Abstract
Objectives
To determine whether joints affected by gout are also affected by osteoarthritis (OA).
Methods
A postal questionnaire was sent to all adults aged over 30 years registered with two general practices. The questionnaire assessed a history of gout (doctor diagnosed, or episodes suggestive of acute crystal synovitis) and medication use. Patients who possibly had gout attended for clinical assessment to verify the diagnosis on clinical grounds and assess the distribution of joints affected by acute attacks of gout and OA. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) were calculated between the history of an acute attack of gout and the presence of OA at an individual joint adjusted for age, gender, body mass index and prior diuretic use in a binary logistic regression model.
Results
A total of 4249 completed questionnaires were returned (32%). From 359 attendees, 164 cases of gout were clinically confirmed. A highly significant association existed between the site of acute attacks of gout and the presence of OA (aOR 7.94; 95% CI 6.27, 10.05). Analysis at individual joint sites revealed a significant association at the first metatarsophalangeal joint (aOR 2.06; 95% CI 1.28, 3.30), mid‐foot (aOR 2.85; 95% CI 1.34, 6.03), knee (aOR 3.07; 95% CI 1.05, 8.96) and distal interphalangeal joints (aOR 12.67; 95% CI 1.46, 109.91).
Conclusion
Acute attacks of gout at individual joint sites are associated with the presence of clinically assessed OA at that joint suggesting that OA may predispose to the localised deposition of monosodium urate crystals.
Gout is one of the most common inflammatory arthritides with a community prevalence of 1–2%.1,2 It displays a characteristic pattern of joint involvement, in particular, a striking tendency to affect the first metatarsophalangeal (1st MTP) joint. It tends to affect also distal peripheral joints such as the mid‐tarsal joints, ankles, knees, interphalangeal (IP) finger joints, wrists and elbows, with axial joints such as the hips, shoulders and spine only very rarely affected. The reason for this characteristic distribution of affected joints is not understood. However, case reports and small hospital‐based case series report the occurrence of gout at joint sites affected by osteoarthritis (OA) especially the 1st MTP joint and distal IP finger joints;3,4,5,6,7 this suggests that changes in the osteoarthritic joint may predispose to monosodium urate (MSU) crystal formation.8 Furthermore, a Polish hospital‐based study of 262 subjects with gout found an association between gout and radiographic evidence of OA at the 1st MTP joints, tarsal joints and knees.9 This study did not, however, consider upper limb joints and also runs the risk of inherent bias as a hospital‐based population, whereas gout is managed largely in primary care.
We undertook this community‐based study in order to determine whether joints affected by gout are also affected by OA, both overall and at individual joint sites.
Methods
The study was undertaken in two general practices in Nottingham, nested within a case–control study. It consisted of two phases: (1) a postal questionnaire survey, and (2) a face‐to‐face clinical assessment. The study was approved by the Nottingham Local Research Ethics Committee 2 and written consent to participate was obtained from participants who attended for clinical assessment.
Postal questionnaire
Each practice drew up a list of all registered adults over the age of 30 years, excluding those with a history of major psychiatric disease, dementia or recently diagnosed malignancy. A questionnaire was then mailed to all listed individuals with a pre‐paid envelope included for their reply. Possible cases of gout were identified by two questions:
Have you ever been diagnosed with or suffered from gout?
Have you ever suffered from an acute attack of arthritis that was severely painful, was associated with a red and swollen joint, came on suddenly reaching its peak severity within 24 h and then went away completely within 3 weeks? (ie, a typical attack of crystal synovitis)?
Age of onset of gout, current use of allopurinol and diuretic use prior to the onset of gout were requested in addition to recording standard demographic information, weight and height.
Following the first round of mailing, in order to enhance a poor response rate, an abbreviated questionnaire was mailed out to non‐respondents in one practice.
Clinical assessment
All subjects that indicated a previous diagnosis of gout or a history of acute self‐limiting attacks of painful, red, swollen joints in their questionnaire (ie, potential cases) were invited to attend a clinical assessment. At this visit, the subject's joint problems were reviewed by a physician with special training in gout (ER) and the most likely diagnosis was made on clinical grounds. The diagnosis of gout was further assessed according to American Rheumatism Association preliminary criteria for the acute arthritis of primary gout.10 Each subject was asked where (ie, individual joint sites) they had ever experienced acute attacks of gout, for example, 1st MTP joint, mid‐foot, ankle, knee, hip, finger and thumb IP joints, thumb‐base (ie, first carpo‐metacarpal joint), wrist, elbow or shoulder (36 joints in total for each subject). Subjects were also asked to specify whether attacks had affected the left or right side at each individual joint site.
Subjects were systematically examined for the presence of OA at all joint sites. OA of the hip was considered to be present if the subject had had a hip replacement or there was restriction of passive internal rotation of the hip with the hip flexed at 90°. The knee joint was examined for five features: joint replacement, restricted passive flexion and extension, crepitus, joint line tenderness and effusion. OA of the knee was defined according to two criteria: (1) the subject had had a knee replacement, or (2) there was restricted passive flexion/extension with either crepitus or joint line tenderness. At other joint sites, OA was defined as the presence of restricted passive movement or bony swelling or crepitus.
Participants were also examined for the presence of tophi. These were classified as definite (asymmetric white/yellow swellings on pulling the overlying skin taut) or possible (asymmetric nodular swellings without white/yellow discoloration). Blood was taken for measurement of serum urate and creatinine.
Statistical analysis
The unit of analysis was considered to be the joint rather than the subject. Two analyses were undertaken.
First, the crude odds ratio (OR) and 95% confidence interval (CI) between the presence of OA at an individual joint and a history of an acute attack of gout at that joint were calculated for all joint sites combined. This analysis included 5904 individual joints (ie, all 36 joints examined in 164 individuals). Whether a joint had been affected by an acute attack of gout was then entered as the dependent variable into a forward step‐wise logistic regression model with age (decades), gender, body mass index (BMI; >30 kg/m2), previous diuretic use and presence of OA at that joint as independent variables and an adjusted OR (aOR) and 95% CI were calculated. In order to investigate the influence of disease duration on the relationship between the site of acute attacks of gout and the presence of OA, subjects were grouped according to tertiles of disease duration and the crude and adjusted ORs calculated for each tertile.
Secondly, crude and adjusted ORs were then calculated at each individual joint site (left and right sides combined). As acute attacks of gout in the finger IP joints were an uncommon occurrence, analyses were performed for three anatomical units: distal IP (DIP) joint row, proximal IP (PIP) joint row and the whole hand (ie, acute attacks of gout and OA occurring anywhere within that unit).
Results
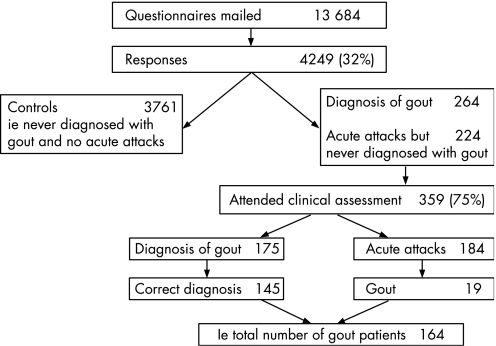
Questionnaires were mailed to a total of 13 684 individuals, and 4249 completed responses were returned; 294 were notified as being sent to the wrong address. The adjusted response rate was, therefore, 32% (fig 1).
Figure 1 Flow of subjects through the study.
A total of 488 respondents reported acute attacks of self‐limiting synovitis and/or a diagnosis of gout: 264 had been given a diagnosis of gout. Three hundred and fifty‐nine attended the clinical assessment (75%). Of these, 175 reported being diagnosed with gout by a doctor. This was thought to be the correct diagnosis in 145 (83%). A further 19 subjects were thought to have gout but had never consulted a doctor. Therefore, 164 subjects were identified as having gout. Characteristics of the 164 subjects with gout are shown in table 1.
Table 1 Characteristics of subjects with gout.
| Subjects with gout (n = 164) | |
|---|---|
| Age: years, mean (SD) | 63.4 (11.2) |
| Gender: male (%) | 133 (81) |
| Height: cm, mean (SD) | 172 (8) |
| Weight: kg, mean (SD) | 83 (15) |
| BMI: kg/m2, mean (SD) | 27.8 (4.0) |
| Age at first attack (years), mean (SD) | 50.5 (13.7) |
| Disease duration (years), median (range) | 10 (0–50) |
| Tophus | |
| Total (%) | 10 (6) |
| Definite (%) | 5 (3) |
| Possible (%) | 5 (3) |
| Fulfil American Rheumatism Association preliminary criteria for primary gout (%) | 150 (92) |
| Serum urate μmol/l, mean (SD) (normal range 100–400) | 399 (107) |
| Serum creatinine μmol/l, median (range) (normal range 60–120) | 94 (63–180) |
| GFR ml/min, median (range) | 78 (19–170) |
| GFR <60 ml/min (%) | 39 (24) |
| Medication use: | |
| Taken diuretics prior to first attack (%) | 25 (15) |
| Low‐dose aspirin (%) | 51 (31) |
| Allopurinol (%) | 44 (30) |
| Uricosuric agents (%) | 0 |
| Possible secondary gout (ie, GFR <60 ml/min or taking diuretics prior to first attack) | 50 (30) |
SD, standard deviation; BMI, body mass index; GFR, glomerular filtration rate.
The most common site of acute attacks of gout was the 1st MTP joint followed by the mid‐foot, ankle and knee (table 2). Upper limb involvement was uncommon. Involvement of left and right sides was similar at all joint sites.
Table 2 frequency of acute attacks of gout at each joint site.
| Joint | Left (% of subjects) | Right (% of subjects) |
|---|---|---|
| 1st MTP joint | 61 | 66 |
| Mid‐foot | 13 | 20 |
| Ankle | 12 | 15 |
| Knee | 7 | 12 |
| Hip | 0 | 0 |
| DIP joints | 2 | 2 |
| PIP joints | 1 | 1 |
| Thumb‐base | 1 | 1 |
| Hand | 4 | 3 |
| Wrist | 3 | 4 |
| Elbow | 3 | 4 |
| Shoulder | 1 | 1 |
MTP, metatarsophalangeal; DIP, distal interphalangeal; PIP, proximal interphalangeal.
Analysis of all 5904 individual joint sites combined found a highly significant association between the site of acute attacks of gout and the presence of OA after adjustment for confounding factors (aOR 7.94; 95% CI 6.27, 10.05) (table 3).
Table 3 Odds ratio (OR) and 95% confidence interval (CI) between the site of acute attacks of gout and presence of OA.
| Variable | Crude OR (95% CI) | Adjusted OR (95% CI)* | p |
|---|---|---|---|
| Presence of OA in the index joint | 6.76 (5.39, 8.46) | 7.94 (6.27, 10.05) | <0.001 |
*Adjusted for age (decades), gender, BMI (>30 kg/m2) and prior diuretic use using a forward step‐wise binary logistic regression model with gout in the index joint as the dependent variable.
On indirect comparison of the three tertiles of disease duration, the strength of association between sites of acute attacks of gout and the presence of OA appeared similar (table 4). ORs were numerically smaller with increasing disease duration, although there was considerable overlap of 95% CI.
Table 4 Odds ratios (OR) and 95% confidence interval (CI) between attacks of gout and presence of OA stratified by disease duration (tertiles) .
| Disease duration (years), mean (SD) | Crude OR (95% CI) | Adjusted OR (95% CI)* | |
|---|---|---|---|
| Tertile 1 | 2.9 (2.2) | 7.39 (4.80, 11.37) | 10.12 (6.29, 16.29) |
| Tertile 2 | 10.2 (2.1) | 7.19 (4.97, 10.41) | 8.22 (5.61, 12.06) |
| Tertile 3 | 25.9 (8.4) | 6.02 (4.11, 8.80) | 6.45 (4.38, 9.48) |
*Adjusted for age (decades), gender, BMI (>30 kg/m2) and prior diuretic use using a forward step‐wise binary logistic regression model with gout in the index joint as the dependent variable.
Analysis at individual joint sites revealed a significant association between acute attacks of gout and the presence of OA at the 1st MTP joint (aOR 2.06; 95% CI 1.28, 3.30), mid‐foot (aOR 2.85; 95% CI 1.34, 6.03), knee (aOR 3.07; 95% CI 1.05, 8.96) and DIP joints (aOR 12.67; 95% CI 1.46, 109.91) after adjustment for confounding variables (table 5).
Table 5 Odds ratio (OR) and 95% confidence interval (CI) between acute attacks of gout and presence of OA for each joint site (left and right combined).
| Joint | Crude OR (95% CI) | Adjusted OR (95% CI)* | p |
|---|---|---|---|
| 1st MTP joint | 1.73 (1.10, 2.73) | 2.06 (1.28, 3.30) | 0.003 |
| Mid‐foot | 2.58 (1.24, 5.35) | 2.85 (1.34, 6.03) | 0.006 |
| Ankle | 4.08 (0.94, 17.73) | NS | – |
| Knee | 2.98 (1.02, 8.68) | 3.07 (1.05, 8.96) | 0.040 |
| Hip | – | – | – |
| DIP joints | 12.50 (1.44, 108.46) | 12.67 (1.46, 109.91) | 0.021 |
| PIP joints | – | – | – |
| Thumb‐base | – | – | – |
| Hand | 2.90 (0.86, 9.83) | NS | – |
| Wrist | 1.16 (0.14, 9.37) | NS | – |
| Elbow | – | – | – |
| Shoulder | – | – | – |
*Adjusted for age (decades), gender, BMI (>30 kg/m2) and prior diuretics using a forward step‐wise binary logistic regression model with gout in the index joint as the dependent variable.
NS, not significant; MTP, metatarsophalangeal; DIP, distal interphalangeal; PIP, proximal interphalangeal.
Discussion
A strong association was seen between joint sites affected by acute attacks of gout and the presence of clinically assessed OA. When this relationship was assessed at individual joint sites, significant associations were seen at the 1st MTP, mid‐foot, knee and finger DIP joints after adjustment for confounding variables. These data support the hypothesis that the presence of OA at an individual joint site predisposes to the formation of urate crystals at that site as suggested by a number of case reports and small case series3,4,5,6,7 and one Polish hospital‐based study.9 This latter study found a significant correlation between gout and radiographic presence of OA at the 1st MTP joint, mid‐foot and knee in 262 subjects with gout. The current study is the first to evaluate this question in a community‐based sample and to consider the upper limb joints.
A number of mechanisms have been suggested to explain an association between the sites of acute attacks of gout and OA, including: mechanical shock;11 changes in cartilage and synovial proteoglycans;12 epitaxial MSU crystal formation on cartilage fragments;13 and transient increases in the urate concentration of resolving synovial effusions owing to the differential permeability of synovium to urate and water,8 which might encourage nucleation and precipitation of MSU crystals. An association between OA and calcium crystal deposition is well recognised. Community studies have confirmed a positive association between knee OA and chondrocalcinosis.14 There is an increased incidence of calcium pyrophosphate dihydrate and basic calcium phosphate crystals in synovial fluid and cartilage from OA joints,15 and also local mechanical factors (including joint hypermobility16 and instability,17 joint motion,18 ligamentous laxity19 and joint damage from surgery20,21 and trauma22) are known to predispose to localised secondary deposition of calcium pyrophosphate dihydrate crystals in the context of joint damage. It is possible that OA results in changes in tissue factors, either an increase in promoters or a reduction in inhibitors of crystal nucleation and growth, which might predispose not just to calcium crystal but also to MSU crystal deposition. It has also been suggested that the predilection of gouty arthritis and tophi for cooler extremities such as the hallux, fingers and ears and the rarity of gout at axial joints such as the hip and shoulder could be explained by the reduction seen in the solubility of urate with decreasing temperature.23,24 However, this does not explain why gout should preferentially affect the MTP joint of the great toe rather than the lesser toes or the great toe IP joint.
The major limitation to our study is the low overall response rate to the postal questionnaire. In addition to 164 gout cases identified, there were a further 89 subjects who reported suffering from gout in the questionnaire but did not consent to clinical assessment. Another possible limitation is the use of a clinical case definition for gout. The recent EULAR (the European League Against Rheumatism) recommendations for the diagnosis of gout found that the rapid development of severe pain, swelling, tenderness and erythema is highly suggestive of crystal inflammation, although not specific for gout;25 however, 1st MTP joint involvement markedly increases the specificity of this clinical composite. Although it is possible that some of the acute attacks that were presumed to be due to gout were, in fact, due to other crystals, most commonly calcium pyrophosphate; this is unlikely to have accounted for much misclassification given the sites of involvement observed. Without doubt, the gold standard for the diagnosis of gout is the identification of crystals on aspirates from synovial fluid or tophus. However, while intercritical joint aspiration is a useful diagnostic technique,26 the requirement to undergo aspiration may have reduced further the number of individuals consenting to the clinical assessment, particularly as only 4% had experienced joint aspiration previously (data not shown). Many studies of gout have used the 1977 American Rheumatism Association preliminary criteria as diagnostic criteria.10 However, these classification criteria are limited to the acute arthritis of primary gout, for example, they are not helpful for either chronic gout or secondary gout, and have never undergone further validation as suggested by the authors in the original report. We have, however, included them as a secondary diagnostic criterion. A further similar caveat is that the presence of OA at an individual joint site was diagnosed on the basis of clinical examination findings. These are not specific for OA and may have introduced misclassification bias. Clinical diagnosis of OA may be valid in certain joints, for example, the knee, 1st MTP joint, DIP joints and hip, but may be less appropriate at other sites, for example, the mid‐foot, shoulder, wrist or ankle. Undertaking radiographs of all sites of interest would have been impractical; furthermore, standard radiographs are relatively insensitive, especially at certain joint sites. Radiographic evidence of an association between gout and OA at the 1st MTP joint, mid‐foot and knee has been reported by Kawenoki‐Minc et al,9 although this hospital‐based study did not consider upper limbs. Although clinically detectable bony swelling is characteristic of OA and correlates with radiographic OA,27,28,29 crepitus and reduced range of movement may also occur as a result of joint damage secondary to gout. It could also be argued that this explains the observed association between the sites of acute attacks of gout and the clinical examination findings of OA. However, the strength of this association was not influenced by the chronicity of gout suggesting that joint damage secondary to gout does not explain the observed association, although a prospective study is required to confirm this. A final caveat is that the observer was not blinded to which joints had been affected by gout at the time of the clinical examination. Further work using blind radiographic assessment to determine the presence of OA is warranted.
In summary, the deposition of MSU crystals at individual joint sites is associated with the presence of OA at that joint, particularly at the 1st MTP, mid‐foot, knee and finger DIP joints suggesting that OA may predispose to the localised deposition of MSU crystals and in part determine which joints are affected by acute attacks of gout.
Acknowledgements
We would like to thank the staff and patients of Arnold Health Centre and The Calverton Practice in Nottingham, UK.
Abbreviations
aOR - adjusted odd ratio
BMI - body mass index CI, confidence interval
DIP - distal interphalangeal
IP - interphalangeal
MSU - monosodium urate
MTP - metatarsophalangeal
OA - osteoarthritis
OR - odds ratio
PIP - proximal interphalangeal
Footnotes
Funding: We are grateful for funding from the Arthritis Research Campaign, UK (ICAC grant 14851) and unrestricted financial support from Astra‐Zeneca‐UK, Glaxo‐Smith‐Kline‐USA and Ipsen, France
Competing interests: None.
References
- 1.Mikuls T R, Farrar J T, Bilker W B, Fernandes S, Schumacher H R, Jr, Saag K G. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 200564267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris C M, Lloyd D C, Lewis J. The prevalence and prophylaxis of gout in England. J Clin Epidemiol 1995481153–1158. [DOI] [PubMed] [Google Scholar]
- 3.Simkin P A, Campbell P M, Larson E B. Gout in Heberden's nodes. Arthritis Rheum 19832694–97. [DOI] [PubMed] [Google Scholar]
- 4.O'Dell J R. Gout in Heberden's nodes. Arthritis Rheum 1983261413–1414. [DOI] [PubMed] [Google Scholar]
- 5.Foldes K, Petersilge C A, Weisman M H, Resnick D. Nodal osteoarthritis and gout: a report of four new cases. Skeletal Radiol 199625421–424. [DOI] [PubMed] [Google Scholar]
- 6.Fam A G, Stein J, Rubenstein J. Gouty arthritis in nodal osteoarthritis. J Rheumatol 199623684–689. [PubMed] [Google Scholar]
- 7.Lally E V, Zimmermann B, Ho G, Jr, Kaplan S R. Urate‐mediated inflammation in nodal osteoarthritis: clinical and roentgenographic correlations. Arthritis Rheum 19893286–90. [DOI] [PubMed] [Google Scholar]
- 8.Simkin P A. The pathogenesis of podagra. Ann Intern Med 197786230–233. [DOI] [PubMed] [Google Scholar]
- 9.Kawenoki‐Minc E, Eyman E, Leo W, Werynska‐Przybylska J. [Osteoarthrosis and spondylosis in gouty patients. Analysis of 262 cases of gout]. Reumatologia 197412267–7 (In Polish with English abstract). [PubMed] [Google Scholar]
- 10.Wallace S L, Robinson H, Masi A T, Decker J L, McCarty D J, Yu T F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 197720895–900. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox W R, Khalaf A A. Nucleation of monosodium urate crystals. Ann Rheum Dis 197534332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perricone E, Brandt K D. Enhancement of urate solubility by connective tissue. I. Effect of proteoglycan aggregates and buffer cation. Arthritis Rheum 197821453–460. [DOI] [PubMed] [Google Scholar]
- 13.Pascual E, Ordonez S. Orderly arrayed deposit of urate crystals in gout suggest epitaxial formation. Ann Rheum Dis 199857255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neame R L, Carr A J, Muir K, Doherty M. UK community prevalence of knee chondrocalcinosis: evidence that correlation with osteoarthritis is through a shared association with osteophyte. Ann Rheum Dis 200362513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton E, Pattrick M, Hornby J, Derrick G, Doherty M. Synovial fluid calcium pyrophosphate dihydrate crystals and alizarin red positivity: analysis of 3000 samples. Br J Rheumatol 199029101–104. [DOI] [PubMed] [Google Scholar]
- 16.Bird H A, Tribe C R, Bacon P A. Joint hypermobility leading to osteoarthrosis and chondrocalcinosis. Ann Rheum Dis 197837203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Settas L, Doherty M, Dieppe P. Localised chondrocalcinosis in unstable joints. Br Med J (Clin Res Ed) 1982285175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fam A G, Schumacher H R, Jr, Clayburne G, Villanueva T, Baker D, Jimenez S A. Effect of joint motion on experimental calcium pyrophosphate dihydrate crystal induced arthritis. J Rheumatol 199017644–655. [PubMed] [Google Scholar]
- 19.Scott D, Bird H, Wright V. Joint laxity leading to osteoarthrosis. Rheumatol Rehabil 197918167–169. [DOI] [PubMed] [Google Scholar]
- 20.Doherty M, Watt I, Dieppe P A. Localised chondrocalcinosis in post‐meniscectomy knees. Lancet 1982i1207–1210. [DOI] [PubMed] [Google Scholar]
- 21.Ellman M H, Vazques L T, Brown N L, Mandel N. Calcium pyrophosphate dihydrate deposition in lumbar disc fibrocartilage. J Rheumatol 19818955–958. [PubMed] [Google Scholar]
- 22.de Lange E E, Keats T E. Localized chondrocalcinosis in traumatized joints. Skeletal Radiol 198514249–256. [DOI] [PubMed] [Google Scholar]
- 23.Kippen I, Klinenberg J R, Weinberger A, Wilcox W R. Factors affecting urate solubility in vitro. Ann Rheum Dis 197433313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeb J N. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum 197215189–192. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Doherty M, Pascual E, Bardin T, Barskova V, Conaghan P.et al EULAR evidence based recommendations for gout—Part I Diagnosis: Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2006651301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual E, Batlle‐Gualda E, Martinez A, Rosas J, Vela P. Synovial fluid analysis for diagnosis of intercritical gout. Ann Intern Med 1999131756–759. [DOI] [PubMed] [Google Scholar]
- 27.Claessens A A M C, Schouten J S A G, Van Ouwelan F A, Valkenburg H A. Do clinical findings associate with radiographic osteoarthritis of the knee? Ann Rheum Dis 199049771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern A G, Moxley G, Sudha Rao T P, Disler D, McDowell C, Park M. Utility of digital photographs of the hand for assessing the presence of hand osteoarthritis. Osteoarthritis Cartilage 200412360–365. [DOI] [PubMed] [Google Scholar]
- 29.Thaper A, Zhang W, Wright G, Doherty M. Relationship between Heberden's nodes and underlying radiographic change of osteoarthritis. Ann Rheum Dis 2005641214–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]