Abstract
Objectives
To study the effects of short‐term intermediate dose glucocorticoid (GC) therapy in patients with active rheumatoid arthritis (RA) on circulating endothelial progenitor cells (EPC), which are known to influence cardiovascular risk, and to elucidate mechanisms potentially responsible for the reduction of EPCs in patients with active RA.
Methods
EPCs were quantified in 29 patients with active RA by flow cytometry, colony forming unit (CFU) and circulating angiogenic cell (CAC) assays before and after 7 days of intermediate dose GC therapy. CFU from patients with RA and from healthy referents (HR) were cultured in vitro in the absence or presence of dexamethasone (Dex) and/or TNF.
Results
After 1 week of GC therapy, EPC increased from 0.026 (SD 0.003)% to 0.053 (SD 0.010)% (p<0.01), and from 12 (SD 4) to 27 (SD 7) CFU/well (p<0.02); CAC also increased from 7 (SD 2) to 29 (SD 8) cells/high power field (p<0.05). In parallel, disease activity decreased significantly after GC treatment. TNF serum levels also decreased from 36 (SD 10) to 14 (SD 6) pg/ml (p<0.0001). Addition of Dex to the RA CFU led to a significant increase of mean CFU counts, whereas addition of TNF induced a decrease of CFU.
Conclusions
Our data indicate that TNF may be at least partly responsible for the reduction of EPC seen in patients with RA. Intermediate doses of GCs for a short period of time, apart from reducing disease activity, significantly increase circulating EPC.
Endothelial progenitor cells (EPC) contribute to new vessel formation (vasculogenesis) in adults.1 Their reduction has been shown to be predictive of cardiovascular outcomes,2,3 to correlate with other risk factors of coronary artery disease4 and therefore a surrogate biological marker for increased cardiovascular risk.5 Another cell population, circulating angiogenic cells (CAC), which are derived from the monocyte/macrophage lineage and carry particular surface markers, contribute to angiogenesis by their ability to secrete proangiogenic factors.6
In rheumatoid arthritis (RA) patients, the severity of inflammation is not only associated with the degree of subsequent joint damage, but also with an increased cardiovascular risk.7,8,9,10 Consequently, improvement or normalisation of the inflammatory changes in the course of effective treatment of RA with traditional disease‐modifying antirheumatic drugs (DMARDs) or biological agents leads to a reduction of the patients' cardiovascular morbidity.11,12 EPC (and also CAC) levels, in line with the increased cardiovascular risk in RA, are decreased in the peripheral blood of patients with active RA; however, patients with inactive disease or patients receiving anti‐tumour necrosis factor (TNF) therapy, even those with active RA, show EPC levels in the normal range.13 The migratory capacity of EPC, isolated from patients with RA, has been shown to be significantly reduced.14 Furthermore, the endothelial dysfunction associated with RA, has been shown to improve after TNF blockade.15 These findings indicated that mediators of inflammation, such as TNF, may be responsible for the reduction in EPC, but this has not been investigated hitherto.
Glucocorticoids (GCs) very efficiently reduce disease activity.16 In RA, they are mostly used until DMARDs can reach adequate efficacy17 and to treat flares of the disease. Moreover, combination therapy that includes intermediate doses of GCs was shown to have superior benefit when compared with therapies that did not include GCs.18,19 Like TNF blockers, but contrasting traditional DMARDs, GCs have rapid anti‐inflammatory effects as seen by a fast improvement of clinical signs and symptoms, decrease of acute phase reactants, and reduction of proinflammatory cytokines, including TNF, a pivotal cytokine in RA pathogenesis.20 On the other hand, GC therapy itself is related to accelerated atherosclerosis by virtue of its metabolic effects.21 Therefore, it is not clear if and how GCs affect EPC.
In the present study, we investigated the effects of short‐term intermediate dose GC therapy on EPC levels; it was shown that EPC levels normalise. In addition, we will show that TNF inhibits EPC generation and that this effect can be reversed by GCs.
Methods
Patients and study protocol
This study included 29 patients with RA according to the 1987 American College of Rheumatology criteria.22 After giving informed consent, patients were enrolled as part of a short‐term clinical outcome study termed BELIRA (BEst LIfe with Rheumatoid Arthritis), which had been approved by the Ethics Committee. During this 7‐day study, patients received 50 mg prednisolone/day for the first 3 days, followed by 25 mg/day from day 4 to day 7. Most patients (n = 25) already received 600 mg calcium and 400 IU vitamin D/day before starting the study medication. This therapy was continued and also instituted in the remaining four patients to prevent possible steroid effects on bone. In addition, pantoprazole 40 mg/day, was given to all patients.
For inclusion, disease activity had to be moderate to high according to the disease activity score (DAS28),23 reflected by a DAS28 >3.2. Significant hypertension or diabetes as well as a history of cardiovascular and cerebrovascular events were exclusion criteria as they are associated with decreased EPC levels.4,24,25 Patients were allowed low‐dose steroids (⩽10 mg prednisolone/day) before enrolment into the study as it was previously shown that low‐dose GC treatment did not affect EPC levels.13 None of the patients received peroxisome proliferator‐activated receptor‐γ agonists or erythropoietin (EPO) or had impaired renal function, which influence circulating EPC quantity and mobilisation.26,27 The mean body mass index was 26.9 (SD 0.7). The patients investigated here were a random subgroup of the BELIRA study population, and their characteristics are summarised in table 1. On the basis of previously described differences of EPC levels in patients with active versus inactive RA,13 power calculations indicated that significant effects on EPC levels would be seen in a study of 16 patients. As surrogates for disease activity, DAS28 and C‐reactive protein levels were determined at baseline and at the end of the treatment. Healthy referents (HR, n = 19) served as the reference population. They were matched in terms of age and sex and had neither a history of ischaemic cardiovascular events or diabetes mellitus nor of inflammatory or autoimmune diseases.
Table 1 Characteristics of patients and controls at baseline. Data presented are mean±SD, where appropriate.
| Patients with RA n = 29 | HR n = 19 | |
|---|---|---|
| Age (years) (SD) | 55 (SD 12) | 52 (SD 4) |
| Female (%) | 83 | 79 |
| Smokers (%) | 17 | 37 |
| Statin therapy (%) | 7 | 5 |
| Cholesterol (mg/dl) | 212 | |
| Triglycerides (mg/dl) | 121 | |
| Disease duration (years) (SD) | 10 (SD 8) | |
| Disease Activity Score (SD) | 5.2 (SD 1.2) | |
| Erythrocyte (SD) sedimentation rate (mm/h) | 36 (SD 27) | |
| C‐reactive protein (SD) (mg/dl) | 2.8 (SD 2.9) | |
| Rheumatoid factor positive (%) | 76 | |
| No DMARD (%) | 7 | |
| MTX (%) | 52 | |
| Other DMARDs (%) | 28 | |
| TNF‐blockers (±MTX) (%) | 14 | |
| Low‐dose glucocorticoids (%) | 66 |
Quantification of endothelial progenitor cells by flow cytometry
Fluorescence‐activated cell sorter analyses of the EPC were performed as previously described.4 Briefly, 100 μl of peripheral blood were incubated with a biotinylated monoclonal antibody against human KDR (kinase domain insert containing receptor) (Becton Dickinson, San Jose, California, USA), followed by staining with Streptavidin‐Quantum‐Red Conjugate (Sigma, St Louis, Missouri, USA). Samples were then incubated with fluorescein isothiocyanate conjugated monoclonal anti‐CD34 (BD Pharmingen, San Diego, California, USA) and phycoerythrin conjugated anti‐CD133 PE (Miltenyi Bergisch, Gladbach, Germany) antibodies. Control stainings were performed with isotype matched irrelevant monoclonal antibodies. After lysis of red cells and fixation with BD Lysing solution, acquisition was performed on a Becton Dickinson “FACScan” flow cytometer and EPC were counted as CD34/VEGF‐R2/CD133‐positive cells within the lymphocyte gate (1–3×105 events per sample). In the patients with RA, these analyses were studied at two time points: 1 day before the first prednisolone dose (day 0) and on day 7 after the last prednisolone dose. Furthermore, we analysed changes in the total leukocyte, and lymphocyte counts before and after prednisolone therapy.
Circulating angiogenic cell culture assay
CAC were quantified in six patients before and after GC therapy, employing a CAC culture assay as described in detail previously.13
Colony forming unit assay and in vitro effects of tumour necrosis factor, infliximab and glucocorticoids on colony forming unit
Circulating EPC were also quantified using the colony forming unit (CFU) assay as described in detail previously.5,13
To test if EPC and colony formation would be affected by dexamethasone (Dex) and/or TNF, we cultured peripheral blood mononuclear cells of 12 patients with RA in the presence of Dex (at a dose of 10−9 M) or TNF at a dose of 10 ng/ml or both. The doses employed resulted from preliminary experiments (data not shown) and are in line with the literature.28 Dex and/or TNF were added to the CFU cultures after 48 h, when non‐adherent peripheral blood mononuclear cells were seeded from six‐well plates to fibronectin coated 24‐well plates (day 3), and again when medium was changed on day 5 and day 8 of culture. In the same series of experiments on peripheral blood mononuclear cells of patients with RA, TNF (10 ng/ml), infliximab (1 mg/ml) and both TNF and infliximab were added in the same manner as described above. CFU were then counted as described above. We also tested the CFU behaviour of six HRs in the presence of Dex and anti‐TNF with or without the addition of TNF.
ELISAs
Vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), TNF, soluble TNF receptors 1 and 2 (sTNF‐R I and II), interleukin (IL)‐1β, IL‐6 and EPO were quantified in the sera of patients with RA and in controls using commercial ELISA kits (TNF: Biosources, Nivelles, Belgium; all other: R&D Systems, Minneapolis, Minnesota, USA). The minimum levels of detection were as follows: 5.0 pg/ml for VEGF, 0.22 pg/ml for bFGF, 3.0 pg/ml for TNF, 0.77 pg/ml for TNF‐R I, 0.6 pg/ml for TNF‐R II, 3.0 pg/ml for IL‐1β, 3.0 pg/ml for IL‐6 and 0.6 mU/ml for EPO.
Statistical evaluation
Statistical evaluations were performed using SPSS for Windows v 8.0 (SPSS Inc., Chicago, Illinois, USA). Data were evaluated using normality test, equal variance test and Student's t‐test. Data not normally distributed were analysed using non‐parametric methods. All data are presented as mean±SEM.
Results
Treatment of active rheumatoid arthritis with intermediate doses of glucocorticoids leads to significant reduction of interleukin‐6 and tumour necrosis factor levels in line with disease activity
Treatment of active RA with intermediate GC doses led to a decrease of serum TNF levels from 36 (SD 10) to 14 (SD 6) pg/ml (p<0.0001) and of serum IL‐6 levels (27 (SD 7) vs. 5 (SD 2) pg/ml, p<0.0005). However, this treatment had no significant influence on the serum levels of TNF‐R I and TNF‐R II, VEGF, bFGF and EPO (table 2). HR had significantly lower cytokine levels than patients with RA (data not shown). After steroid treatment, IL‐1β was below the detection limit in all serum samples tested, while it had been detectable in three patients before therapy.
Table 2 Serum levels of the cytokines and growth factors tested.
| Rheumatoid arthritis | |||
|---|---|---|---|
| Before GC | After GC | p‐value (before GC versus after GC) | |
| VEGF (pg/ml) (SD) | 659 (SD 1.2) | 521 (SD 52 | NS |
| bFGF (pg/ml) (SD) | 11.0 (SD 1.1) | 8.9 (SD 1.7) | NS |
| TNF (pg/ml) (SD) | 36 (SD 10) | 14 (SD 6) | p<0.0001 |
| IL‐6 (pg/ml) (SD) | 27 (SD 7) | 5 (SD 2) | p<0.0005 |
| TNF‐R I (pg/ml) (SD) | 2452 (SD 2.8) | 2715 (SD 2.9) | NS |
| TNF‐R II (pg/ml) (SD) | 4219 (SD 1.1) | 4347 (SD 1.4) | NS |
| Epo (mU/ml) (SD) | 20.4 (SD 4.4) | 18.0 (SD 1.9) | NS |
NS, not significant.
As expected, disease activity in patients with RA decreased by intermediate dose GC therapy; the mean DAS28 of the patients with RA was 5.2 (SD 0.2) before and 3.7 (SD 0.1) (p<0.0001) at the end of the 1‐week prednisolone treatment. Subanalysis of the DAS components (ie, swollen and tender 28 joint count, erythrocyte sedimentation rate and patient global assessment) showed a decrease of all four components (not shown). Total leucocyte counts significantly increased from 8.8 (SD 0.7) to 10.6 (SD 0.7) G/l (p<0.05), whereas absolute lymphocyte counts (3.0 (SD 1.2) vs. 2.9 (SD 0.8)) remained unchanged after GC therapy.
Glucocorticoid treatment of active rheumatoid arthritis induces an increase of circulating endothelial progenitor cells, circulating angiogenic cell and colony forming unit
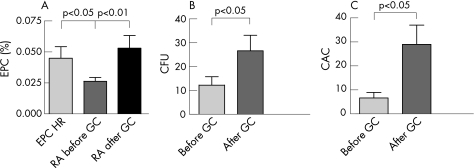
At baseline, levels of circulating EPC determined by flow cytometry were lower in patients with active RA than in HR (0.026 (SD 0.003)% vs. 0.045 (SD 0.009)% within the lymphocyte population, p<0.05, fig 1A, in line with previous data on the reduction of EPC in patients with active RA.18 Low levels of EPC in RA were observed irrespective of low‐dose GC therapy (0.025 (SD 0.004)% without versus 0.027 (SD 0.004)% with low‐dose GC treatment). However, after 7 days of treatment with intermediate doses of GCs according to the study protocol, levels of circulating EPC normalised (0.053 (SD 0.010)%, p<0.01 compared with baseline, fig 1A). To account for possible methotrexate‐mediated effects, we divided patients with RA into methotrexate and non‐methotrexate users. Neither before nor after GC treatment significant differences with regard to EPC levels were seen among these subgroups, irrespective of disease activity. EPC changes showed no significant correlation with reduction of disease activity, and even patients who continued to have high disease activity mostly normalised their EPC levels.
Figure 1 GC treatment increases and normalises endothelial progenitor cells (EPCs) in patients with active RA. (A) Normalisation of EPC frequencies. Patients with active RA, when treated with glucocorticoids (GCs) for 7 days, showed a significant increase in levels of circulating EPC (0.026±0.003 vs. 0.053±0.010% within the lymphocyte population, p<0.01), which then were comparable with healthy referents (HR) (0.045±0.009%). (B,C) Increase of colony forming unit (CFU) and circulating angiogenic cell (CAC) by GC therapy. Treatment of active RA with GCs induced a significant increase of CFU (B: 27±7 vs. 12±4/well, p<0.02) as well as of CAC (C: 29±8 vs. 7±2 CAC/high power field, p<0.05).
Importantly, however, when we excluded the smokers or patients receiving statins among the patients with RA, the results obtained within the RA group before and after GC therapy were very similar. Thus, the fact that we included a few smokers and patients taking statins in this study, does not reduce the validity of the results, although smoking is associated with lower circulating EPC levels and statins with higher circulating EPC levels.29,30
Analysis of CFU and CAC before and after GC therapy confirmed the flow cytometric data: the mean CFU counts doubled from 12 (SD 4) to 27 (SD 7) CFU/well (p<0.02, figs 1B and 2A,B) and CAC increased fourfold from 7 (SD 2) to 29 (SD 8) acetylated low‐density lipoprotein and lectin‐positive cells/high power field (p<0.05, fig 1C).
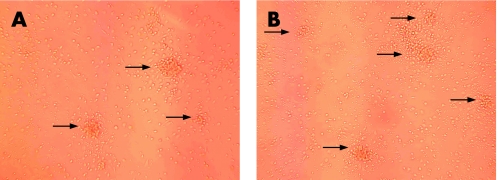
Figure 2 Confocal microscopy of colony forming unit (CFU) in patients with RA. Representative images of CFU by confocal microscopy show an increase in CFU after GC therapy (B) when compared with baseline (A). For numeric data see fig 1B.
Tumour necrosis factor decreases colony forming unit of rheumatoid arthritis and controls, and glucocorticoids and anti‐tumour necrosis factor increase rheumatoid arthritis–colony forming unit in vitro
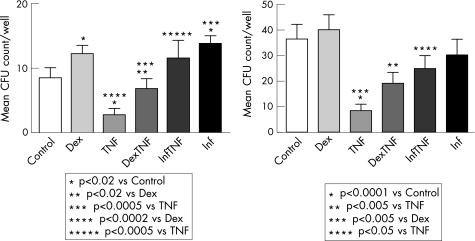
Given that GCs decreased TNF levels and increased EPC levels in vivo, we next asked if there could be a relationship between these two findings. To this end, we first performed EPC cultures in the presence of TNF, which in fact, led to a significant decrease in CFU formation (9 (SD 2) vs. 3 (SD 1) CFU/well p<0.02, fig 3A). Addition of Dex in the presence of TNF reversed most of these effects (7 (SD 2) CFU/well, p<0.0005 compared with TNF alone). The invalidation of the TNF‐mediated inhibition of in vitro CFU formation seen in the presence of an anti‐TNF antibody (12 (SD 3) CFU/well, p<0.0005 compared with TNF alone) confirmed that the effect observed was due to TNF. In line with these observations and the low CFU counts in patients with RA at baseline, addition of Dex or infliximab to EPC cultures of patients with RA, in the absence of added TNF, increased CFUs (from 9 (SD 2) to 12 (SD 1) CFU/well with Dex, p<0.02, and to 14 (SD 2) CFU with infliximab, p<0.05, fig 3A). In contrast to RA, addition of Dex or anti‐TNF to EPC cultures of HR did not induce a significant increase of colony formation (fig 3B), whereas addition of TNF reduced the CFU counts of HR from 36 (SD 6) to 8 (SD 3) (p<0.0001), an effect that could be inhibited by concomitant incubation with Dex (19 (SD 4) and anti‐TNF (25 (SD 6) (p<0.005 compared with anti‐TNF alone).
Figure 3 (A) Glucocorticoids (GCs) increase and TNF decreases colony forming unit (CFU) in RA. Addition of Dexamethasone (Dex) (10−9) to the CFU cultures of patients with RA led to a significant increase of the mean CFU counts/well vs. medium alone (from 9±2 to 12±1 CFU/well, p<0.02). Addition of TNF (10 ng/ml) led to a significant decrease of the CFU counts (3±1 versus control experiments, p<0.02) that could mostly be reversed by the addition of Dex (7±2 CFU/well versus TNF alone, p<0.0005). Addition of infliximab (Inf) fully reversed the CFU reduction induced by TNF (12±3 vs. 3±1 CFU/well, p<0.005). (B) Dex and anti‐TNF reverses the TNF‐induced CFU depletion in controls. Addition of TNF to the CFU cultures of HC led to a significant decrease of colonies (36±6 to 8±3 CFU/well, p<0.0001). This effect could be reversed upon addition of Dex (19±4 CFU/well) or anti‐TNF (25±6 CFU/well) (p<0.005 vs TNF).
These in vitro data, in conjunction with the in vivo observations, suggest that TNF‐mediated effects are responsible for the decrease of circulating EPC in patients with RA, while GCs and anti‐TNF reverse these effects.
Discussion
Active RA, when compared with healthy individuals or inactive disease, is associated with a decrease in circulating EPC levels.13,14 The data obtained in the present study reveal that treatment with intermediate doses of prednisolone over 7 days leads to normalisation of circulating EPC levels in patients with active RA. This improvement was not only accompanied by a reduction of clinical disease activity, but also by a significant decrease of TNF and IL‐6 serum levels. Importantly, these effects of GCs on EPC were seen in all three assays utilised in this study.
The fact that intermediate dose GC therapy reduced TNF levels by >60% while EPC frequencies increased suggested that TNF may be an important factor leading to EPC depletion. When cells from patients with RA with active disease were cultured in vitro, addition of GCs or anti‐TNF increased the frequencies of CFU. To distinguish if these effects were direct ones or possibly due to interference with proinflammatory cytokines, we incubated cells in vitro with TNF. While TNF led to a further decrease of CFU of patients with RA (and also to a decrease of CFU formation of HRs) in vitro, the addition of GCs reversed this inhibitory capacity. Thus, the effects of GCs on EPC levels ex vivo and in vitro are compatible with an inhibition of the actions of TNF on EPC and are further corroborated by the in vivo decrease of TNF during GC therapy. Not surprisingly, anti‐TNF neutralised the CFU reduction induced by TNF in vitro and is also associated with normalisation of EPC in vivo.13 Our conclusions are further supported by the lack of effect of Dex and anti‐TNF on the EPC formation of HRs, while the TNF‐induced decrease in their in vitro EPC formation was reversed by the addition of Dex or anti‐TNF to the CFU assays.
Although the doses necessary to achieve this effect, as employed in the present study, are higher than serum levels and synovial fluid levels measured in patients with RA,31,32 it is conceivable that similar concentrations occur locally within the tissues. In line with our results, Seeger et al33 recently made similar observations in patients with coronary artery disease.
An increase in circulating EPC levels upon successful interference with the inflammatory process may improve the cardiovascular risk of patients with RA, which is strongly associated with the extent of inflammation.8 Conversely, GC therapy itself constitutes a cardiovascular risk factor.21 In fact, a recent publication, showed that supraphysiological GC doses led to an inhibition of angiogenesis.34 However, the CFU studied here reflect the vasculogenic rather than angiogenic capacity, and levels of circulating EPC have been shown to predict cardiovascular outcomes.2,4 Likewise, the association of low‐dose GC over the long term with increased mortality21,35 actually supports our overall conclusions, as chronic low‐dose steroid therapy had no apparent effect on EPC levels and did not dissociate the inverse relationship between EPC levels and disease activity in this and a previous study. Rather, irrespective of prior low‐dose GC treatment, it was the addition of short‐term intermediate dose GC that led to an increase in EPC. Moreover, this increase was even seen in patients who continued to have high disease activity, suggesting some dissociation of inflammation from its effects on EPC—at least in the short term.
Remarkably, the other growth factors and cytokines determined in the patients' sera, some of them known to promote angiogenesis, showed no significant reduction in the course of GC therapy. This might be a consequence of a slower response of these cytokines to the GC‐induced anti‐inflammatory effects, to the need for higher doses of GCs than employed in this study in achieving their reduction, or refractoriness to GCs.
It is a limitation of our study that we did not perform a long‐term analysis of cardiovascular outcomes in the context of intermediate dose GC therapy of RA. On the other hand, it has been unequivocally shown, that the cardiovascular morbidity in general is highly related to the decrease in EPC levels2,4 and that in RA in particular this risk is highly related to disease activity,13 which can significantly be reduced by short‐term intermediate dose GC therapy. Also, the results obtained cannot and do not claim a beneficial effect on cardiovascular outcome in RA. Such claims can only come from long‐term follow‐up, which includes studying cardiovascular events and survival. However, given the significant association of EPC levels with adverse cardiovascular outcome2 and the results obtained here, such studies will be of significant interest.
In this study we have subjected patients with RA only to a short course of GC therapy, and their long‐term effects on EPC have not been studied. However, it is particularly the relatively short‐term application of GCs that is important to rapidly reduce disease activity in the context of initiation of DMARD therapy.18,36 As effective DMARD treatment is associated with normalisation of EPC, the addition of intermediate dose GCs, which leads to rapid reduction of disease activity, may support these effects and, in fact, may induce earlier and more rapid reversal of the EPC depletion; however, some of the effects seen may be due to demargination.
Taken together, our data reveal that a short course of intermediate dose GCs not only leads to a reduction in disease activity, but also rapidly normalises EPC levels. Thus, GCs might bring about protective cardiovascular effects, at least in the short term, by interfering with the inflammatory response and the TNF pathways; however, such effects will have to be investigated separately in a prospective outcome study.
Acknowledgements
This work was supported by grant number 2244 of the “Medizinisch‐Wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien” and the Center of Molecular Medicine of the Austrian Academy of Sciences. We thank Elisabeth Höfler, Brigitte Mayer, Susanne Moser and Irene Radda for excellent technical assistance. Furthermore, the authors are also grateful to Karin Frisch and Young Sook Steiner, the nurses in our outpatient clinic.
Abbreviations
bFGF - basic fibroblast growth factor
CAC - circulating angiogenic cell
CFU - colony forming unit
Dex - dexamethasone
DMARD - disease‐modifying antirheumatic drugs
EPC - endothelial progenitor cell
EPO - erythropoietin
GC - glucocorticoids
HR - healthy referents
IL - interleukin
VEGF - vascular endothelial growth factor
Footnotes
Funding: This work was supported by “Medizinisch‐Wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien” and the Center of Molecular Medicine of the Austrian Academy of Sciences.
Competing interests: None.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Z R, Li T.et al Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997275964–967. [DOI] [PubMed] [Google Scholar]
- 2.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A.et al Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005353999–1007. [DOI] [PubMed] [Google Scholar]
- 3.Rosenzweig A. Circulating endothelial progenitors‐cells as biomarkers. N Engl J Med 20053531055–1057. [DOI] [PubMed] [Google Scholar]
- 4.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H.et al Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 200189E1–E7. [DOI] [PubMed] [Google Scholar]
- 5.Hill J M, Zalos G, Halcox J P, Schenke W H, Waclawiw M A, Quyyumi A A.et al Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003348593–600. [DOI] [PubMed] [Google Scholar]
- 6.Rehman J, Li J, Orschell C M, March K L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 20031071164–1169. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe F, Mitchell D M, Sibley J T, Fries J F, Bloch D A, Williams C A.et al The mortality of rheumatoid arthritis. Arthritis Rheum 199437481–494. [DOI] [PubMed] [Google Scholar]
- 8.Wallberg‐Jonsson S, Johansson H, Ohman M L, Rantapaa‐Dahlqvist S. Extent of inflammation predicts cardiovascular disease and overall mortality in seropositive rheumatoid arthritis. A retrospective cohort study from disease onset. J Rheumatol 1999262562–2571. [PubMed] [Google Scholar]
- 9.Ridker P M, Rifai N, Rose L, Buring J E, Cook N R. Comparison of C‐reactive protein and low‐density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 20023471557–1565. [DOI] [PubMed] [Google Scholar]
- 10.Szmitko P E, Wang C H, Weisel R D, de Almeida J R, Anderson T J, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation 20031081917–1923. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti‐tumor necrosis factor therapy. Am J Med 2004116305–311. [DOI] [PubMed] [Google Scholar]
- 12.Choi H K, Hernan M A, Seeger J D, Robins J M, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet 20023591173–1177. [DOI] [PubMed] [Google Scholar]
- 13.Grisar J, Aletaha D, Steiner C W, Kapral T, Steiner S, Seidinger D.et al Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation 2005111204–211. [DOI] [PubMed] [Google Scholar]
- 14.Herbrig K, Haensel S, Oelschlaegel U, Pistrosch F, Foerster S, Passauer J. Endothelial dysfunction in patients with rheumatoid arthritis is associated with a reduced number and impaired function of endothelial progenitor cells. Ann Rheum Dis 200665157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurlimann D, Forster A, Noll G, Enseleit F, Chenevard R, Distler O.et al Anti‐tumor necrosis factor‐alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation 20021062184–2187. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs J W, Geenen R, Evers A W, van Jaarsveld C H, Kraaimaat F W, Bijlsma J W. Short term effects of corticosteroid pulse treatment on disease activity and the wellbeing of patients with active rheumatoid arthritis. Ann Rheum Dis 20016061–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Everdingen A A, Jacobs J W, Siewertsz Van Reesema D R, Bijlsma J W. Low‐dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease‐modifying properties, and side effects: a randomized, double‐blind, placebo‐controlled clinical trial. Ann Intern Med 20021361–12. [DOI] [PubMed] [Google Scholar]
- 18.Boers M, Verhoeven A C, Markusse H M, van de Laar M A, Westhovens R, van Denderen J C.et al Randomised comparison of combined step‐down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis: a randomised trial. Lancet 1997350309–318. [DOI] [PubMed] [Google Scholar]
- 19.Goekoop‐Ruiterman Y P, de Vries‐Bouwstra J K, Allaart C F, van Z D, Kerstens P J, Hazes J M.et al Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005523381–3390. [DOI] [PubMed] [Google Scholar]
- 20.Feldmann M, Brennan F M, Foxwell B M, Maini R N. The role of TNF alpha and IL‐1 in rheumatoid arthritis. Curr Dir Autoimmun 20013188–199. [DOI] [PubMed] [Google Scholar]
- 21.Nashel D J. Is atherosclerosis a complication of long‐term corticosteroid treatment? Am J Med 198680925–929. [DOI] [PubMed] [Google Scholar]
- 22.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 23.Prevoo M L, 't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 24.Tepper O M, Galiano R D, Capla J M, Kalka C, Gagne P J, Jacobowitz G R.et al Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 20021062781–2786. [DOI] [PubMed] [Google Scholar]
- 25.Choi J H, Kim K L, Huh W, Kim B, Byun J, Suh W.et al Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler Thromb Vasc Biol 2004241246–1252. [DOI] [PubMed] [Google Scholar]
- 26.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C.et al Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 20031021340–1346. [DOI] [PubMed] [Google Scholar]
- 27.Wang C H, Ciliberti N, Li S H, Szmitko P E, Weisel R D, Fedak P W.et al Rosiglitazone facilitates angiogenic progenitor cell differentiation toward endothelial lineage: a new paradigm in glitazone pleiotropy. Circulation 20041091392–1400. [DOI] [PubMed] [Google Scholar]
- 28.Redlich K, Kiener H P, Schett G, Tohidast‐Akrad M, Selzer E, Radda I.et al Overexpression of transcription factor Ets‐1 in rheumatoid arthritis synovial membrane: regulation of expression and activation by interleukin‐1 and tumor necrosis factor alpha. Arthritis Rheum 200144266–274. [DOI] [PubMed] [Google Scholar]
- 29.Michaud S E, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis 2006187423–432. [DOI] [PubMed] [Google Scholar]
- 30.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher A M.et al Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation 20011032885–2890. [DOI] [PubMed] [Google Scholar]
- 31.Steiner G, Tohidast‐Akrad M, Witzmann G, Vesely M, Studnicka‐Benke A, Gal A.et al Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology (Oxford) 199938202–213. [DOI] [PubMed] [Google Scholar]
- 32.Partsch G, Steiner G, Leeb B F, Dunky A, Broll H, Smolen J S. Highly increased levels of tumor necrosis factor‐alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol 199724518–523. [PubMed] [Google Scholar]
- 33.Seeger F H, Haendeler J, Walter D H, Rochwalsky U, Reinhold J, Urbich C.et al p38 mitogen‐activated protein kinase downregulates endothelial progenitor cells. Circulation 20051111184–1191. [DOI] [PubMed] [Google Scholar]
- 34.Small G R, Hadoke P W, Sharif I, Dover A R, Armour D, Kenyon C J.et al Preventing local regeneration of glucocorticoids by 11beta‐hydroxysteroid dehydrogenase type 1 enhances angiogenesis. Proc Natl Acad Sci USA 200510212165–12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sihvonen S, Korpela M, Mustonen J, Huhtala H, Karstila K, Pasternack A. Mortality in patients with rheumatoid arthritis treated with low‐dose oral glucocorticoids. A population‐based cohort study. J Rheumatol 2006331740–1746. [PubMed] [Google Scholar]
- 36.Smolen J S, Aletaha D, Machold K P. Therapeutic strategies in early rheumatoid arthritis. Best Pract Res Clin Rheumatol 200519163–177. [DOI] [PubMed] [Google Scholar]