Abstract
Rationale: Epidemiologic data indicate an increased incidence of asthma in the obese.
Objectives: To determine whether obese mice exhibit augmented pulmonary responses after allergen sensitization and challenge.
Methods: Lean, wild-type (C57BL/6), obese ob/ob, and obese db/db mice were sensitized to ovalbumin (OVA), and then challenged with aerosolized OVA or phosphate-buffered saline (PBS). Changes in total pulmonary resistance (Rl) induced by intravenous methacholine were measured by forced oscillation. Blood was collected, bronchoalveolar lavage (BAL) was performed, and lungs were harvested for measurement of cytokine expression by real-time reverse transcription–polymerase chain reaction.
Measurements and Main Results: OVA challenge increased baseline Rl in ob/ob, but not wild-type, mice, and airway responsiveness was greater in ob/ob than wild-type mice, regardless of the challenge. Compared with PBS, OVA challenge caused an increase in the number of BAL fluid (BALF) cells, an increase in lung Th2 cytokine expression, and an increase in serum IgE. Significantly fewer BALF cells were recovered from OVA-challenged ob/ob versus wild-type mice, whereas serum IgE levels were elevated significantly more in ob/ob versus wild-type mice. BALF and lung Th2 cytokine expression was not different in ob/ob versus wild-type mice. Airway responsiveness was greater in db/db versus wild-type mice, regardless of the challenge, and OVA caused airway hyperresponsiveness in db/db but not wild-type mice, despite reduced BALF cells in OVA-challenged db/db versus wild-type mice.
Conclusions: These results demonstrate that obesity enhances OVA-induced changes in pulmonary resistance and serum IgE and that these changes are not the result of increased Th2 type airway inflammation.
Keywords: airway responsiveness, eosinophil, immunoglobulin E, interleukin-13, pulmonary resistance
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Obesity is a risk factor for the development of asthma. However, the mechanistic basis for this relationship is not known.
What This Study Adds to the Field
Obese mice exhibit enhanced airway responsiveness to methacholine and augmented IgE production and/or release following allergen sensitization and challenge.
Obesity and asthma are both important public health issues. Epidemiologic data indicate an association between obesity and asthma (1–4). It is likely that the relationship between obesity and asthma is a causal one. Longitudinal studies indicate that obesity antedates asthma and that the relative risk of incident asthma increases with increasing body mass index (5–8). In addition, obese persons with asthma studied after weight loss demonstrate decreased severity and symptoms of asthma (9–12). Obesity may be particularly important for severe asthma because more than 75% of individuals visiting the emergency room for asthma are obese or overweight (13).
We have recently reported that innate airway hyperresponsiveness (AHR) was observed in mice that were obese as a result of a genetic deficiency in leptin, a satiety hormone (ob/ob mice) (14, 15); in mice obese because of a genetic deficiency in the long form of the leptin receptor (db/db mice) (16); and in mice obese due to a genetic deficiency in carboxypeptidase E (Cpe) (17), an enzyme involved in processing prohormones and proneuropeptides involved in satiety and energy expenditure (Cpefat mice) (18). In addition, inhalation of ozone (O3), a common environmental pollutant and an asthma trigger (19, 20), augments airway responsiveness and pulmonary inflammation to a greater extent in obese versus lean mice (14–17). Because innate AHR and augmented pulmonary responses to O3 are observed regardless of the modality of obesity induction (14–17), this suggests that obese mice may be useful tools to enhance our understanding of the relationship between obesity and asthma.
Several epidemiologic studies have reported an increased risk of atopy in the overweight and/or obese (6, 21–23), although these observations have not been consistent (24, 25). Nevertheless, because atopy is an important risk factor for the development of asthma, it is plausible that allergic airway responses could be enhanced in the overweight and/or obese. Therefore, the purpose of this study was to determine whether pulmonary responses to allergen are also augmented in obese versus lean mice. To that end, we sensitized lean, wild-type, and obese ob/ob mice with ovalbumin (OVA). Four weeks after the initial OVA sensitization, the mice were challenged with aerosols of either phosphate-buffered saline (PBS) or OVA. Baseline pulmonary resistance (Rl) and airway responsiveness to intravenous methacholine were measured, blood was collected and serum isolated for measurement of IgE and markers of systemic inflammation, bronchoalveolar lavage (BAL) was performed for cytokine/chemokine analysis, and lungs were either harvested for RNA extraction or fixed in situ for histologic analysis. To determine if obesity-related changes in pulmonary responses to OVA sensitization and challenge were dependent on the modality of obesity induction, similar experiments were performed in obese db/db mice and their lean, wild-type controls.
Some of the results of this study have been previously reported in the form of abstracts (26, 27).
METHODS
Animals
This study was approved by the Harvard Medical Area Standing Committee on Animals. Obese ob/ob and db/db mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Age- and sex-matched wild-type control mice were purchased at the same time. Because both ob/ob and db/db mice were on a C57BL/6J background, wild-type C57BL/6J mice were used as controls.
Allergen Sensitization and Challenge
Wild-type and ob/ob mice were sensitized to chicken egg albumin (OVA, grade V; Sigma-Aldrich Co., St. Louis, MO) on Days 0 and 14 by an intraperitoneal injection of 20 μg of OVA and adjuvant, 2 mg of aluminum hydroxide (J.T. Baker, Phillipsburg, NJ) dispersed in 0.2 ml of PBS. On Days 28 through 34, mice were challenged for 30 minutes with an aerosol of either PBS containing 6% OVA (weight/volume) or PBS as previously described (28). Mice were studied 24 hours after the last aerosol challenge. Because we did not observe increases in airway responsiveness in wild-type mice challenged in this manner, we used the same sensitization but a different challenge protocol in our study with db/db mice. Challenge of db/db mice and their controls was performed using 1% rather than 6% solutions of OVA for aerosolization. We also challenged the mice for only 3 days (Days 28, 29, and 30), and we studied the mice at 48 hours rather than 24 hours after the last aerosol challenge.
Measurement of Airway Responsiveness
Mice were anesthetized, instrumented for mechanical ventilation and ventilated with a specialized ventilator (flexiVent; SCIREQ, Inc., Montreal, PQ, Canada) that uses forced oscillation to measure pulmonary mechanics, as previously described (14, 15, 29). The animals were studied open-chested, at a fixed positive end-expiratory pressure (3 cm H2O). Dose–response curves to intravenously administered methacholine were then obtained as reported previously (15, 16).
Blood Collection and BAL
Mice were killed with an overdose of pentobarbital sodium. Blood was drawn, and serum was isolated and stored at −20°C. BAL was performed and the BAL fluid (BALF) cells and differentials were counted as described previously (30). BALF supernatant was stored at −80°C. BALF and/or serum was subsequently analyzed by ELISA for eotaxin, IgE, IL-4, IL-5, IL-13, and soluble tumor necrosis factor receptors 1 and 2 (sTNFR1 and sTNFR2) (BD Biosciences, San Jose, CA, for IgE; R&D Systems, Inc., Minneapolis, MN, for all others). The levels of sTNFR1 and sTNFR2 were measured in the serum of wild-type and obese mice. Both of these soluble cytokine receptors are markers of systemic inflammation (31, 32), a condition that is manifest in both obese humans and mice (16, 31–35).
RNA Extraction and Real-Time Reverse Transcription–Polymerase Chain Reaction
RNA was extracted from lung tissue and real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed as described previously (28). Primer sets and product sizes for murine IL-4, IL-5, IL-13, and β-actin have been reported previously (28, 30). Primer sets and product sizes for IFN-γ and acidic mammalian chitinase (AMCase) were as follows: IFN-γ (forward 5′-TCA AGT GGC ATA GAT GTG GAA GAA-3′ and reverse 5′-TGG CTC TGC AGG ATT TTC ATG-3′ [92 bp]); AMCase (forward 5′-TGG ACA CAC CTT CAT CCT GA-3′ and reverse 5′-AAC AAG CCC TGC TTG ACA AT-3′ [586 bp]). Changes in lung cytokine mRNA transcript copy number were assessed relative to changes in β-actin mRNA transcript copy number.
Histologic Examination of Lung Tissue
After BAL, lungs were fixed in situ with 10% formalin (J.T. Baker) at a pressure of 23 cm H2O. The lungs were then embedded with paraffin, cut into 5- to 6-μm sections, and stained with hematoxylin and eosin. Sections were examined blindly under light microscopy to determine the inflammation score, a product of the severity and prevalence of inflammation, as described by Hamada and colleagues (36). Severity was assigned a numerical value based on the number of inflammatory cell infiltrate layers around the airways and blood vessels (0, no cells; 1, ⩽3 cell layers; 2, 4–9 cell layers; 3, ⩾10 cell layers). The prevalence of inflammation was assigned a numerical value according to the percentage of airways and blood vessels in each section encompassed by inflammatory cells (0, no airways; 1, ⩽25%; 2, 25–50%; 3, >50%). In addition, separate sections from the same animals were subjected to the periodic acid-Schiff reaction to identify the prevalence of carbohydrates and mucoproteins. In this instance, prevalence was assigned based on the aforementioned criteria used to determine the prevalence of inflammation.
Statistical Analysis
Serum eotaxin, sTNFR1, sTNFR2, and total blood leukocytes were analyzed by an unpaired Student t test. All other data were analyzed by analysis of variance (ANOVA) or factorial ANOVA using Statistica software (StatSoft, Tulsa, OK). For BALF cell counts and cytokine mRNA expression, data were logarithmically transformed before analysis because untransformed data were not normally distributed. A P value of less than 0.05 was considered statistically significant.
RESULTS
Baseline Rl and Airway Responsiveness to Methacholine in ob/ob Mice
The total body mass of ob/ob mice was substantially greater than that of age- and sex-matched wild-type C57BL/6 mice (54.8 ± 0.7 and 21.6 ± 0.4 g, respectively). ANOVA indicated a significant effect of treatment group on baseline Rl (P < 0.05). Compared with PBS challenge, OVA challenge resulted in a significant increase in baseline Rl in ob/ob mice (0.88 ± 0.04 and 0.75 ± 0.02 cm H2O/ml/s in OVA- and PBS-challenged mice, respectively; P < 0.05). In contrast, there was no effect of OVA challenge on baseline Rl in wild-type mice (0.73 ± 0.05 and 0.72 ± 0.06 cm H2O/ml/s in OVA- and PBS-challenged mice, respectively).
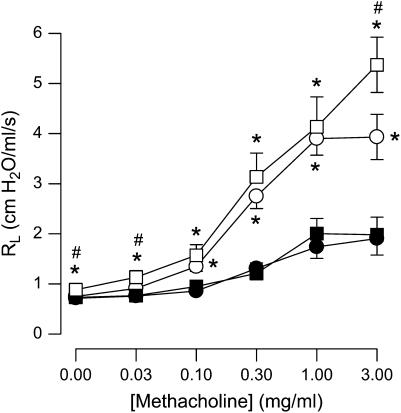
Intravenous administration of methacholine resulted in robust increases in Rl (Figure 1). For all concentrations of methacholine greater than 0.03 mg/ml, ob/ob mice had significantly greater responses to methacholine than wild-type mice regardless of whether the mice were treated with aerosolized PBS or OVA. There was no significant effect of OVA on airway responsiveness in wild-type mice. In ob/ob mice, Rl was greater in OVA-challenged versus PBS-challenged mice at 0.03 and 3 mg/ml but not at other concentrations of methacholine.
Figure 1.
Changes in pulmonary resistance (Rl) induced by intravenous methacholine in ovalbumin (OVA)-sensitized wild-type (C57BL/6) and ob/ob mice challenged with aerosols of either phosphate-buffered saline (PBS) or OVA once per day for 7 consecutive days. Responses were measured 24 hours after the cessation of the final aerosol challenge. n = 9–10 mice for each group. Solid circles, wild-type (PBS); solid squares, wild-type (OVA); open circles, ob/ob (PBS); open squares, ob/ob (OVA). *P < 0.05 compared with wild-type (C57BL/6) mice with an identical exposure; #P < 0.05 compared with genotype-matched, PBS-challenged controls.
Serum IgE after OVA Challenge in ob/ob Mice
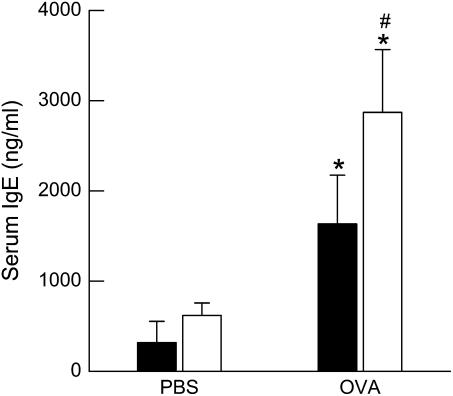
In PBS-challenged mice, there was no difference in total serum IgE between wild-type and ob/ob mice. OVA challenge caused a significant increase in serum IgE in wild-type and ob/ob mice (P < 0.01). The magnitude of the effect of OVA challenge depended on genotype such that ob/ob mice had greater total serum IgE after OVA challenge than wild-type mice (Figure 2).
Figure 2.
Concentration of total IgE in the serum of ovalbumin (OVA)-sensitized wild-type (C57BL/6) and ob/ob mice challenged with aerosols of either phosphate-buffered saline (PBS) or OVA once per day for 7 consecutive days. Blood was collected and serum was isolated 24 hours after the cessation of the final aerosol challenge. n = 6–10 mice for each group. Solid bars, wild-type; open bars, ob/ob. *P < 0.05 compared with genotype-matched, PBS-challenged controls. #P < 0.05 compared with wild-type (C57BL/6) mice with an identical exposure.
BALF Cell Profile and Histopathology after OVA Challenge in ob/ob Mice
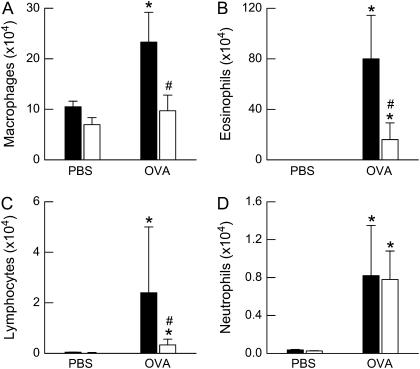
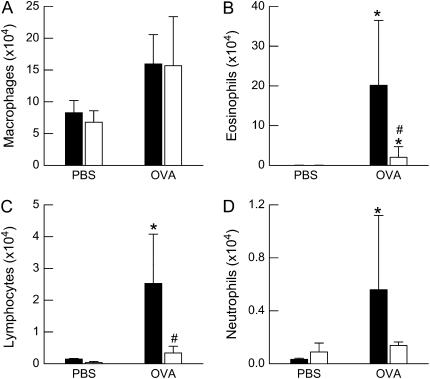
Factorial ANOVA indicated a significant effect of OVA challenge on all BALF cell types (P < 0.01 in each case; Figure 3). There was also a significant effect of genotype on BALF macrophages (P < 0.01), eosinophils (P < 0.01), and lymphocytes (P < 0.02), but no effect on BALF neutrophils, such that after OVA challenge, cell numbers were higher in wild-type than in ob/ob mice.
Figure 3.
Total number of (A) macrophages, (B) eosinophils, (C) lymphocytes, and (D) neutrophils in the bronchoalveolar lavage fluid (BALF) of ovalbumin (OVA)-sensitized wild-type (C57BL/6) and ob/ob mice challenged with aerosols of either phosphate-buffered saline (PBS) or OVA once per day for 7 consecutive days. BALF was collected 24 hours after the cessation of the final aerosol challenge. n = 10–14 mice for each group. Solid bars, wild-type; open bars, ob/ob. *P < 0.05 compared with genotype-matched, PBS-challenged controls; #P < 0.05 compared with wild-type (C57BL/6) mice with an identical exposure.
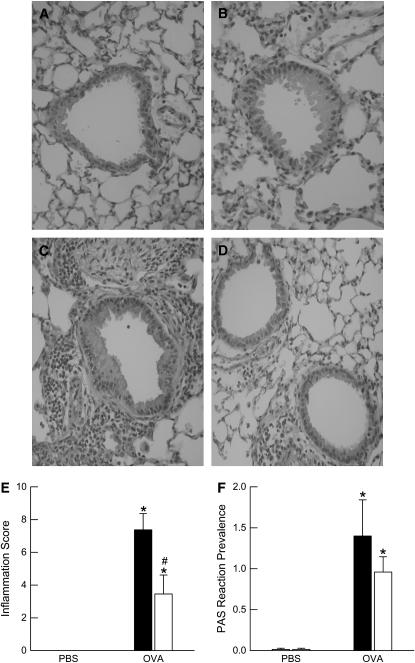
To determine whether the differences we observed in the BALF inflammatory cell profile between wild-type and ob/ob mice were also observed in the lung tissue, we performed histopathologic analysis on sections of lung tissue from PBS- and OVA-challenged wild-type and ob/ob mice. Representative hematoxylin-and-eosin–stained histologic lung sections are shown in Figures 4A–4D to illustrate the differences in pulmonary inflammation between PBS- and OVA-challenged wild-type and ob/ob mice. Compared with PBS challenge, OVA increased the inflammation score in both wild-type and ob/ob mice (Figure 4E). However, the inflammation score was significantly greater in wild-type versus ob/ob mice after OVA challenge, consistent with the BALF cell data. In addition, we determined the prevalence of carbohydrates and mucoproteins in the lung tissue of these same animals (Figure 4F). Although OVA challenge significantly increased carbohydrate and mucoprotein staining in the lungs of both wild-type and ob/ob mice when compared with their genotype-matched, PBS-challenged controls, there were no differences in the degree of staining between genotypes after OVA challenge.
Figure 4.
(A–D) Representative hematoxylin-and-eosin–stained histologic sections, (E) inflammation score, and (F) periodic acid-Schiff (PAS) reaction prevalence of carbohydrates and mucoproteins from the lungs of ovalbumin (OVA)-sensitized wild-type (C57BL/6) and ob/ob mice challenged with aerosols of either phosphate-buffered saline (PBS) or OVA once per day for 7 consecutive days. A and B are sections from PBS-challenged wild-type and ob/ob mice, respectively. C and D are sections from OVA-challenged wild-type and ob/ob mice, respectively. Lungs were fixed in situ with 10% formalin 24 hours after the cessation of the final aerosol challenge. n = 6–7 mice for each group. Solid bars, wild-type; open bars, ob/ob. *P < 0.05 compared with genotype-matched, PBS-challenged controls; #P < 0.05 compared with wild-type (C57BL/6) mice with an identical exposure.
BALF Cytokine/Chemokine Profile and Lung Tissue Cytokine mRNA Expression after OVA Challenge in ob/ob Mice
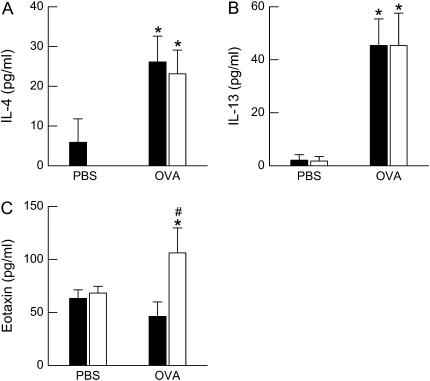
Compared with PBS challenge, OVA challenge caused a significant increase in the BALF concentrations of both IL-4 and IL-13 (Figures 5A and 5B). However, there was no significant effect of genotype on BALF IL-4 or IL-13 and no interaction between genotype and challenge. Compared with PBS challenge, OVA challenge significantly increased BALF eotaxin in ob/ob but not wild-type mice, and BALF eotaxin concentrations were greater in OVA-challenged ob/ob versus wild-type mice (Figure 5C). IL-5 was undetectable in the BALF of wild-type and ob/ob mice challenged with either PBS or OVA (data not shown).
Figure 5.
Concentration of total (A) IL-4, (B) IL-13, and (C) eotaxin in the bronchoalveolar lavage fluid (BALF) of ovalbumin (OVA)-sensitized wild-type (C57BL/6) and ob/ob mice challenged with aerosols of either phosphate-buffered saline (PBS) or OVA once per day for 7 consecutive days. BALF was collected 24 hours after the cessation of the final aerosol challenge. n = 4–10 mice for each group. Solid bars, wild-type; open bars, ob/ob. *P < 0.05 compared with genotype-matched, PBS-challenged controls; #P < 0.05 compared with wild-type (C57BL/6) mice with an identical exposure.
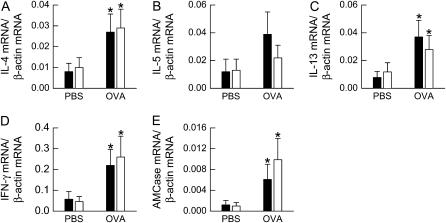
To examine cytokine expression in lung tissue, we measured lung IL-4, IL-5, IL-13, and IFN-γ mRNA transcript copy number by real-time RT-PCR (Figure 6) and normalized expression of each of these genes by β-actin mRNA transcript copy number. Compared with PBS challenge, OVA challenge caused a significant increase in the mRNA expression of IL-4, IL-13, and IFN-γ (P < 0.05 in each cases); however, there was no effect of genotype on gene expression and no interaction between genotype and challenge. Although there were no obesity-related changes in IL-4 and IL-13 expression, we wished to determine whether there were differences in the downstream effects of IL-4 and IL-13 signaling in the lungs of ob/ob mice that may explain the enhanced effect of OVA challenge on changes in airway responsiveness and serum IgE in these mice. Therefore, we examined the pulmonary expression of AMCase, which promotes allergen-induced AHR in mice and whose expression is IL-13 dependent (37). Compared with PBS challenge, OVA challenge increased AMCase expression, but the magnitude of the increase was not different between wild-type and ob/ob mice (Figure 6E).
Figure 6.
mRNA expression of (A) IL-4, (B) IL-5, (C) IL-13, (D) IFN-γ, and (E) acidic mammalian chitinase (AMCase) in the lung tissue of ovalbumin (OVA)-sensitized wild-type (C57BL/6) and ob/ob mice challenged with aerosols of either phosphate-buffered saline (PBS) or OVA once per day for 7 consecutive days. Lung tissue was collected 24 hours after the cessation of the final aerosol challenge. mRNA transcript copy number was normalized to β-actin transcript copy number. n = 4–10 mice for each group. Solid bars, wild-type; open bars, ob/ob. *P < 0.05 compared with genotype-matched, PBS-challenged controls.
Effect of Obesity on Systemic Markers of Inflammation
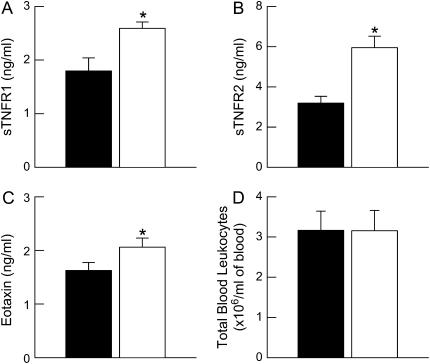
In both humans and mice, obesity is associated with chronic, low-grade systemic inflammation characterized by increases in the serum concentrations of cytokines, soluble cytokine receptors, chemokines, and acute-phase proteins as well as the number of blood leukocytes (16, 17, 31, 35, 38–41). To assess systemic inflammation in ob/ob versus wild-type mice, we measured the serum levels of eotaxin, sTNFR1, and sTNFR2 by ELISA and enumerated the total number of blood leukocytes from mice of both genotypes challenged with PBS. Eotaxin, sTNFR1, and sTNFR2 were significantly increased in serum from ob/ob compared with wild-type mice (Figure 7). There were no differences in the total number of blood leukocytes between wild-type and ob/ob mice.
Figure 7.
Concentrations of total serum (A) soluble tumor necrosis factor receptor 1 (sTNFR1), (B) sTNFR2, (C) eotaxin, as well as (D) total number of blood leukocytes from ovalbumin (OVA)-sensitized wild-type (C57BL/6) and ob/ob mice challenged with aerosols of phosphate-buffered saline (PBS) once per day for 7 consecutive days. Blood was collected and serum was isolated 24 hours after the cessation of the final aerosol challenge. n = 5–14 for each group. Solid bars, wild-type; open bars, ob/ob. *P < 0.05 compared with wild-type (C57BL/6) mice.
Responses to OVA in db/db Mice
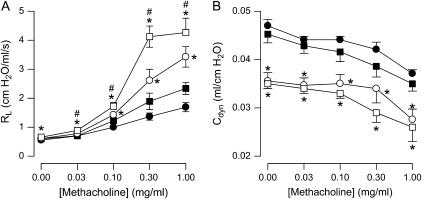
Because our data indicated only very limited effects of OVA on airway responsiveness (Figure 1) and because the ability of OVA to induce AHR in C57BL/6 mice can vary with the precise nature of the OVA-challenge protocol (42), we repeated a limited number of experiments using a different aerosol challenge protocol involving a lower concentration of OVA and fewer challenge days, which has been shown by others to elicit significant increases in airway responsiveness to methacholine in wild-type C57BL/6 mice (43, 44). These experiments were performed in db/db mice, which have a phenotype very similar to that of ob/ob mice. The db/db mice were substantially obese, weighing 47.6 ± 1.5 g versus 25.3 ± 0.5 g for age- and sex-matched wild-type mice. Airway responsiveness to methacholine was significantly greater in db/db than wild-type mice regardless of whether the mice were challenged with PBS or with OVA (Figure 8A). Compared with PBS challenge, OVA challenge caused a significant increase in airway responsiveness in db/db mice. A similar trend was observed in the wild-type mice, but it was smaller in magnitude and did not reach statistical significance. We also measured dynamic compliance (Cdyn) in these mice. Baseline Cdyn was significantly lower in db/db versus wild-type mice in both PBS- and OVA-challenged animals (Figure 8B), consistent with the smaller lungs of db/db mice (16). ANOVA indicated no effect of OVA versus PBS challenge on Cdyn at any dose of methacholine in either genotype.
Figure 8.
Changes in (A) pulmonary resistance (Rl) and (B) dynamic compliance (Cdyn) induced by intravenous methacholine in ovalbumin (OVA)-sensitized wild-type (C57BL/6) and db/db mice challenged with aerosols of either phosphate-buffered saline (PBS) or OVA once per day for 3 consecutive days. Responses were measured 48 hours after the cessation of the final aerosol challenge. n = 6–7 mice for each group. Solid circles, wild-type (PBS); solid squares, wild-type (OVA); open circles, db/db (PBS); open squares, db/db (OVA). *P < 0.05 compared with wild-type (C57BL/6) mice with an identical exposure; #P < 0.05 compared with genotype-matched, PBS-challenged controls.
For the cohort of db/db mice and their wild-type controls, factorial ANOVA indicated a significant effect of OVA challenge on all BALF cell types (P < 0.02 in each case; Figure 9). There was also a significant effect of genotype on BALF eosinophils (P < 0.05) and lymphocytes (P < 0.01), such that cell numbers for these cell types were higher in OVA-challenged wild-type versus OVA-challenged db/db mice.
Figure 9.
Total number of (A) macrophages, (B) eosinophils, (C) lymphocytes, and (D) neutrophils in the bronchoalveolar lavage fluid (BALF) of ovalbumin (OVA)-sensitized wild-type (C57BL/6) and db/db mice challenged with aerosols of either phosphate-buffered saline (PBS) or OVA once per day for 3 consecutive days. BALF was collected 48 hours after the cessation of the final aerosol challenge. n = 4–5 mice for each group. Solid bars, wild-type; open bars, db/db. *P < 0.05 compared with genotype-matched, PBS-challenged controls; #P < 0.05 compared with wild-type (C57BL/6) mice with an identical exposure.
DISCUSSION
Our results confirm previous observations of innate AHR in obese mice (Figures 1 and 8). In addition, our results show that OVA challenge caused changes in baseline Rl and airway responsiveness to methacholine to a greater extent in obese than wild-type mice (Figures 1 and 8). Serum IgE levels were also increased to a greater extent in obese versus lean mice after OVA challenge (Figure 2). These changes occurred despite the absence of any differences in Th2 cytokine expression between obese and lean mice (Figures 5 and 6) and in the face of decreased inflammatory cell emigration to the lungs of the obese versus lean mice (Figures 3, 4, and 9).
Airway responsiveness to methacholine was increased in ob/ob and db/db mice compared with wild-type controls even in the absence of OVA challenge (Figures 1 and 8). We have also reported increased airway responsiveness in nonsensitized ob/ob or db/db versus wild-type mice (15, 16), suggesting that this innate AHR did not develop as a result of sensitization. As previously discussed (15–17, 45, 46), this innate AHR is unlikely to be related to the lower functional residual capacity and spontaneous tidal volumes extant in ob/ob and db/db mice, because measurements of pulmonary mechanics were made with the chest wall open and the mice artificially ventilated at the same tidal volume and the same positive end-expiratory pressure. We cannot rule out the possibility that breathing chronically at low lung volume or low tidal volume results in remodeling of the airways in such a manner as to promote AHR, or that there are developmental changes in the airways of ob/ob and db/db mice that lead to AHR. However, as previously discussed, we favor the hypothesis that this innate AHR may be related to chronic, low-grade systemic inflammation. In obese humans, there are increased serum concentrations of cytokines, cytokine receptors, chemokines, and acute-phase proteins that correlate with the presence of diseases common to obesity, including type II diabetes and atherosclerosis (33, 47–49). Our results (Figure 7) also indicate increased serum concentrations of eotaxin, sTNFR1, and sTNFR2 in ob/ob versus wild-type mice, supporting our previous data showing increased markers of inflammation in the serum of other types of obese mice (16, 17), and extending those data to include increased serum eotaxin. Vasudevan and colleagues (41) have also shown increased serum eotaxin in mice with diet-induced obesity. The relationship between airway inflammation and AHR is certainly complex, but it is conceivable that the AHR observed in PBS-challenged ob/ob and db/db mice may be the result of this low-grade systemic inflammation. For example, TNF-α, which is increased in the serum of obese mice (39), has been shown to augment calcium fluxes and force development in airway smooth muscle (50). Indeed, preliminary data from our laboratory demonstrate that neutralization of TNF-α with an anti–TNF-α antibody attenuates the innate AHR of obese db/db and Cpefat mice (51).
Baseline pulmonary resistance increased in ob/ob but not wild-type mice after OVA challenge. There was also an effect of OVA on responses to some doses of methacholine in ob/ob but not wild-type mice (Figure 1). As previously described, the ability of OVA to induce AHR in C57BL/6 mice is quite variable and depends on the exact challenge protocol used (42). When we used a slightly different OVA-challenge protocol, we observed OVA-induced AHR in db/db mice but not in wild-type controls (Figure 8A). We also examined the impact of OVA challenge on methacholine-induced changes in Cdyn in db/db and wild-type mice. Baseline Cdyn was significantly lower in db/db versus wild-type mice (Figure 8B), which is consistent with the smaller lungs of db/db mice (16). However, there was no effect of OVA challenge on methacholine-induced changes in Cdyn in either genotype. These data suggest that the locus of the AHR observed in db/db mice after OVA challenge is likely the airways rather than the lung parenchyma.
IL-13 has been shown to be particularly important for the generation of AHR in mouse models of allergic asthma (52, 53). However, differences in IL-13 expression do not appear to account for the effects of obesity on OVA-induced changes in Rl or AHR. IL-13 expression did increase with OVA challenge, but the change was virtually identical in wild-type and ob/ob mice (Figures 5 and 6). It is conceivable that there were effects of obesity on IL-4Rα and/or IL-13Rα1 expression and/or their downstream signaling events, but our data suggest that this is unlikely. The pulmonary mRNA expression of AMCase, an IL-13 regulated gene, increased with allergen exposure, as described by others (37), but changes in its expression were not affected by obesity (Figure 6). Instead, obesity-related differences in pulmonary mechanics after allergen exposure appear to occur independently of differences in Th2 cytokine expression or signaling.
There are conflicting data in the literature concerning the role of eosinophils in the development of AHR in murine models of allergic asthma (29, 54, 55). However, it is unlikely that eosinophils contribute to the increased effects of OVA on airway responsiveness observed in obese mice (Figures 1 and 8), because the number of eosinophils was actually reduced in ob/ob versus wild-type mice (Figure 3), and this was confirmed by histopathologic analysis of the lung tissue (Figure 4). Similarly, OVA challenge resulted in enhanced airway responsiveness in db/db versus wild-type mice (Figure 8), even though OVA-induced increases in BALF eosinophils were substantially lower in db/db than wild-type mice (Figure 9). To determine whether differences in the generation of eosinophil chemotactic factors might contribute to the reduced influx of eosinophils observed in the lungs of ob/ob mice, we measured BALF and serum eotaxin. BALF eotaxin levels were actually greater in ob/ob versus wild-type mice after OVA challenge, but serum levels of eotaxin were also slightly greater in ob/ob versus wild-type mice (Figure 7), a condition that would favor eosinophils remaining in the blood rather than migrating to the airways if the serum eotaxin was derived from a source other than the lung. Indeed, this is a plausible scenario because eotaxin expression and secretion are significantly enhanced in adipose tissue of mice with diet-induced obesity (41). IL-5 is important in eosinophil recruitment (56, 57), but OVA challenge did not significantly increase IL-5 mRNA expression (Figure 6), although there may have been alterations in IL-5 receptor expression and/or signaling that negatively impacted eosinophil recruitment in ob/ob mice. Finally, it is possible that the reduced migration of inflammatory cells to the lungs of OVA-challenged ob/ob and db/db mice could be due to interrupted leptin signaling. For example, Bellmeyer and colleagues have recently reported that db/db mice manifest diminished pulmonary inflammation in response to exposure to prolonged hyperoxia, whereas exogenous leptin promotes hyperoxia-induced inflammation (58). However, we think that alterations in leptin signaling are unlikely to explain the reduced emigration of inflammatory cells to the lungs of ob/ob and db/db mice in this study because we have previously reported that exogenous leptin administration has no effect on the recruitment of inflammatory cells after OVA sensitization and challenge (28). Similarly, we do not think that leptin deficiency is the basis for the OVA-induced changes in AHR in ob/ob and db/db mice, because exogenously administered leptin has no effect on baseline airway responsiveness and augments, rather than inhibits, OVA-induced AHR in BALB/c mice (28).
It is possible that the enhanced ability of OVA to augment airway responsiveness in obese mice is related to some aspect of their phenotype other than obesity. For example, ob/ob and db/db mice demonstrate fasting hyperglycemia and hyperinsulinemia (18), and we cannot rule out the possibility that these differences might have influenced their response to OVA. The ob/ob and db/db mice also have increased serum corticosterone (18). Corticosteroids have been shown to attenuate OVA-induced increases in inflammatory cell emigration to the lungs in rats and mice (59, 60), suggesting that obesity-related increases in corticosterone may be the cause of the reduced cell migration into the airways of the obese mice (Figures 3, 4, and 9). However, the effect of obesity-related increases in corticosterone on the development of AHR is more difficult to assess, because previous reports have demonstrated that either increases or decreases in serum corticosterone can attenuate the development of OVA-induced pulmonary mechanical responses (59, 60).
The augmentation of airway responsiveness after OVA challenge observed in obese mice may be related to their enhanced IgE production/release. We observed increased serum IgE after OVA challenge of ob/ob versus wild-type mice (Figure 2). The OVA-induced increase in IgE is consistent with other reports in mice that indicate that allergen challenge is an important stimulus for IgE production (61). Cross-linking FcεRI receptors on mast cells upon binding of allergen to IgE results in the secretion of a panel of mediators, such as leukotrienes, which have the capacity to elicit AHR, and others have reported increased mast cell numbers in the tracheal mucosa of obese mice upon sensitization by OVA (62). The relationship between allergen-induced AHR and allergen-induced IgE production in mice is controversial (for review, see Shore [42]), but it is interesting to note that the expression of 5-lipoxygenase activating protein is substantially increased in the adipose tissue of ob/ob mice (63). This could lead to greater leukotriene synthesis and subsequent release from activated mast cells and ultimately enhance airway responsiveness. In this context, Peters-Golden and colleagues recently reported a more beneficial effect of the leukotriene antagonist montelukast in obese versus lean individuals with asthma (64).
Finally, it is possible that, in obese mice, reductions in the adipokine adiponectin could result in augmentation of OVA-induced AHR. Serum adiponectin levels are reduced in obese mice (16). Furthermore, administration of exogenous adiponectin has been demonstrated to abrogate the development of OVA-induced AHR in mice (65).
The relationship between obesity and atopy has been less well studied in humans than the relationship between obesity and asthma. There are some epidemiologic studies indicating that higher body mass index is associated with increased prevalence of atopy (22, 23), but this is not always observed (24, 25). In this study, we observed increased serum IgE in ob/ob compared with wild-type mice challenged with OVA, whereas there was no significant effect of genotype on IgE in PBS-challenged mice (Figure 2). Because the ob/ob mice were obese even at the time of sensitization, these results indicate that obesity is exerting its effect during the allergen challenge rather than the sensitization phase of the response. We do not think that lack of leptin was the cause of the difference in IgE, because we have previously reported that exogenous leptin augments rather than inhibits OVA-induced increases in serum IgE in BALB/c mice (28). Th2 cytokines, particularly IL-4, impact B-cell class switching to IgE, but we did not observe any difference in BALF or lung tissue IL-4 expression between ob/ob and wild-type mice. It is possible that obesity has other effects that impact on IgE synthesis, such as effects on B-cell function.
In conclusion, we report, that after OVA sensitization and challenge, obese mice exhibit enhanced airway responsiveness to methacholine as well as greater synthesis/release of IgE than lean wild-type controls. These increased effects of OVA are observed in the absence of any differences in Th2 cytokines and even in the face of reduced pulmonary cellular inflammation. Taken together with our results from obese mice exposed to O3, these data suggest that obesity augments mechanical responses of the airways to multiple asthma triggers.
Supported by National Heart, Lung, and Blood Institute grant HL-33009, National Institute of Environmental Health Sciences grants ES-013307 and ES-00002, American Lung Association Research Training Fellowship RT-41-N (to R.A.J.), and a generous gift from Paul and Mary Finnegan.
Originally Published in Press as DOI: 10.1164/rccm.200702-323OC on July 19, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gidding SS, Nehgme R, Heise C, Muscar C, Linton A, Hassink S. Severe obesity associated with cardiovascular deconditioning, high prevalence of cardiovascular risk factors, diabetes mellitus/hyperinsulinemia, and respiratory compromise. J Pediatr 2004;144:766–769. [DOI] [PubMed] [Google Scholar]
- 2.Mishra V. Effect of obesity on asthma among adult Indian women. Int J Obes Relat Metab Disord 2004;28:1048–1058. [DOI] [PubMed] [Google Scholar]
- 3.Ronmark E, Andersson C, Nystrom L, Forsberg B, Jarvholm B, Lundback B. Obesity increases the risk of incident asthma among adults. Eur Respir J 2005;25:282–288. [DOI] [PubMed] [Google Scholar]
- 4.Wickens K, Barry D, Friezema A, Rhodius R, Bone N, Purdie G, Crane J. Obesity and asthma in 11–12 year old New Zealand children in 1989 and 2000. Thorax 2005;60:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camargo CA Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med 1999;159:2582–2588. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med 2001;163:1344–1349. [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Mott J, Ferdinands JM, Camargo CA, Friedman M, Greves HM, Redd SC. Boys with high body masses have an increased risk of developing asthma: findings from the National Longitudinal Survey of Youth (NLSY). Int J Obes (Lond) 2006;30:6–13. [DOI] [PubMed] [Google Scholar]
- 8.Nystad W, Meyer HE, Nafstad P, Tverdal A, Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol 2004;160:969–976. [DOI] [PubMed] [Google Scholar]
- 9.Dixon JB, Chapman L, O'Brien P. Marked improvement in asthma after Lap-Band surgery for morbid obesity. Obes Surg 1999;9:385–389. [DOI] [PubMed] [Google Scholar]
- 10.Stenius-Aarniala B, Poussa T, Kvarnstrom J, Gronlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 2000;320:827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aaron SD, Fergusson D, Dent R, Chen Y, Vandemheen KL, Dales RE. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 2004;125:2046–2052. [DOI] [PubMed] [Google Scholar]
- 12.Spivak H, Hewitt MF, Onn A, Half EE. Weight loss and improvement of obesity-related illness in 500 US patients following laparoscopic adjustable gastric banding procedure. Am J Surg 2005;189:27–32. [DOI] [PubMed] [Google Scholar]
- 13.Thomson CC, Clark S, Camargo CA Jr. Body mass index and asthma severity among adults presenting to the emergency department. Chest 2003;124:795–802. [DOI] [PubMed] [Google Scholar]
- 14.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol 2004;96:2200–2206. [DOI] [PubMed] [Google Scholar]
- 15.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 2003;95:938–945. [DOI] [PubMed] [Google Scholar]
- 16.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 2006;290:L856–L865. [DOI] [PubMed] [Google Scholar]
- 17.Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol 2006;290:R126–R133. [DOI] [PubMed] [Google Scholar]
- 18.Leibel RL, Chung WK, Chua SC Jr. The molecular genetics of rodent single gene obesities. J Biol Chem 1997;272:31937–31940. [DOI] [PubMed] [Google Scholar]
- 19.Fauroux B, Sampil M, Quenel P, Lemoullec Y. Ozone: a trigger for hospital pediatric asthma emergency room visits. Pediatr Pulmonol 2000;30:41–46. [DOI] [PubMed] [Google Scholar]
- 20.Gent JF, Triche EW, Holford TR, Belanger K, Bracken MB, Beckett WS, Leaderer BP. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 2003;290:1859–1867. [DOI] [PubMed] [Google Scholar]
- 21.Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, McLachlan CR, Cowan JO, Flannery EM, Herbison GP, Sears MR. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med 2005;171:440–445. [DOI] [PubMed] [Google Scholar]
- 22.Huang SL, Shiao G, Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy 1999;29:323–329. [DOI] [PubMed] [Google Scholar]
- 23.Schachter LM, Peat JK, Salome CM. Asthma and atopy in overweight children. Thorax 2003;58:1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis D, Chinn S, Potts J, Burney P. Association of body mass index with respiratory symptoms and atopy: results from the European Community Respiratory Health Survey. Clin Exp Allergy 2002;32:831–837. [DOI] [PubMed] [Google Scholar]
- 25.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP). Thorax 2003;58:1036–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera-Sanchez YM, Johnston RA, Theman TA, Shore SA. Impact of obesity on allergic airway responses in mice [abstract]. Am J Respir Crit Care Med 2004;169:A762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu M, Williams ES, Lang JE, Johnston RA, Shore SA. Allergic airway responses in obese db/db mice [abstract]. Am J Respir Crit Care Med 2007;175:A912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol 2005;115:103–109. [DOI] [PubMed] [Google Scholar]
- 29.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 30.Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 2005;288:L390–L397. [DOI] [PubMed] [Google Scholar]
- 31.Bullo M, Garcia-Lorda P, Salas-Salvado J. Plasma soluble tumor necrosis factor alpha receptors and leptin levels in normal-weight and obese women: effect of adiposity and diabetes. Eur J Endocrinol 2002;146:325–331. [DOI] [PubMed] [Google Scholar]
- 32.Hotamisligil GS, Arner P, Atkinson RL, Spiegelman BM. Differential regulation of the p80 tumor necrosis factor receptor in human obesity and insulin resistance. Diabetes 1997;46:451–455. [DOI] [PubMed] [Google Scholar]
- 33.Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab 2002;87:2084–2089. [DOI] [PubMed] [Google Scholar]
- 34.Johnston RA, Theman TA, Terry RD, Williams ES, Shore SA. Pulmonary responses to acute ozone exposure in fasted mice: effect of leptin administration. J Appl Physiol 2007;102:149–156. [DOI] [PubMed] [Google Scholar]
- 35.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282:2131–2135. [DOI] [PubMed] [Google Scholar]
- 36.Hamada K, Suzaki Y, Goldman A, Ning YY, Goldsmith C, Palecanda A, Coull B, Hubeau C, Kobzik L. Allergen-independent maternal transmission of asthma susceptibility. J Immunol 2003;170:1683–1689. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004;304:1678–1682. [DOI] [PubMed] [Google Scholar]
- 38.Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha: effect of weight loss in obese men. Eur J Endocrinol 2003;148:535–542. [DOI] [PubMed] [Google Scholar]
- 39.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91. [DOI] [PubMed] [Google Scholar]
- 40.Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, Fagoaga OR. Influence of obesity on immune function. J Am Diet Assoc 1999;99:294–299. [DOI] [PubMed] [Google Scholar]
- 41.Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. Eotaxin and obesity. J Clin Endocrinol Metab 2006;91:256–261. [DOI] [PubMed] [Google Scholar]
- 42.Shore SA. Asthma and chronic bronchitis: animal models. In: Barnes P, Drazen JM, Rennard S, Thompson NC, editors. Asthma and COPD: basic mechanisms. London: Academic Press; 2002. pp. 79–88.
- 43.Hamelmann E, Takeda K, Haczku A, Cieslewicz G, Shultz L, Hamid Q, Xing Z, Gauldie J, Gelfand EW. Interleukin (IL)-5 but not immunoglobulin E reconstitutes airway inflammation and airway hyperresponsiveness in IL-4-deficient mice. Am J Respir Cell Mol Biol 2000;23:327–334. [DOI] [PubMed] [Google Scholar]
- 44.Makela MJ, Kanehiro A, Borish L, Dakhama A, Loader J, Joetham A, Xing Z, Jordana M, Larsen GL, Gelfand EW. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci USA 2000;97:6007–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther 2006;110:83–102. [DOI] [PubMed] [Google Scholar]
- 46.Shore SA. Obesity and asthma: lessons from animal models. J Appl Physiol 2007;102:516–528. [DOI] [PubMed] [Google Scholar]
- 47.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997;40:1286–1292. [DOI] [PubMed] [Google Scholar]
- 48.Teramoto S, Yamamoto H, Ouchi Y. Increased C-reactive protein and increased plasma interleukin-6 may synergistically affect the progression of coronary atherosclerosis in obstructive sleep apnea syndrome. Circulation 2003;107:E40. [DOI] [PubMed] [Google Scholar]
- 49.Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res 2001;9:414–417. [DOI] [PubMed] [Google Scholar]
- 50.Amrani Y, Panettieri RA Jr. Cytokines induce airway smooth muscle cell hyperresponsiveness to contractile agonists. Thorax 1998;53:713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang JE, Williams ES, Shore SA. TNFα contributes to innate airway hyperresponsiveness in murine obesity [abstract]. Am J Respir Crit Care Med 2007;175:A912. [Google Scholar]
- 52.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261. [DOI] [PubMed] [Google Scholar]
- 54.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773–1776. [DOI] [PubMed] [Google Scholar]
- 55.Wills-Karp M, Karp CL. Biomedicine: eosinophils in asthma: remodeling a tangled tale. Science 2004;305:1726–1729. [DOI] [PubMed] [Google Scholar]
- 56.Hamelmann E, Cieslewicz G, Schwarze J, Ishizuka T, Joetham A, Heusser C, Gelfand EW. Anti-interleukin 5 but not anti-IgE prevents airway inflammation and airway hyperresponsiveness. Am J Respir Crit Care Med 1999;160:934–941. [DOI] [PubMed] [Google Scholar]
- 57.Kaminuma O, Mori A, Suko M, Kikkawa H, Naito K, Okudaira H. Development of lung eosinophilic inflammation by the infusion of IL-5-producing T cell clones. Int Arch Allergy Immunol 1997;114:10–13. [DOI] [PubMed] [Google Scholar]
- 58.Bellmeyer A, Martino JM, Chandel NS, Scott Budinger GR, Dean DA, Mutlu GM. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am J Respir Crit Care Med 2007;175:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eum SY, Maghni K, Hamid Q, Eidelman DH, Campbell H, Isogai S, Martin JG. Inhibition of allergic airways inflammation and airway hyperresponsiveness in mice by dexamethasone: role of eosinophils, IL-5, eotaxin, and IL-13. J Allergy Clin Immunol 2003;111:1049–1061. [DOI] [PubMed] [Google Scholar]
- 60.Turner DJ, Myron P, Powell WS, Martin JG. The role of endogenous corticosterone in the late-phase response to allergen challenge in the brown Norway rat. Am J Respir Crit Care Med 1996;153:545–550. [DOI] [PubMed] [Google Scholar]
- 61.Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med 1999;160:1150–1156. [DOI] [PubMed] [Google Scholar]
- 62.Mito N, Kitada C, Hosoda T, Sato K. Effect of diet-induced obesity on ovalbumin-specific immune response in a murine asthma model. Metabolism 2002;51:1241–1246. [DOI] [PubMed] [Google Scholar]
- 63.Back M, Sultan A, Ovchinnikova O, Hansson GK. 5-Lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res 2007;100:946–949. [DOI] [PubMed] [Google Scholar]
- 64.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J 2006;27:495–503. [DOI] [PubMed] [Google Scholar]
- 65.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 2006;118:389–395. [DOI] [PubMed] [Google Scholar]