Abstract
CART (cocaine- and amphetamine-regulated transcript) peptides appear to be mediators or modulators of psychostimulant drugs. An interesting result in the nucleus accumbens has been that injection of CART peptide has no effect by itself on locomotor activity, but it reduces the locomotor activity induced by cocaine or amphetamine. However, in the ventral tegmental area (VTA), injections of CART peptide have been shown to increase locomotor activity, although to a lesser degree (Kimmel et al. 2000). This study was carried out to clarify the interaction of intra-VTA CART 55-102 and systemic cocaine on locomotor activity. The CART-cocaine interaction has been examined using the rigorous isobolographic approach. This type of analysis permits an assessment of additivity, subadditivity, or synergism of two substances. By measuring locomotor activity and using a range of doses of CART peptide and cocaine, both alone and together, with different dosing strategies, clear evidence of subadditivity was found. CART reduced the locomotor activating effects of systemic cocaine, especially at higher doses of CART. These results imply that intra-VTA CART is not simply acting in the same manner as cocaine, and is likely to oppose the action of cocaine. This has implications for the physiological significance of CART-DA (dopamine) interactions and for medications development.
Keywords: Cocaine, CART, peptides, dopamine, ventral tegmental area, psychostimulant, medications development, cocaine-CART interaction
INTRODUCTION
CART (cocaine- and amphetamine-regulated transcript) peptides are brain-gut neurotransmitters and neuroendocrine factors involved in a variety of functions including reward and reinforcement, feeding and body weight, stress, anxiety and endocrine control (Hunter and Kuhar, 2003; Kuhar et al., 2002). There is strong evidence for reciprocal interactions between CART and mesolimbic dopamine, and that CART is involved in the action of psychostimulants. CART is found in nerve terminals in the ventral tegmental area (VTA) and in neuronal cell bodies and processes in the nucleus accumbens (Dallvechia-Adams et al., 2002; Koylu et al., 1998; Smith et al., 1997). Injection of CART peptide into the cerebral ventricles results in changes in DA (dopamine) turnover in the accumbens (Shieh, 2003; Yang et al., 2004). In some studies, more often those using binge dosing of cocaine, CART mRNA levels are altered in the accumbens (Brenz Verca et al., 2001; Douglass et al., 1995; Fagergren and Hurd, 1999), and some acutely and chronically administered DA-related drugs can alter CART levels in the accumbens (Hunter and Kuhar, 2003). In human postmortem brain tissue from cocaine addicts, CART mRNA levels are changed (Albertson et al., 2004; Tang et al., 2003). At a behavioral level, direct injection of CART peptide into the nucleus accumbens has no effect on locomotor activity when used alone, but it blunts the locomotor activating effects of cocaine when coadministered with cocaine or amphetamine (Jaworski et al., 2003a; Kim et al., 2003). It also blunted the locomotor activating effect of dopamine (Jaworski et al., 2003b).
The lack of effect of CART alone in the accumbens appeared opposite to the effects in the VTA, where it has been shown to slightly increase locomotor activity by a dopaminergic mechanism and to produce a conditioned place preference (Kimmel et al., 2000). Since systemic administration of psychostimulants also causes these effects, it was suggested that CART in the VTA may be part of the mechanism of action of cocaine or amphetamine. In order to more fully understand the interaction of CART peptide and cocaine, we have carried out an analysis of the locomotor activating effects of intra-VTA CART peptide and systemic cocaine individually and together.
Isobolographic analysis can be used to determine if the effect of a combination of two qualitatively similar drugs is subadditive, additive or synergistic. First, detailed dose-response curves for the individual drugs are obtained. Combinations of these drugs are then chosen based on the relative potencies of the drugs. In these studies, we tested two different series of dose combinations. In the first analysis, dose combinations were based on the individual drug doses that yielded the theoretical maximum response for each drug. In the second analysis the dose combinations were based on an effect common to both drugs (an increase of 1500 cm locomotion over baseline). In both analyses, several other diluted dose combinations with the same fixed ratio between the drugs were also tested. Finally, the experimental results of these various drug combinations were compared to theoretical results (which assumes additivity between the drugs) based on the potency information for each drug alone (Grabovsky and Tallarida, 2004; Tallarida, 2000; Tallarida, 2001; Tallarida, et al. 1997).
METHODS
Animals
Male Sprague-Dawley rats (Charles River labs, Charlestown, SC) weighing between 275–400 g at the time of the experiment were used. Separate group of rats were used for each experiment. The rats were maintained on a 12h light-dark cycle (lights on at 7 a.m.) and all experiments were carried out during the light phase. Rats were group housed prior to surgery (for CART only & cocaine + CART groups) and individually housed thereafter. All experiments were carried out according to the National Institute of Health Guide for the Care and Use of Laboratory Animals and approval was given by the Emory Institutional Animal Care and Use Committee.
Surgical and infusion procedures
Rats which had injection cannula implanted were anesthetized with ketamine HCl (70 mg/kg i.p; Henry Schein, Inc., Melville, NY) and medetomidine (0.5 mg/kg i.p; Pfizer, New York, NY). A 26 ga bilateral guide cannula assembly (Plastics One, Roanoke, VA) was implanted 2 mm above the VTA (the injection cannula extended 2 mm past the guide cannula to the target region). Stereotaxic coordinates relative to bregma for the guide cannula were: A/P = −6.0 mm, M/L = ± 0.75 mm, and D/V = −6.2 mm (Paxinos and Watson, 1986). The guide cannula assembly was secured to the skull with small stainless steel screws and dental cement. Dummy cannula extending 0.5 mm beyond the guide cannula tips were inserted and a dust cap was attached to the top of the cannula assembly. After surgery, the rats were given 0.025 mg/kg (sc) buprenorphine (Reckitt Benckiser Pharmaceuticals, Inc., Richmond, VA) once to minimize pain and discomfort and allowed to recover for at least 7 days before experiments.
Stainless steel injection cannula (33 ga) cut to extend 2 mm beyond the guide cannula were used for infusions. PE-10 tubing was used to connect the injection cannula to 25 μl syringes (Hamilton; Reno, NV) which were attached to infusion pumps (Harvard Apparatus; Cambridge, MA). For the infusions, the rats were confined to a small polyethylene box. Each infusion was given in a volume of 0.5 μl/side over 30 sec. The injector cannula was left in place for an additional 30 sec after the infusion, then the injector cannula was removed, and the dummy cannula and dust cap were reinserted. A separate group of rats were used for each of the 6 experiments (above). For the rats that received both CART peptide and cocaine, the i.p. cocaine injection was given 1 minute after the CART infusion. Animals given cocaine alone (i.p.) were not subjected to surgery to keep discomfort to a minimum.
Measurement of locomotor activity
Locomotor activity was measured in photocell cages (Omnitech Electronics; Columbus OH) measuring 40 × 40 × 30 cm. Each cage had 32 photocells (16 front to back and 16 side to side) positioned 5 cm off the cage floor. Each cage was isolated in a stainless steel box equipped with a ventilated fan, 10 W light bulb and a keyhole to observe the rats. The distance traveled (in cm) was calculated by measuring the consecutive breaks of adjacent photocell beams. Operation of the photocell cages and data collection was done by an IBM computer.
For the infusions, each rat was put into the photocell cage for 1 hr for habituation, given the infusion, then returned to the cages for an additional hour. Prior to the start of the experiments, each rat was habituated to the testing procedure and environment by receiving daily sham injections for 3 days. The sham procedure was identical to the testing procedure except injector cannula cut flush to the guide cannula were used and no infusion was given. Separate groups of animals were used for cocaine only, for CART only and for both cocaine and CART. In many experiments over the years we have never seen animals who have successfully recovered from surgery produce significantly different cocaine-induced locomotor responses compared to those with out surgery.
Drugs
Drugs used in this study were rlCART 55-102 (Peptide Institute; Lexington KY) and cocaine hydrochloride (NIDA; Bethesda MD). The numbering of rlCART peptide 55-102 corresponds to that for the long (l) form of proCART protein with 102 amino acids as found in the rat (r). This peptide begins with the amino acids IPIYE and ends at the terminal leucine. Cocaine doses are expressed as the base. Both drugs were dissolved in sterile 0.9% saline.
Statistical analysis
Time course data was analyzed with a 2-way ANOVA with repeated measures on treatment dose and time. The 1 hour data collapsed across time was analyzed with a one-way ANOVA with repeated measures on treatment. Tukey’s post-hoc testing was done for the follow-up comparisons.
Isobolographic analysis of CART peptide and cocaine data
Analysis of the individual dose-response curves for CART peptide and cocaine as well as the simultaneous CART + cocaine dose-response curve and the expected additive effect of both drugs in combination were performed (Grabovsky and Tallarida, 2004; Tallarida, 2000; Tallarida, 2001; Tallarida et al., 1997) using the software package PharmToolsPro (The McCary Group, Elkins Park, PA). The analysis of the combination of two drugs is based on, but generalizes, the conventional concept of isobolar analysis (Grabovsky and Tallarida, 2004). In this method equi-effective doses of drugs A and B are determined from their respective dose-effect equations and this procedure allows the conversion of the dose of one in terms of the equivalent dose of the other. Thus, the B equivalent of the dose of drug A is added to the actual dose of drug B and that sum is used in drug B’s dose effect equation to calculate the additive effect. This is the same concept that is used in the conventional isobolar analysis, but in that approach, one uses dose effect relations that have a constant potency ratio. This can only hold if the dose-effect curves attain the same maximum, and this process leads to linear isoboles. When, however, the drugs differ in efficacy (as in the current application) the calculation still uses equieffective doses, but the isoboles are no longer linear and the additive effect must be determined by getting the equivalent total dose of the higher efficacy drug and using that value in its dose-effect equation to get the additive effect. Stated another way, we match the effects to get equivalent doses. That equivalent (say from drug A in dose a) is used, along with the actual dose of drug B, to get the total equivalent of drug B. Then we put that total in B’s dose-effect equation to get the expected effect. The expected effect is then compared with the actual combination effect as in Fig. 5. This approach applied here and an illustrative example is provided in the appendix.
Figure 5.
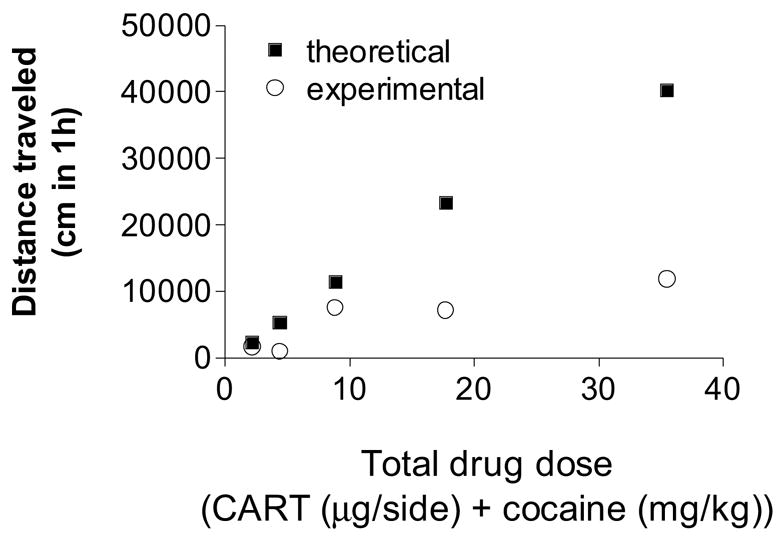
A plot of the theoretical (additive) effect and the observed effect in terms of the locomotive distance traveled after a drug combination whose total is plotted on the horizontal scale. The effect is the locomotive distance over the saline control value. The observed effect is the mean from 8 animals. The two curves (linear regressions) were examined in an overall comparison (ANOVA) of the two regressions (based on the cocaine component) and were found to differ significantly ( P<0.05, F = 73.1).
RESULTS
The locomotor response to several doses of systemic cocaine is shown in Figure 1. Each rat received each of the 9 treatments over 9 separate experimental days in a counterbalanced order. Experimental days were spaced at least 3 days apart. To ensure that the dose-response curve was well-characterized, we tested many doses of cocaine (saline, 1, 2, 3, 4, 6, 10, 20 and 30 mg/kg, i.p.). The time courses of the locomotor response to various doses of cocaine are shown in Figure 1A. A two-way ANOVA revealed that there was a significant main effect for cocaine [F(8,48)=16.01, P < 0.0001] and for time [F(11,66)=10.11, P < 0.0001], as well as a significant cocaine x time interaction [F(88,528)=2.37, P <0.0001]. The total distance traveled in 1 hour by the various doses of cocaine is shown in Figure 1B. Administration of 6.0 and 10 mg/kg of cocaine roughly doubled the distance traveled by the rats given saline, and treatment with 20 and 30 mg/kg cocaine increased locomotion over saline treatment by roughly 8- and 10-fold, respectively. After an overall effect of cocaine was found with a repeated measures one-way ANOVA [F(8,62)=16.18, P < 0.0001], Tukey’s post hoc testing revealed a significant increase (versus saline) in locomotion caused by both 20 and 30 mg/kg cocaine.
Figure 1.
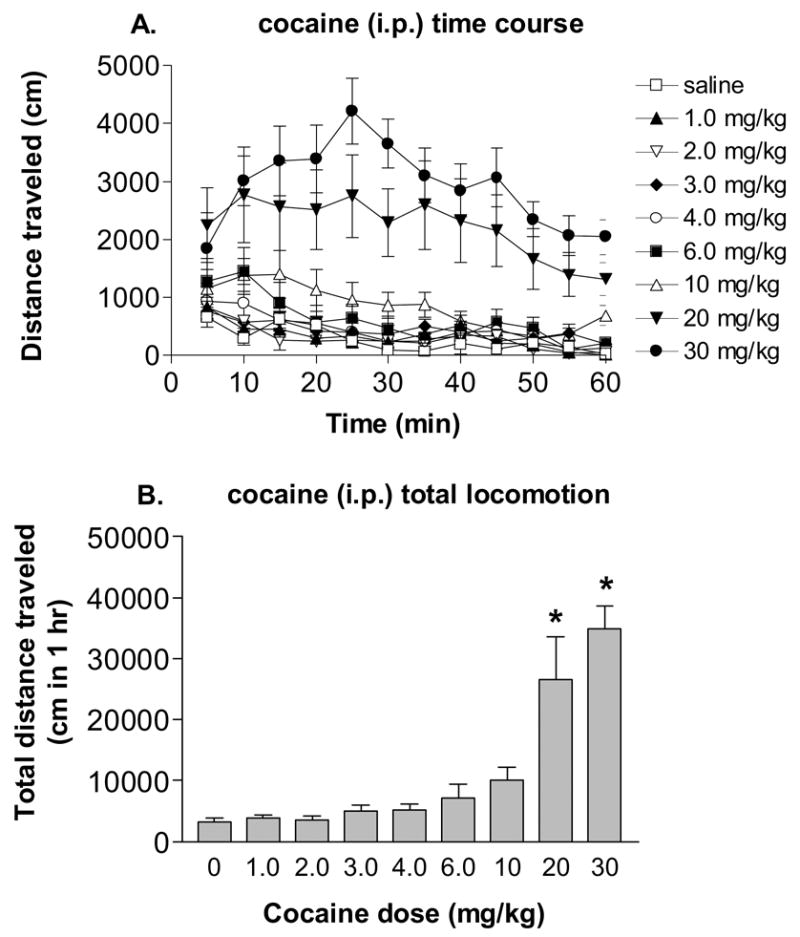
Cocaine (i.p.) dose-dependently increased locomotor activity in rats (mean ± SEM, n = 7). A. Temporal data for the first hour after cocaine administration. B. The same data summed across time (1 hr) for each dose. * - significantly different from saline treatment, p<0.05.
The locomotor response to bilateral intra-VTA injection of rlCART 55-102 peptide is shown in Figure 2. Five doses of CART peptide plus saline were tested (0.04, 0.2, 0.6, 1.0 and 2.5 μg/side) to produce a well-characterized locomotor dose-response curve. The time course data for intra-VTA CART is shown in Figure 2A and is nearly identical to previous results in our lab (Kimmel et al., 2000). Higher doses of CART peptide were not tested here because of the production of seizures (Kimmel et al 2000) A two-way ANOVA revealed that there was a significant main effect for CART peptide [F(5,25)=6.61, P < 0.0005] and for time [F(11,55)=12.24, P < 0.0001], as well as a significant CART peptide x time interaction [F(55,275)=2.37, P <0.0001]. The total distance traveled in 1 hour after these intra-VTA CART injections is shown in Figure 2B. After an overall effect of intra-VTA CART was found with a repeated measures one-way ANOVA [F(5,35)=6.609, P < 0.0005], Tukey’s post hoc testing revealed a significant increase (versus saline) in locomotion caused by the 3 highest doses of CART peptide (0.6, 1.0 and 2.5 μg/side).
Figure 2.
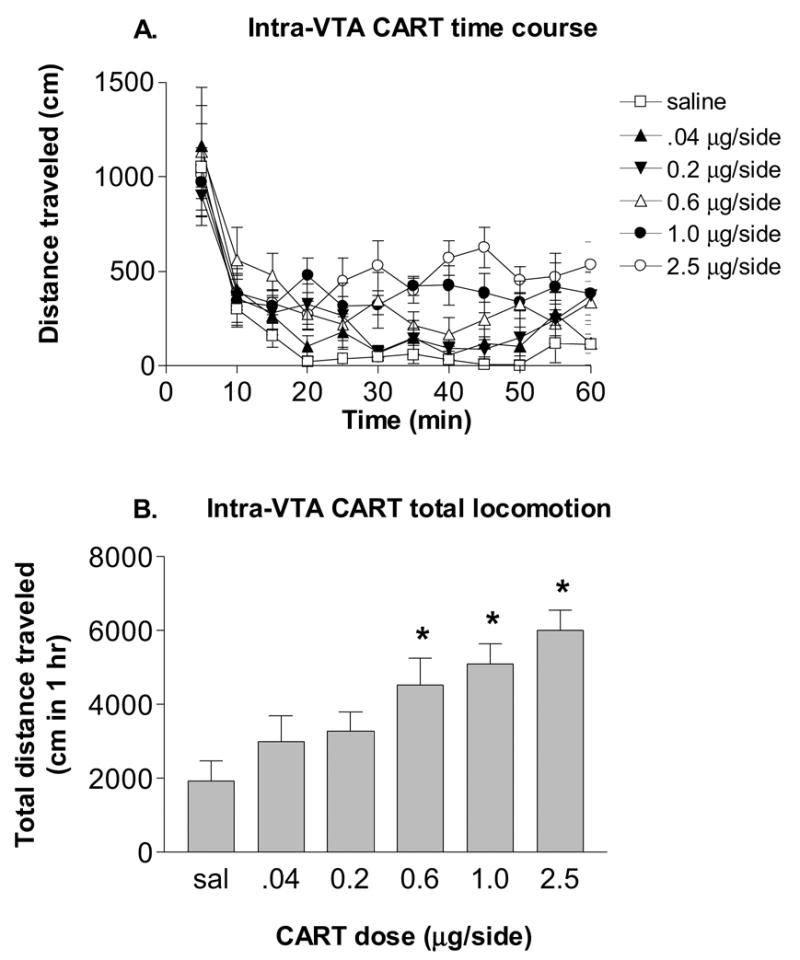
Bilateral intra-VTA CART 55-102 dose-dependently increased locomotor activity in rats (mean ± SEM, n = 6). A. Temporal data for the first hour after CART administration. B. The same data summed across time (1 hr) for each dose. * - significantly different from saline treatment, p<0.05.
The locomotor data for each dose of the respective agent yielded the drug effect after subtraction of the saline control. It is seen that cocaine produces large locomotor effects compared to those of CART. The consequent dose-effect data allow the generation of dose-effect curves that are obtained by nonlinear regression analysis. These curves, derived from group means, are shown in Figure 3 and the parameters describing these curves (given in the legend) lead to nonlinear additive isoboles (not shown). Terms are as follows: For cocaine, E = effect, EB = maximum (theoretical ) effect for this drug, B = dose, B50 = dose for 50% of the maximum effect, and p is a parameter related to the slope of the curve. For CART, E = effect, EC = its maximum (theoretical) effect, A = dose, AC = dose of CART that gives E = EC/2, and q is its curve fitting parameter. The locomotor response to a simultaneous administration of bilateral intra-VTA rlCART 55-102 peptide and systemic cocaine is shown in Figure 4. A fixed-ratio dose combination may use virtually any ratio. In our experiment that ratio was determined from examination of the fitted curves of the individual agents that are shown in Fig. 3. [ More specifically, we chose the combination proportions to be approximately the same as the parameter ratio AC : B50]. In our experiment that ratio was determined from the parameters (B50 and AC: see appendix) of the respective nonlinear regression equations and resulted in proportions (CART 1.00 : cocaine 37.99). The time course data for the simultaneous administration treatment is shown in Figure 4A. A two-way ANOVA revealed that there was a significant main effect of drug treatment [F(5,35)=4.37, P < 0.005] and time [F(11,77)=19.53, P < 0.0001], as well as a significant drug treatment x time interaction [F(55,385)=1.17, P < 0.0001]. The total distance traveled in 1 hour after the treatment combinations is shown in Figure 4B. After an overall effect of the drug combination was found with a repeated measures one-way ANOVA [F(5,47)=4.742, P < 0.005], Tukey’s post hoc testing revealed a significant increase (versus saline, saline) in locomotion caused by the highest dose combination of CART peptide and cocaine (i.p.).
Figure 3.
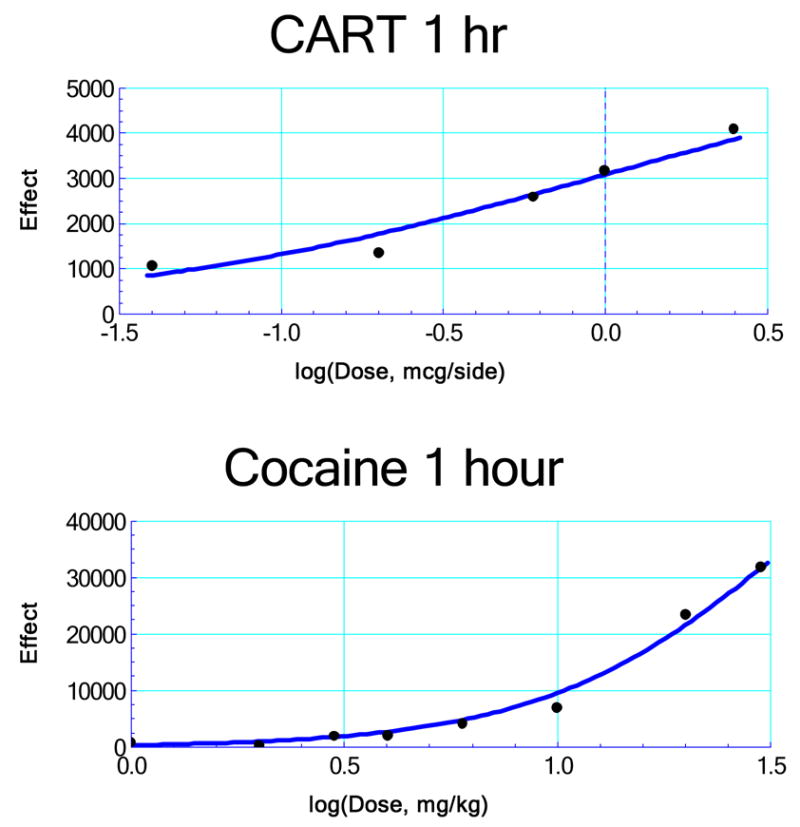
Dose-effect relations for the individual agents, where the drug effect is the difference between the observed and the saline control value. For cocaine the data yielded an equation given by, , EB = 73030, B50 = 34.55, p = 1.596. For CART the equation is given by, , EC = 6114, AC = 0.924, q = 0.688. The use of these in calculating the additive (predicted) effect of any combination is illustrated by an example in the appendix. See Results section for definition of symbols.
Figure 4.
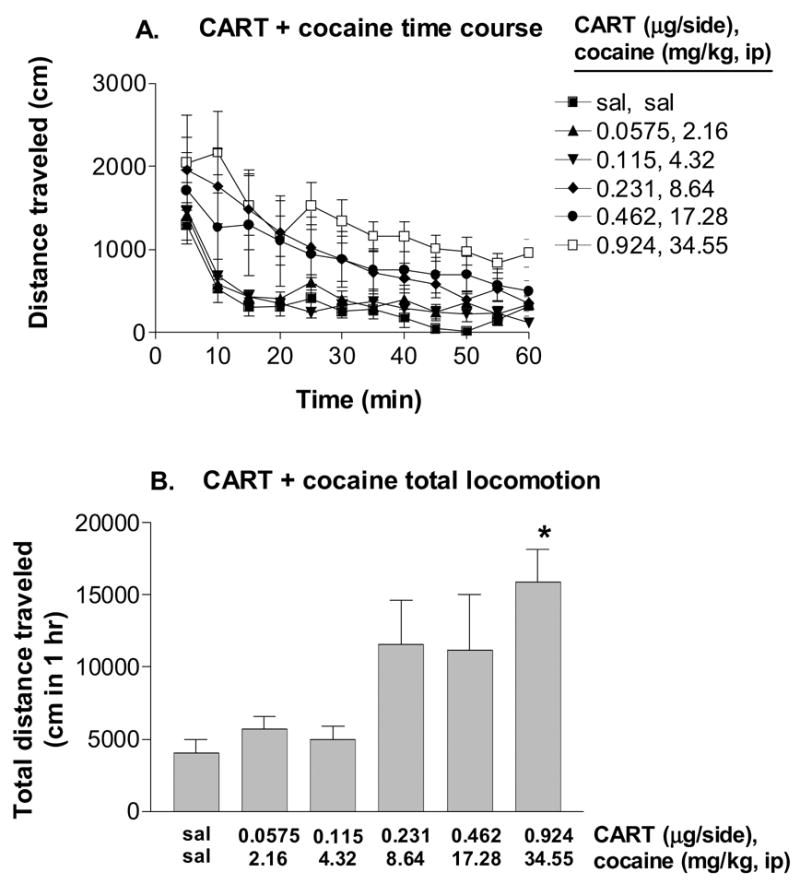
Effects of a combination of doses of bilateral intra-VTA CART 55-102 and cocaine (i.p.) on locomotor activity in rats (mean ± SEM, n = 8). A. Time course data for the first hour after simultaneous administration of CART and cocaine. The doses that were combined are given on the right side of the figure. B. The same data as in A but the distance summed across the full time course is added up foe wach combination of doses. The doses that were combined for simultaneous administration aer given on the two lines of the x-axis. * - significantly different from saline treatment, p<0.05.
The calculated additive effect of each dose combination is obtained by determining (from their respective equations) the dose of one agent that is equivalent to the dose of the other. Accordingly, any dose combination is converted to its equivalent in terms of one of the drugs (in this case, cocaine). From this procedure the additive (expected) effect is calculated. A sample calculation is provided in the appendix and a display of the results is given in Table 1 and Figure 5. An ANOVA analysis of the two curves using regression analysis confirmed that the predicted additive curve differed significantly from the observed curve ( F=73.1, P<0.05) (Figure 5). Stated differently, the presence of a fixed proportion of CART (CART 1.00: cocaine 37.99) reduces the locomotor effect of cocaine. Accordingly, this drug combination is sub-additive for the enhancement of locomotion.
Table 1.
Theoretically additive and observed effects of combinations of CART and Cocaine on locomotion
| CART ug/side | Cocaine, mg/kg | Effect (additive) | Effect (observed) |
|---|---|---|---|
| 0.924 | 34.55 | 40164 | 11801 |
| 0.462 | 17.28 | 23149 | 7086 |
| 0.231 | 8.64 | 11311 | 7463 |
| 0.115 | 4.32 | 5246 | 908 |
| 0.0575 | 2.16 | 2295 | 1625 |
Data are total distance traveled (cm in 1 hour). See text for additional details.
Because this result was based on the ratio 1:38 of CART to cocaine, we conducted an additional (though limited) combination study that used different proportions of the constituents, viz. (1: 2.19), the latter ratio determined by comparing the respective doses for an effect = 1500 (saline values subtracted). This effect level, attained by each drug individually, is clearly within the range of doses (of each) that were actually employed.
Specifically, we employed (CART, cocaine) combinations as follows: (0.079, 0.174), (0.159, 0.348) and (0.318, 0.696). Calculation of the additive effect for each of these combinations yields 1067, 1671 and 2594, respectively. However, the observed effects of these were 0, 600 and 200, respectively, values that are all appreciably less than the additive effect. Because this confirming experiment was limited to just three dose combinations there is no accompanying statistical analysis.
DISCUSSION
CART peptides may act as modulators of mesolimbic dopamine as described in the introduction. Jaworski and colleagues (Jaworski et al., 2003a) recently found that intra-accumbal injection of CART peptide had no locomotor effect by itself, but blunted the locomotor effects of systemic cocaine when coadministered with cocaine. This lack of effect when used alone in the accumbens was different from small apparent cocaine-like effects after intra-VTA injections of CART (Kimmel et al., 2000). Also, in the VTA, CART produced a conditioned place preference (Kimmel et al., 2000). Because of the interesting cocaine-like effect of CART in the VTA, a further analysis of the interaction of CART and cocaine was undertaken.
The method (Grabovsky and Tallarida, 2004) applied here begins with curve fitting to yield equations of effect against dose of the individual compounds. The equations result from the use of monotonically increasing effects of each agent. The two equations allow a determination of equally effective doses and thereby lead to the equivalent dose of one (CART) in terms of the other (cocaine). Thus, any combination of the compounds results in equivalent dose of cocaine from which the effect is calculated from its equation. [This same concept is used in conventional isobolar analysis where the potency ratio is assumed constant, an assumption that does not hold here.] While any dose combination could be used we chose the combinations that insure a monotone increasing relation for cocaine. To accomplish this, we specifically chose the combination doses based on the parameters B50 and Ac, and thus the quantities shown in Table 1 show progressive dilutions that preserve the dose ratio. The set of additive effects were seen to exceed the observed effects of each combination as shown in Table 1 and Figure 5
The approach leading to (Figures 1 – 5) showed that while both cocaine and CART peptide had increasing dose-response curves over the range of doses used, a combination of the peptide and drug did not produce additive effects. From the perspective of CART affecting cocaine, CART blunted or reduced the locomotor effects of cocaine, which is more like what was found in the accumbens for both cocaine and amphetamine (Jaworski et al., 2003a; Kim et al., 2003) and supports the general idea that CART is a modulator of mesolimbic dopamine.
Because drug interactions may depend on the ratio of the constituents we carried out (a limited number of) trials with different dose ratio. Selection of those doses was based on equieffective doses for effect = 1500 cm of locomotion, an arbitrary choice. This led to the three low dose combinations (indicated in the Results) in which the ratio CART: cocaine = 1: 2.19 was used. These combinations were also seen to produce effects that were less than the calculated additive; they were too few for statistical analysis, but they nevertheless confirmed the previous findings. While additional experiments could be carried out with additional doses and strategies, the present data seem strong and adequate to show that CART and cocaine are not simply additive, at least with regard to locomotor effects. Even ignoring the formal isobolographic approach, the data in Table 1 show that the co-administration of both drugs is consistently below the expected additive activity. The mechanism underlying the “sub-additive” effect in these intact animals is unknown but presumably involves anatomical and neurochemical interactions. However, additional experiments will be needed to elucidate the cellular and anatomical mechanisms underlying these data. Further, it is often difficult to determine the role of endogenous compounds by exogenous administration, and this caution must be kept in mind.
At first glance, one might be concerned that the blunting effect of intra-VTA CART peptide on cocaine-induced locomotion may be due to producing additive effects that result in going over an inverted “U” dose-response curve. However, we do not think this is the case for the following reasons. All of the individual doses used were on the ascending limb of the dose-response curve, most on the lowest part of the limb. Most of the doses of cocaine used to show subadditivity were far below the inversion point in the dose-response curve. Our study utilizing drug dosing ratios based on an effect common to both (a dose which produced 1500 cm of locomotion over baseline) used very low doses of drugs which were not near the peak of the inverted U, yet the results were the same – subadditivity, particularly as the doses were increased. Inspection of the coinjection data in Figure 4B reveals no hint of surpassing the peak of an inverted U dose-response curve, even though the two highest doses were strongly subadditive (Figure 5). At the end of the locomotor test there was never any rebound increase in locomotion which would be expected if the rats were initially on the descending limb of the “inverted U” dose-response curve.
It is clear that the different ways of choosing doses did not reveal additivity, but rather we see subadditivity. An isobolographic approach to the interaction of psychostimulants has been carried out previously. For example, the dopamine uptake inhibitor GBR12909 produced either additivity or synergism with amphetamine or cocaine (Holtzman, 2001). Thus, the effects of CART are different from those of a dopamine uptake inhibitor, which suggests that CART is not acting simply like a psychostimulant. It could even be questioned if CART has psychostimulant-like effects, since other kinds of drugs such as ethanol can also increase locomotion (June and Lewis, 1994). Indeed, the locomotor activating effect of CART could possibly be more of a drug-induced perturbation of behavior than a true psychomotor-stimulant effect. However, CART peptide does seem to produce a conditioned place preference (Kimmel et al., 2000), and the possibility that CART is functioning as an apparent partial agonist seems reasonable.
Our findings imply that CART in the VTA and accumbens may oppose or reduce the effects of cocaine. This is perhaps not surprising given that other peptides, such as neurotensin, CCK, and NPY have been shown to have similar effects on stimulant-induced locomotion (Binder et al., 2001). Also, other factors are known to oppose the actions of cocaine. cAMP response element-binding protein (CREB) tends to oppose the rewarding effects of cocaine (Carlezon et al., 1998; Pliakas et al., 2001). Interestingly, CREB activates expression of the CART gene (Barrett et al., 2002; Dominguez et al., 2002). Another factor, NAC-1, which is upregulated by cocaine, tends to oppose behavioral sensitization caused by cocaine (Wang et al., 2003). Thus, a variety of evidence suggests that the large changes in mesolimbic dopamine caused by psychostimulants may have opposing mechanisms in the nucleus accumbens.
In summary, intra-VTA CART and systemic cocaine produced locomotion as previously reported. However, when CART and cocaine were coadministered, the response was substantially less than additive, suggesting that CART in the VTA does not act in the same manner as cocaine and instead actually opposes the action of cocaine. This is similar to the finding where CART was injected into the nucleus accumbens and blunted the effect of systemic cocaine. Taken together, these findings suggest that CART peptide acts in a manner different from that of cocaine.
Acknowledgments
The authors acknowledge the support of NIH grants RR00165, DA00418, DA009793, and DA10732. These experiments comply with the current laws of the USA. Principles of laboratory animal care were followed, as well as specific national laws.
APPENDIX
The dose-effect data for the partial agonist, CART, and the full agonist, cocaine, were fitted to equations of the form and respectively, where p and q are curve-fitting parameters (“Hill coefficients”). The parameter estimates from these are as follows. Ec: 6114.4 ± 1051.5, Ac: 0.924 +/− 0.487 and q: 0.688 for CART, and EB: 73030.2 ± 13722.7, B50: 34.550 +/− 6.472 and p: 1.596 for cocaine.
A combination dose (a,b) is equivalent (under additivity) to dose B of drug B (Grabovsky and Tallarida, 2004) given below.
Accordingly, this calculated B is used to get the expected additive effect. For example, the combination dose (a = 0.462, b = 17.28) and the parameters given in the text, when used in the above equation, yield B = 21.36 from which E = 23149 as shown in the table. This expected additive effect was not attained experimentally for this dose combination. The observed effect was much less, 7086, as noted in the table. Similar calculations produced the other additive values shown in the table from which it is obvious that the interaction is subadditive. See the Results section for additional explanation and definitions of symbols.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–9. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P, Davidson J, Morgan P. CART gene promoter transcription is regulated by a cyclic adenosine monophosphate response element. Obes Res. 2002;10:1291–8. doi: 10.1038/oby.2002.175. [DOI] [PubMed] [Google Scholar]
- Binder E, Kinkead B, Nemeroff C. Neuropeptides. In: Breier A, Tran P, Herrea J, Tollefson G, Bymaster F, editors. Current Issues in the Psychopharmacology of Schizophrenia. Lippincott, Williams & Wilkins; Philadelphia, PA: 2001. pp. 349–371. [Google Scholar]
- Brenz Verca MS, Widmer DA, Wagner GC, Dreyer J. Cocaine-induced expression of the tetraspanin CD81 and its relation to hypothalamic function. Mol Cell Neurosci. 2001;17:303–16. doi: 10.1006/mcne.2000.0942. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–5. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Dallvechia-Adams S, Kuhar MJ, Smith Y. Cocaine- and amphetamine-regulated transcript peptide projections in the ventral midbrain: Colocalization with gamma-aminobutyric acid, melanin-concentrating hormone, dynorphin, and synaptic interactions with dopamine neurons. J Comp Neurol. 2002;448:360–72. doi: 10.1002/cne.10268. [DOI] [PubMed] [Google Scholar]
- Dominguez G, Lakatos A, Kuhar MJ. Characterization of the cocaine- and amphetamine-regulated transcript (CART) peptide gene promoter and its activation by a cyclic AMP-dependent signaling pathway in GH3 cells. J Neurochem. 2002;80:885–93. doi: 10.1046/j.0022-3042.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–81. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagergren P, Hurd YL. Mesolimbic gender differences in peptide CART mRNA expression: effects of cocaine. Neuroreport. 1999;10:3449–52. doi: 10.1097/00001756-199911080-00034. [DOI] [PubMed] [Google Scholar]
- Grabovsky Y, Tallarida RJ. Isobolographic analysis for combinations of a full and partial agonist: curved isoboles. J Pharmacol Exp Ther. 2004;310:981–6. doi: 10.1124/jpet.104.067264. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Differential interaction of GBR 12909, a dopamine uptake inhibitor, with cocaine and methamphetamine in rats discriminating cocaine. Psychopharmacology (Berl) 2001;155:180–6. doi: 10.1007/s002130100684. [DOI] [PubMed] [Google Scholar]
- Hunter R, Kuhar M. Dopaminergic regulation of CART mRNA in the nucleus accumbens. Society for Neuroscience Abstracts. 2003:889.21. [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003a;307:1038–44. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Vicentic A, Hunter RG, Kimmel HL, Kuhar MJ. CART peptides are modulators of mesolimbic dopamine and psychostimulants. Life Sciences. 2003b;27:741–747. doi: 10.1016/s0024-3205(03)00394-1. [DOI] [PubMed] [Google Scholar]
- June HL, Lewis MJ. Interactions of Ro15-4513, Ro15-1788 (flumazenil) and ethanol on measures of exploration and locomotion in rats. Psychopharmacology (Berl) 1994;116:309–16. doi: 10.1007/BF02245334. [DOI] [PubMed] [Google Scholar]
- Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–73. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine- regulated transcript peptide 55-102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–92. [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–32. [PubMed] [Google Scholar]
- Kuhar MJ, Adams S, Dominguez G, Jaworski J, Balkan B. CART peptides. Neuropeptides. 2002;36:1–8. doi: 10.1054/npep.2002.0887. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; New York: 1986. [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh K. Effects of the cocaine- and amphetamine-regulated transcript peptide on the turnover of central dopaminergic neurons. Neuropharmacology. 2003;44:940–948. doi: 10.1016/s0028-3908(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Koylu EO, Couceyro P, Kuhar MJ. Ultrastructural localization of CART (cocaine- and amphetamine- regulated transcript) peptides in the nucleus accumbens of monkeys. Synapse. 1997;27:90–4. doi: 10.1002/(SICI)1098-2396(199709)27:1<90::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and Dose-Effect Data Analysis. Chapman-Hall/CRC; Boca Raton: 2000. [Google Scholar]
- Tallarida RJ. Drug synergism: its detection and applications. J Pharmacol Exp Ther. 2001;298:865–72. [PubMed] [Google Scholar]
- Tallarida RJ, Kimmel HL, Holtzman SG. Theory and statistics of detecting synergism between two active drugs: cocaine and buprenorphine. Psychopharmacology (Berl) 1997;133:378–82. doi: 10.1007/s002130050417. [DOI] [PubMed] [Google Scholar]
- Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–24. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PJ, Stromberg M, Replenski S, Snyder-Mackler A, Mackler SA. The relationship between cocaine-induced increases in NAC1 and behavioral sensitization. Pharmacol Biochem Behav. 2003;75:49–54. doi: 10.1016/s0091-3057(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Yang SC, Pan JT, Li HY. CART peptide increases the mesolimbic dopaminergic neuronal activity: A microdialysis study. Eur J Pharmacol. 2004;494:179–182. doi: 10.1016/j.ejphar.2004.05.018. [DOI] [PubMed] [Google Scholar]


