesis that lungs with bleomycin-induced injury stimulate a late increase in the expression of SDF-1, which can be implicated in the mobilizing CXCR4+ expressing BMDMSC. In the fibrogenic environment of the injured lung, CXCR4+ cells may assume a fibroblast phenotype and contribute to fibrogenesis.
Current concepts of tissue repair implicate recruitment of BMDMSC to sites of injury. Several mechanisms have been implicated in the recruitment of these cells, one of them being the SDF-1/CXCR4 axis, which increases late after 3 d of injury (as distinct from other soluble factors like G-CSF, which peaks in the first 24 h). SDF-1 is a chemokine implicated in the recruitment of HSC principally; only recently has it been described as an important element in the recruitment of mesenchymal stem cells (23–25). After injury, SDF-1 is up-regulated in many tissues and thus is positioned to recruit progenitor cells to the site of injury to effect repair (26–30). For example, SDF-1 is both necessary and sufficient to promote proliferative retinopathy (31), and SDF-1 signals mobilization and homing of CXCR4+ cells to the kidney after ischemic injury (32).
CXCR4 is a G protein–linked seven transmembrane spanning receptor that was first identified as a co-factor for T cell–tropic HIV-1 and -2 viral entry into cells (33). A variety of stem cells express CXCR4 (34–36), including hematopoietic stem cells (23) as well as progenitor cells committed to neural (2), myocardial (37), and endothelial (3, 38) differentiation pathways. Thus, CXCR4+ stem cells recruited to the lungs after injury may be precursors of inflammatory cells (hematopoietic lineage) as well as of mesenchymal-derived profibrotic cells.
The CXCR4 antagonist TN14003 we used is a peptide with specificity for the receptor that, like other similar agents, has anti–HIV-1 activity (17, 39, 40) and inhibits metastases of breast cancer in animal models (15, 41, 42). Treatment of mice with either the CXCR4 antagonist that we used in the present study or with a neutralizing antibody to SDF-1 (14) attenuates bleomycin-induced lung fibrosis, although the fibrosis was not completely prevented in either case. This could be because some of the fibrotic response is due to activation of fibroblasts already present in the lungs.
Mechanisms involved in stem cell differentiation in the lung are not well understood. We reported earlier that BM cells are required for lung repair after bleomycin instillation. Myelosuppressed mice treated with bleomycin had mortality closer to 75%. When BMDMSC were administered to those animals, there was no mortality, similar to results seen with the nonimmunosuppressed mice (43). In those studies, 14 d after bleomycin was detected in the lung, donor-derived cells expressing specific markers were evenly distributed among type I and type II epithelium. In addition, the presence of fibroblasts derived from donor BMDMSC (the donor cells were vimentin-negative) demonstrates that these cells have the capacity for fibroblastic transformation, and thus could contribute to fibrogenesis. Recently Lama and collaborators (44) had reported the presence of bone marrow–derived and resident mesenchymal stem cells, isolated from the lower respiratory tract of transplanted lungs; this observation reaffirms the relevance of these cells in process of adaptive and maladaptive lung repair.
Others have reported similar results. For example, BMDMSC can develop markers specific for type II pneumocytes and cell types of many other tissues after bone marrow transplantation in mice (45). MSC-derived type II pneumocytes have been identified by the presence of a Y chromosome in male donor–female recipient studies, and by expression of surfactant protein B mRNA (21). Kotton and colleagues (46) concluded that plastic adherent cultured BM-derived stem cells given to mice after bleomycin administration differentiated directly into type I pneumocytes. Some controversy persists surrounding the techniques used to identify differentiated donor stem cells (47), but there is considerable evidence that such cells can participate in responses of the lungs to injury, although the nature of their participation is not fully understood.
We and others reported earlier that transfer of BMDMSC to mice early (but not late) after administration of bleomycin protected against bleomycin-induced fibrosis (21, 43). In the present study we find that inhibition of late signals can inhibit the mobilization of subpopulations of BMDMSC, which markedly decreases bleomycin-induced fibrosis. We believe that this is a demonstration that BMDMSC is not a homogenous population and is required in the normal process of repair, which includes anti-inflammatory effect, fibrosis, and tissue regeneration. Our data suggest that the subpopulation involved in fibrosis are CXCR4-expressing cells, and based on our results we cannot rule out that some of these cells can participate in the other two phases. In the process of repair, an initial flux of BMDMSC will diminish inflammation by their anti-inflammatory effect, the number of inflammatory cells recruited into the lung will determine the levels of SDF-1, because the sources of SDF-1 are principally macrophages and neutrophils. If the inflammation is severe, large numbers of profibrotic CXCR4+ cells will be recruited to the injured lung, resulting in fibrosis. Our data suggest that in patients with pulmonary fibrosis there is a continuous inflammatory process that results in disrepair by the persistent recruitment of pro-fibrotic cells.
The studies we report here implicate the SDF-1/CXCR4 axis in the pathogenesis of lung fibrosis produced by intratracheal infusion of bleomycin to mice. The fact that the lungs of patients with idiopathic pulmonary fibrosis contain increased numbers of cells expressing SDF-1 and CXCR4 is consistent with the idea that chronic active injury in the lungs is accompanied by continued expression of SDF-1 and continued recruitment of CXCR4+ stem cells that may be a perpetual reservoir for new fibroblasts.
This study was supported by grants 5 P01 HL0669496-02 (to K.B.) and HL080284-01 (to A.S.) from the National Heart, Lung, and Blood Institute and the McKelvey Center for Lung Transplantation.
Originally Published in Press as DOI: 10.1165/rcmb.2006-0187OC on April 26, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996;382:635–638. [DOI] [PubMed] [Google Scholar]
- 2.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998;393:595–599. [DOI] [PubMed] [Google Scholar]
- 3.Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N, Margalit R, Zsak M, Nagler A, Hardan I, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol 2005;6:1038–1046. [DOI] [PubMed] [Google Scholar]
- 4.Vianello F, Kraft P, Mok YT, Hart WK, White N, Poznansky MC. A CXCR4-dependent chemorepellent signal contributes to the emigration of mature single-positive CD4 cells from the fetal thymus. J Immunol 2005;175:5115–5125. [DOI] [PubMed] [Google Scholar]
- 5.Narducci MG, Scala E, Bresin A, Caprini E, Picchio MC, Remotti D, Ragone G, Nasorri F, Frontani M, Arcelli D, et al. Skin homing of Sezary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood 2006;107:1108–1115. [DOI] [PubMed] [Google Scholar]
- 6.Petit I, Goichberg P, Spiegel A, Peled A, Brodie C, Seger R, Nagler A, Alon R, Lapidot T. Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J Clin Invest 2005;115:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone 2005;36:840–853. [DOI] [PubMed] [Google Scholar]
- 8.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells 2005;23:1324–1332. [DOI] [PubMed] [Google Scholar]
- 9.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 2006;12:657–664. [DOI] [PubMed] [Google Scholar]
- 10.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994;150:967–972. [DOI] [PubMed] [Google Scholar]
- 11.Perez A, Rogers RM, Dauber JH. The prognosis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S19–S26. [PubMed] [Google Scholar]
- 12.Sheppard D. Pulmonary fibrosis: a cellular overreaction or a failure of communication? J Clin Invest 2001;107:1501–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, Nie S, Umbreit J, Shim H. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res 2004;64:4302–4308. [DOI] [PubMed] [Google Scholar]
- 16.Tamamura H, Omagari A, Hiramatsu K, Gotoh K, Kanamoto T, Xu Y, Kodama E, Matsuoka M, Hattori T, Yamamoto N, et al. Development of specific CXCR4 inhibitors possessing high selectivity indexes as well as complete stability in serum based on an anti-HIV peptide T140. Bioorg Med Chem Lett 2001;11:1897–1902. [DOI] [PubMed] [Google Scholar]
- 17.Tamamura H, Fujisawa M, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, Otaka A, Fujii N. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett 2004;569:99–104. [DOI] [PubMed] [Google Scholar]
- 18.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961;93:440–447. [DOI] [PubMed] [Google Scholar]
- 19.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med 1998;157:1301–1315. [DOI] [PubMed] [Google Scholar]
- 20.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004;10:858–864. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang IV, Burch LH, Steele MP, Savov JD, Hollingsworth JW, McElvania-Tekippe E, Berman KG, Speer MC, Sporn TA, Brown KK, et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med 2007;175:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med 1997;185:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma 2003;44:575–582. [DOI] [PubMed] [Google Scholar]
- 25.Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, Slav MM, Nagler A, Lider O, Alon R, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 2000;95:3289–3296. [PubMed] [Google Scholar]
- 26.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003;362:697–703. [DOI] [PubMed] [Google Scholar]
- 27.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 2004;110:3300–3305. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999;5:434–438. [DOI] [PubMed] [Google Scholar]
- 29.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 2002;105:732–738. [DOI] [PubMed] [Google Scholar]
- 30.Kijowski J, Baj-Krzyworzeka M, Majka M, Reca R, Marquez LA, Christofidou-Solomidou M, Janowska-Wieczorek A, Ratajczak MZ. The SDF-1-CXCR4 axis stimulates VEGF secretion and activates integrins but does not affect proliferation and survival in lymphohematopoietic cells. Stem Cells 2001;19:453–466. [DOI] [PubMed] [Google Scholar]
- 31.Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest 2005;115:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 2005;67:1772–1784. [DOI] [PubMed] [Google Scholar]
- 33.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996;272:872–877. [DOI] [PubMed] [Google Scholar]
- 34.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999;283:845–848. [DOI] [PubMed] [Google Scholar]
- 35.Rosu-Myles M, Gallacher L, Murdoch B, Hess DA, Keeney M, Kelvin D, Dale L, Ferguson SS, Wu D, Fellows F, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci USA 2000;97:14626–14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 2002;16:1992–2003. [DOI] [PubMed] [Google Scholar]
- 37.Damas JK, Eiken HG, Oie E, Bjerkeli V, Yndestad A, Ueland T, Tonnessen T, Geiran OR, Aass H, Simonsen S, et al. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res 2000;47:778–787. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003;107:1322–1328. [DOI] [PubMed] [Google Scholar]
- 39.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, et al. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med 1997;186:1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med 1997;186:1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells 2005;23:879–894. [DOI] [PubMed] [Google Scholar]
- 42.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 2004;64:8604–8612. [DOI] [PubMed] [Google Scholar]
- 43.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol 2005;33:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 2007;117:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 46.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 47.Chang JC, Summer R, Sun X, Fitzsimmons K, Fine A. Evidence that bone marrow cells do not contribute to the alveolar epithelium. Am J Respir Cell Mol Biol 2005;33:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]