Abstract
Objectives
The degree to which treatment with tumour necrosis factor (TNF) antagonists may be associated with increased risks for serious infections is unclear. An observational cohort study was performed using prospectively collected data from the Swedish Biologics Register (ARTIS) and other national Swedish registers.
Methods
First, in the ARTIS, all 4167 rheumatoid arthritis (RA) patients starting TNF antagonist treatment between 1999 and 2003 were identified. Secondly, in the Swedish Inpatient Register, all individuals hospitalised for any reason and who also carried a diagnosis of RA, between 1964 and 2003 (n = 44 946 of whom 2692 also occurred in ARTIS), were identified. Thirdly, in the Swedish Inpatient Register, all hospitalisations listing an infection between 1999 and 2003 were identified. By cross‐referencing these three data sets, RRs for hospitalisation with infection associated with TNF antagonist treatment were calculated within the cohort of 44 946 RA patients, using Cox regression taking sex, age, geography, co‐morbidity and use of inpatient care into account.
Results
Among the 4167 patients treated with TNF antagonists, 367 hospitalisations with infections occurred during 7776 person‐years. Within the cohort of 44 496 RA patients, the RR for infection associated with TNF antagonists was 1.43 (95% CI 1.18 to 1.73) during the first year of treatment, 1.15 (95% CI 0.88 to 1.51) during the second year of treatment, and 0.82 (95% CI 0.62 to 1.08) for subjects remaining on their first TNF antagonist treatment after 2 years.
Conclusion
Treatment with TNF antagonists may be associated with a small to moderate increase in risk of hospitalisation with infection, which disappears with increasing treatment duration.
While the clinical efficacy of tumour necrosis factor (TNF) antagonists in rheumatoid arthritis (RA) and several other chronic inflammatory conditions is well documented, several aspects of their safety profile with respect to infections remain incompletely understood. Previously, we and several others documented an increased occurrence of uncommon intracellular infections such as tuberculosis following treatment with TNF antagonists.1,2 Less is established with respect to the risk of more common, yet serious, infections, which constitute a more frequently occurring clinical problem. Most of the published randomised clinical trials with TNF antagonists have not been powered sufficiently to exclude meaningful increases in the risk for serious infections, but it is of interest to note that the numerical risks of serious infections were elevated in several such trials, and the difference reached statistical significance in at least one of these.3 A recent meta‐analysis of randomised trials with adalimumab and infliximab suggested a statistically significant 2‐fold increase in the occurrence of serious infections with these agents.4 The durations of these randomised controlled trials were 12–54 weeks and they included patients meeting tight inclusion and exclusion criteria characteristic of trials. It is therefore imperative to understand whether (1) the increased risk of serious infections is also mirrored in clinical practice, and (2) whether any increased infection risk also extends beyond the first 6–12 months of treatment. Infection data from observational studies based on biologics registers are in apparent conflict: Listing et al from the German Biologics Register reported an incidence of serious infections of around 6/100 and a significant 2‐ to 3‐fold increased risk associated with TNF antagonists based on a total of 66 serious infections among 986 RA patients treated with biologics and 601 comparison patients.5 A recent study by Dixon et al from the UK Biologics Register showed an overall rate for serious infections of 5.3/100, which did not correspond to any increased risk overall (but an increased risk of skin/soft tissue infections) based on 525 vs 56 infections among 9868 vs 1352 treated and untreated patients, respectively.6 Whereas many of these differences can be explained by differences in design, analytical approach and statistical precision, more data are clearly needed.
In this study, we used one of the largest biologics registers (ARTIS7) and some unique features of the Swedish health care system to assess the occurrence, relative risks (RRs) and predictors for patients with RA to be hospitalised with an infection.
Subjects and methods
Study population
The ARTIS cohort of patients treated with TNF antagonists
The setting and registers used in this study are described in more detail elsewhere.7 Since 1999, patients above 16 years of age with RA (or other rheumatological diseases) starting treatment with TNF antagonists have been entered and followed‐up in the practice‐based national ARTIS register. For each initiated treatment, information on the underlying rheumatological condition including date of onset, date of treatment initiation (and discontinuation), type and dose of biologic, Disease Activity Score 28 (DAS28) and Health Assessment Questionnaire (HAQ), concomitant disease‐modifying anti‐rheumatic drugs (DMARDs), steroids, non‐steroidal anti‐inflammatory drugs (NSAIDs) and analgesics are recorded by the treating rheumatologist at treatment start as well as at pre‐specified follow‐up visits. For this study, we included all 4167 patients with RA starting a TNF antagonist treatment between 1 January 1999 and 31 December 2003. Characteristics of this population are given in table 1. Sixty‐four percent had been exposed to infliximab, 40% to etanercept and 13% to adalimumab (17% had been exposed to more than one TNF antagonist). At the start of the first TNF antagonist treatment, proportions reporting treatment with DMARDs, steroids and NSAIDs were 70, 56 and 68%, respectively.
Table 1 Characteristics at start of first tumour necrosis factor (TNF) antagonist treatment in the Swedish ARTIS register cohort of 4167 patients with rheumatoid arthritis (RA) starting treatment with TNF antagonists in 1999–2003, and in the subset thereof who had a pre‐treatment hospitalisation with RA.
| All anti‐TNF 1998–2003 | Anti‐TNF 1998–2003, also in Inpatient Register RA cohort | |
|---|---|---|
| Overall | 4167 (100%) | 2692 (100%) |
| Sex | ||
| Males | 1015 (24%) | 581 (22%) |
| Females | 3152 (75%) | 2111 (78%) |
| Age at entry (years) | ||
| 0–49 | 1343 (32%) | 727 (27%) |
| 50–74 | 2603 (62%) | 1778 (66%) |
| 75+ | 221 (5.3%) | 187 (7.0%) |
| Year of first anti‐TNF start | ||
| 1998–2000 | 1633 (39%) | 1221 (45%) |
| 2001–2002 | 1390 (33%) | 848 (32% |
| 2003 | 1144 (27%) | 623 (23%) |
| RA duration at start (years) | 12.1 (9.7) | 15.0 (13.3) |
| DAS28 at start | 5.63 (5.72) | 5.74 (5.83) |
| HAQ at start | 1.43 (1.45) | 1.57 (1.63) |
| Year of birth | ||
| 1900–1919 | 38 (0.9%) | 33 (1.2%) |
| 1920–1939 | 1203 (29%) | 924 (34%) |
| 1940–1959 | 2133 (52%) | 1335 (50%) |
| 1960–1979 | 754 (18%) | 388 (14%) |
| 1980– | 39 (0.9%) | 12 (0.5%) |
| Married | 2410 (59%) | 1636 (61%) |
DAS28, Disease Activity Score 28; HAQ, Health Assessment Questionnaire.
The Inpatient Register cohort of RA
Thresholds for hospitalisation have been relatively low in Sweden, especially for rheumatological diseases (see for instance table 2). The nationwide and population‐based Swedish Inpatient Register contains information on all inpatient care (dates, medical discharge diagnoses (coded according to International Classification of Diseases (ICD) versions 7–10),8 department, hospital, personal identification number) since 1964 (nationwide since 1987). Using the ICD codes for RA, all individuals hospitalised with (but not necessarily because of) RA can be identified. For this study, we identified all 44 946 individuals ever discharged from inpatient care, having been admitted for any reason, but also having RA included as a discharge diagnosis, between 1964 and 2003, and alive in 1998. Previous validation surveys against information in the underlying medical files suggest that the diagnostic correctness of these RA diagnoses is around 90%, and that the RA cohort that can be identified corresponds to a substantial proportion of all prevalent Swedish patients with RA (>50% assuming a population RA prevalence of 0.7%).9
Table 2 Co‐morbidity at the start of first tumour necrosis factor (TNF) antagonist treatment in (1) the Swedish ARTIS register cohort of 4167 patients with rheumatoid arthritis (RA) starting treatment with TNF antagonists 1999–2003; (2) in the subset thereof who had a pre‐treatment hospitalisation with RA; and (iii) among sex‐, age‐ and calendar period‐matched bio‐naïve RA controls (4 per RA patient in (2)) from the Swedish Inpatient Register RA cohort of patients hospitalised with RA.
| Inpatient morbidity at TNF antagonist start | (1) All anti‐TNF 1998–2003 | (2) Anti‐TNF 1998–2003, also in Inpatient Register RA cohort | (3) Controls from RA Inpatient Register RA cohort, matched to (2) |
|---|---|---|---|
| Number of patients | 4167 (100%) | 2692 (100%) | 10 295 (100%) |
| RA discharge | 2692 (65%) | 2692 (100%) | 10 295 (100%) |
| Infection | 1087 (26%) | 855 (32%) | 3078 (30%) |
| Cardiac disease | 763 (18%) | 604 (22%) | 2620 (25%) |
| COPD | 157 (3.8%) | 124 (4.6%) | 552 (5.4%) |
| Diabetes | 175 (3.6%) | 141 (5.2%) | 530 (5.2%) |
| Knee replacement | 306 (7.3%) | 299 (11%) | 929 (9.0%) |
| Hip replacement | 668 (16%) | 648 (24%) | 1643 (16%) |
| Shoulder joint replacement | 128 (3.1%) | 126 (4.7%) | 232 (2.3%) |
| Foot surgery | 652 (16%) | 631 (23%) | 1493 (15%) |
| Total days in hospital (mean/median) | 65/30 | 89/53 | 93/44 |
| Number of discharges, all diagnoses | 8.2/5 | 11/8 | 9.8/7 |
CODD, chronic obstructive pulmonary disease.
Of the 4167 RA patients in the TNF antagonist cohort, 2692 (65%) had one or more hospitalisations (listing RA) before their start of TNF antagonist treatment, and were also included in the Inpatient Register RA cohort (table 1). Table 2 displays the co‐morbidity pattern among these 4167 and 2692 subjects, respectively, and among—for illustrative purposes—individually matched RA patients (four bio‐naive subjects per each patient starting TNF antagonist treatment, matched by sex, age, time and county) from the Inpatient Register RA cohort.
Follow‐up and outcome
All individuals in the above RA cohorts were linked through their personal identification10 number to the following national, virtually complete registers: the Cause of Death Register (date of death), the Population Register (residency in Sweden during the study period) and the Emigration register (emigrations during the study period). Through linkage of the ARTIS TNF antagonist RA cohort and of the Inpatient Register RA cohort to the Inpatient Register, we identified all episodes of hospitalisation listing an infection among the discharge codes. Outcome was defined as the first hospitalisation listing an infection as the (or: among the) discharge diagnosis (‐es) during follow‐up. For individuals with more than one hospitalisation for infection during follow‐up, only the first was used in the analyses of infections overall, and only the first per type of infection was used in the analyses of infections by type.
Follow‐up was defined as time on first TNF antagonist (which started at first TNF antagonist treatment start, and ended at the first of 30 days after discontinuation of treatment, date of death, date of emigration or 31 December 2003), or as the corresponding time on a second TNF antagonist, respectively. (+0 and +90 day post‐discontinuation windows were also employed but had little impact on the results, data not shown). For <5% of all patients, exact treatment durations could not be determined. These individuals were considered at risk until the end of the study period. About one‐third of the 4167 TNF antagonist‐treated RA patients contributed less than 1 year of follow‐up, one‐third contributed 1–2 years of follow‐up and one‐third contributed 3–6 years.
Statistics
Occurrence of infection was expressed as the crude incidence of the outcome. RRs of infection associated with TNF antagonists were estimated through internal comparison within the Inpatient Register RA cohort 1999–2003 using Cox regression. In these analyses, TNF antagonist treatment was assessed as a time‐dependent covariate. RRs were assessed for infections overall and by type. All models were stratified for sex, year of birth and county of residence, and adjusted for marital status and combinations of the following time‐dependent co‐morbidity hospitalisation covariates until 1 year before the outcome: hip, knee, shoulder and ankle joint replacements, diabetes, cardiovascular disease, pre‐treatment infection, chronic obstructive pulmonary disease, accumulated number of hospitalisations, accumulated number of RA hospitalisations, accumulated number of medical diagnoses received and accumulated number of days spent in hospital. Proportionality of hazards was assessed by adding time (linear) as an interaction term. Predictors of infection were estimated using uni‐ and multivariate Poisson regression in the cohort of all 4167 TNF antagonist‐exposed RA patients in ARTIS, using the first hospitalisation listing an infection as the dependent variable. The study was approved by the Ethics Committee at the Karolinska Institute.
Results
Occurrence of infections following TNF antagonist treatment
Among all 4167 individuals with RA treated with TNF antagonists, 367 first episodes of infections occurred during 7776 person‐years of follow‐up on TNF antagonist treatment, resulting in a crude incidence of 4.7 (95% CI 4.2 to 5.2) per 100 person‐years. The incidence was 4.5 (95% CI 4.0 to 5.0, n = 320 infections) during time on first TNF antagonist, and 7.0 (95% CI 5.0 to 10, n = 34 infections) during time on second TNF antagonist. In the subset of 2692 individuals who had been hospitalised (not necessarily because, but with, RA) prior to treatment with a TNF antagonist, the incidence of infection during time on first TNF antagonist treatment was 5.4 (95% CI 4.7 to 6.0, n = 261 infections), and 10 (95% CI 7.1 to 16, n = 26 infections) during time on second TNF antagonist treatment.
RR of infection following TNF antagonist treatment
Time on first TNF antagonist
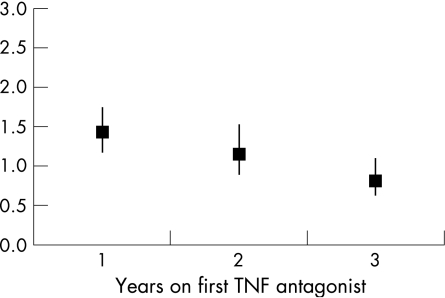
Within the Inpatient Register RA cohort, the overall RR for TNF antagonist‐associated infection adjusted for co‐morbidity and use of inpatient care was increased around 30%, but displayed a statistically significant effect modification by time since treatment start, violating the proportional hazards assumption for all overall models (table 3). Whereas the age‐, sex‐ and county‐stratified but otherwise unadjusted RRs were 1.74, 1.39 and 1.04 for the first, second and third year on treatment, adjustment for co‐morbidity and use of inpatient care resulted in an RR of 1.43 (95% CI 1.18 to 1.73) during the first year of treatment, 1.15 (95% CI 0.87 to 1.50) during the second year of treatment and 0.82 (95% CI 0.62 to 1.08) for subjects remaining two or more years on their first TNF antagonist treatment (fig 1).
Table 3 Numbers of infections and RR (estimated through Cox regression) for hospitalisation with infection during time on first tumour necrosis factor (TNF) antagonist treatment 1999–2003 in the Swedish Inpatient rheumatoid arthritis (RA) cohort, by time since start of TNF antagonist treatment, among 44 946 RA patients of whom 2692 patients started treatment with a first TNF antagonist.
| During first TNF antagonist | TNF antagonist naive | RR by time since treatment start* | ||||
|---|---|---|---|---|---|---|
| No. of events | Crude rate per 100 | No of events | Year 1 | Year 2 | Year 3 | |
| Any infection | 261 | 5.4 | 10 669 | 1.43 (1.18 to 1.72) | 1.15 (0.88 to 1.51) | 0.82 (0.62 to 1.08) |
| Respiratory | 111 | 2.2 | 6046 | 1.24 (0.92 to 1.68) | 1.45 (1.00 to 2.09) | 0.68 (0.44 to 1.05) |
| Pneumonia | 79 | 1.5 | 4704 | 1.11 (0.77 to 1.60) | 1.31 (0.86 to 1.93) | 0.59 (0.35 to 0.99) |
| Gastrointestinal | 24 | 0.5 | 1517 | 1.03 (0.58 to 1.81) | 0.13 (0.02 to 0.91) | 0.94 (0.45 to 1.94) |
| Skin/soft tissue | 21 | 0.4 | 1004 | 0.57 (0.21 to 1.57) | 1.01 (0.48 to 2.16) | 0.82 (0.39 to 1.76) |
| Joint | 25 | 0.5 | 652 | 1.26 (0.66 to 2.40) | 0.17 (0.02 to 1.28) | 1.42 (0.67 to 2.98) |
| Septicaemia | 36 | 0.7 | 1,716 | 1.07 (0.65 to 1.77) | 0.71 (0.33 to 1.55) | 0.62 (0.32 to 1.18) |
*Cox model stratified for sex, year of birth, county of residence and using calendar time as time scale. Adjusted for marital status, chronic obstructive pulmonary disease, any cardiovascular disease, any pre‐treatment infection, diabetes, joint replacements in hip, knee, shoulder and ankle, respectively, total number of days spent in hospital and number of discharges listing RA. All time‐varying covariates were assessed as time‐dependent variables until 1 year before the outcome.
Figure 1 RRs for hospitalisation with infection during time on first tumour necrosis factor (TNF) antagonist treatment 1999–2003 in the Swedish Inpatient rheumatoid arthritis (RA) cohort, by time since start of TNF antagonist treatment, among 44 946 RA patients of whom 2692 patients started treatment with a first TNF antagonist. RRs estimated through Cox regression.
A similar pattern with increased, unelevated, and subsequently decreased risks over time on treatment was observed in models using alternative adjustments for co‐morbidity and use of inpatient care, in which the point of RR estimates ranged from ±10 to 15% compared with the above‐mentioned adjusted RRs. There was no effect modification by age or sex. When infection was assessed separately by type, none of the RRs reached statistical significance apart from a borderline significant increase in respiratory infections (table 3). In absolute terms, the around 30% elevated overall risk corresponded to one additional case of hospitalisation with infection for every 80 one‐year treatments of RA with a first TNF antagonist (one additional case per 66 subjects during year 1, 148 during year 2, and one avoided infection per 123 subjects year 3 and onwards).
Time on second TNF antagonist
Among the 760 RA patients in the Inpatient Register RA cohort who switched to a second TNF antagonist treatment, the RR for infection during this second treatment was 2.10 (95% CI 1.36 to 3.27).
Baseline predictors for infection following TNF antagonist treatment
In univariate analyses among all 4167 TNF antagonist‐treated RA patients in ARTIS, age, duration of RA, HAQ, DMARD use other than methotrexate, and pre‐treatment co‐morbidity all predicted infection risk (table 4). Neither DAS28 not treatment with steroids at baseline were predictive for infection (table 4). In multivariate models adjusted for all the above parameters, age, HAQ and DMARD treatment other than methotrexate remained as significant predictors.
Table 4 RRs for hospitalisation for any infection during first tumour necrosis factor (TNF) antagonist treatment among all 4167 Swedish rheumatoid arthritis (RA) patients in the ARTIS register of TNF antagonists 1998–2003.
| Univariate RR | Multivariate RR* | |
|---|---|---|
| Sex | ||
| Males | 1.10 (0.86 to 1.41) | 1.07 (0.83 to 1.39) |
| Females | 1.0 (reference) | 1.0 (reference) |
| Age at treatment start | ||
| Q1 (<46) | 1.0 (reference) | 1.0 (reference) |
| Q2 (46–55) | 1.29 (0.89 to 1.87) | 1.30 (0.89 to 1.89) |
| Q3 (56–63) | 1.75 (1.21 to 2.50) | 1.51 (1.05 to 2.19) |
| Q4 (>64) | 3.04 (2.17 to 4.26) | 2.12 (1.48 to 3.04) |
| RA duration at treatment start | ||
| Missing | 1.42 (0.96 to 2.10) | 1.29 (0.87 to 1.92) |
| Q1 (<4) | 1.0 (reference) | 1.0 (reference) |
| Q2 (4–9) | 1.50 (0.97 to 2.31) | 1.43 (0.93 to 2.23) |
| Q3 (10–17) | 1.69 (1.10 to 2.59) | 1.41 (0.92 to 2.22) |
| Q4 (<17) | 1.96 (1.31 to 2.98) | 1.41 (0.92 to 2.15) |
| DAS28 at treatment start | ||
| Missing | 1.35 (0.95 to 1.93) | 1.06 (0.65 to 1.72) |
| Q1 (<4.86) | 1.0 (reference) | 1.0 (reference) |
| Q2 (4.86–5.71) | 1.15 (0.79 to 1.70) | 1.04 (0.70 to 1.54) |
| Q3 (5.71–6.53) | 1.21 (0.83 to 1.78) | 0.87 (0.58 to 1.29) |
| Q4 (>6.53) | 1.19 (0.81 to 1.73) | 0.76 (0.51 to 1.14) |
| HAQ at treatment start | ||
| Missing | 1.88 (1.28 to 2.74) | 1.28 (0.73 to 2.25) |
| Q1(<1.00) | 1.0 (reference) | 1.0 (reference) |
| Q2 (1.00–1.49) | 1.35 (0.89 to 2.04) | 1.30 (0.86 to 1.98) |
| Q3 (1.50–1.88) | 1.81 (1.21 to 2.72) | 1.47 (0.96 to 2.27) |
| Q4 (>1.88) | 2.49 (1.73 to 3.57) | 1.86 (1.23 to 2.80) |
| DMARD at treatment start | ||
| Missing | 1.00 (0.70 to 1.44) | 1.24 (0.60 to 2.57) |
| No DMARD | 1.00 (reference) | 1.00 (reference) |
| MTX | 0.72 (0.54 to 0.94) | 0.91 (0.69 to 1.20) |
| Other DMARDs | 1.50 (1.04 to 2.17) | 1.45 (1.00 to 2.12) |
| Steroids at treatment start | ||
| Missing | 1.10 (0.79 to 1.53) | 0.76 (0.41 to 1.42) |
| No | 1.0 (reference) | 1.0 (reference) |
| Yes | 1.07 (0.83 to 1.39) | 0.90 (0.69 to 1.17) |
| Co‐morbidity at treatment start | ||
| COPD | 2.43 (1.56 to 3.79) | 1.52 (0.96 to 2.42) |
| Diabetes | 2.07 (1.37 to 3.10) | 1.43 (0.94 to 2.18) |
| Any CVD | 2.41 (1.91 to 3.05) | 1.61 (1.24 to 2.07) |
| Infection | 2.07 (1.65 to 2.58) | 1.63 (1.28 to 2.07) |
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DAS28, Disease Activity Score 28; DMARD, disease‐modifying anti‐rheumatic drug; HAQ, Health Assessment Questionnaire; MTX, methotrexate.
RRs estimated through Poisson regression. Continuous variables are categorised into quartiles (Q1–Q4).
*Adjusted for all parameters in the table
Discussion
Based on more than 4000 RA patients treated with TNF antagonists in a population‐based setting, close to 400 observed hospitalisations with infections during follow‐up, and some 10 000 corresponding outcomes in the comparator, our assessment of infection risk following TNF antagonist use has substantial statistical power. Our results suggest that when TNF antagonists are used in routine clinical care: (1) the incidence of hospitalisations with infections is of the same order of magnitude as that of serious infections in previous randomised or observational studies; (2) treated individuals are at a statistically significantly increased risk for hospitalisation with infection, but the magnitude of this risk increase is low to moderate, and lower than, for example, in the recent meta‐analysis of trial data4; (3) this excess infection risk is not driven by any particular type of infection, but is made up by an increased risk largely confined to the first year following treatment start; and (4) HAQ and other easily assessed clinical parameters, including past hospitalisations, all predict risk of subsequent hospitalisation with infection during TNF antagonist treatment, whereas baseline disease activity as measured by DAS28 does not.
The infection risk in the randomised controlled trials of TNF antagonists is somewhat difficult to interpret. Whereas some trials have reported similar numbers of serious infections in both arms,11,12 most have observed more serious infections in the TNF antagonist arm than in the placebo arm,3,13,14,15,16 but power restraints have made this difference reach statistical significance in only a few trials.3,16 By and large, no clear pattern with respect to drug, dose or setting has emerged. Of some concern is the recent meta‐analysis including nine of the largest randomised trials of infliximab and adalimumab, in which TNF antagonist treatment (during 6–12 months) was associated with a doubled risk of serious infections, corresponding to one additional case per every 59 treatments of 6–12 months.4 Data on infection risks from observational studies, mainly from biologics registers, are conflicting, with two of the largest registers reporting doubled5 and no overall increase,6 respectively, yet observed incidences of serious infections well in keeping with that reported from trials and that of our study. In both studies, mean follow‐up was around 1 year. Data from the National Data Bank for Arthritis have so far not suggested any increased occurrence of hospitalisation for pneumonia following TNF antagonist treatment.17
Our finding of increased RRs early after treatment start might offer some support for the results from the clinical trials of TNF antagonists. Importantly, however, beyond the first year of follow‐up on first TNF antagonist treatment, we noted no significant increase in infection risk. Any analyses by time on drug should, however, be interpreted with some caution, as the case‐mix is likely to shift over time: following “shedding” of individuals susceptible to infections relatively early following start of treatment, and of non‐responders to the drug, RRs are likely to decline with increasing follow‐up. The impact of selection towards or away from high‐risk patients is highlighted by our analysis of RR during the second treatment, which—despite similar exposure (TNF antagonist)—was markedly higher than during the first treatment episode.
Outcome ascertainment
In randomised trials, serious infections are defined as infections requiring hospitalisation and intravenous antibiotics, or which result in death. In the different observational studies of infections hitherto presented, serious infections have been identified through either patients' or doctors' self‐reporting.16,17 In our study, outcome was defined as hospitalisation with infection and was assessed in a manner independent of the patient or the treating doctor, but by an external and virtually complete prospective register linkage, independently of treatment status. This reduced bias from differential recall and observation, but did not allow a more detailed characterisation of all the 10 000 or so outcomes observed. It may be that RA patients treated with TNF inhibitors have a lower threshold for hospital care as clinicians might be more concerned with their “new” treatment. Differential thresholds for hospitalisation might have biased (presumably inflated) the RRs observed.
Internal validity
In our study, RRs were stratified for sex, age, county of residence, and implicitly for calendar period, thus allowing for substantial heterogeneity in hazards between different subsets of the study population. Furthermore, models were adjusted for time‐dependent data on several co‐morbidities and on hospitalisation patterns, which was of particular importance in light of the fact that our outcome was also defined as hospitalisation. Conversely, although a limitation of our study, the effect of a lack of DAS28 data in the Inpatient Register RA cohort is likely to be of limited magnitude since DAS28 was not a strong predictor for infection following TNF antagonist treatment. Importantly, the adjustments made guarantee neither lack of residual confounding, nor confounding from other factors. Because of the marked decline in RR by time on drug, an overall measure of RR would be difficult to interpret and is hence not presented.
Generalisability
The data sources used and the underlying health care are all largely population based, suggesting that no major uncontrolled selection bias was at hand. However, to maximise comparability, and since our comparator (individuals in the Inpatient Register RA cohort not exposed to TNF antagonists during the study period) had all been hospitalised with RA, we only included in the analyses of RRs those RA patients (n = 2629, 65%) of the TNF antagonist RA cohort who also had a pre‐treatment hospitalisation with RA. Thereby, we essentially performed an internal comparison of those exposed and unexposed, respectively, to TNF antagonists within the Inpatient Register RA cohort. Although analyses comparing the entire ARTIS RA cohort with the Inpatient Register RA cohort yield largely similar results (data not shown), it should be noted that the RRs presented are strictly not generalisable beyond the two‐thirds of all TNF antagonist‐treated patients included in our estimation of RRs.
In our study, baseline HAQ and co‐morbidity variables emerged as important predictors for hospitalisation for infection during TNF antagonist treatment. In contrast, DAS28 at treatment start was not predictive of infection risk. This observation, which is in line with data from, for example, the British Biologics register,6 underscores the fact that whereas activity measures such as DAS28 are important for the evaluation of therapeutic response, prediction of other health outcomes18 requires other parameters. The predictive effect of DMARDs other than methotrexate might, considering that methotrexate is the standard DMARD option in the study population, reflect risks associated with methotrexate failure or concomitant conditions prohibiting use of methotrexate rather than risk associated with other DMARDs per se.
In conclusion, RA patients treated with TNF antagonists in clinical practice are at a slightly increased risk of being hospitalised with an infection, suggesting that a certain clinical alertness for infectious symptoms among treated patients may be warranted. The increase is, however, lower than was recently suggested from trial data,4 and largely occurs during the first year of treatment.
Acknowledgements
We gratefully acknowledge the following centres and their Swedish RA register/ARTIS representatives for allowing us to use their data: Sunderby Hospital, Luleå; Norrland's University Hospital, Umeå; Östersund County Hospital; Sundsvall County Hospital; Hudiksvall's Hospital; Gävle County Hospital; Falu lasarett, Falun; Karlstad's Central Hospital; Akademiska Hospital, Uppsala; Västerås Hospital; University Hospital, Örebro; Karolinska University Hospital, Solna; Karolinska University Hospital, Huddinge; Danderyd's Hospital, Stockholm; Gun Sandahl, Queen Sophia Hospital, Stockholm; Visby lasarett, Visby; Anders Lindblad, Visby Privat, Visby; Mälarsjukhuset, Eskilstuna; University Hospital, Linköping; County Hospital Ryhov, Jönköping; Västervik's Hospital; Oskarshamn's Hospital; Kalmar County Hospital; Växjö's Central Hospital; Olof Börjesson, Växjö Privat; Centrallasarettet Borås; Kärnsjukhuset, Skövde; Uddevalla Hospital; Ingeli Andreasson, Göteborg Privat; Sahlgrenska University Hospital, Gothenburg; University Hospital in Lund; Lasarettet Trelleborg; Spenshult, Oskarström; Helsingborg's Lasarett; Ängelholm's Hospital; Kristianstad's Central Hospital; MAS University Hospital, Malmö.
Abbreviations
DAS - Disease Activity Score
DMARD - disease‐modifying anti‐rheumatic drug
HAQ - Health Assessment Questionnaire
NSAID - non‐steroidal anti‐inflammatory drug
RA - rheumatoid arthritis
RR - relative risk
TNF - tumour necrosis factor
Footnotes
Funding: Financial support for this study was obtained from Wyeth‐Ayerst, Schering‐Plough, Abbott Immunology and Bristol Myer Squibb. The investigators were in charge of and solely responsible for all data collection, analysis and writing of the manuscript, without any constraints exerted from the agencies or companies that helped to sponsor the study. The South Swedish Anti‐TNF Register has received funding from King Gustav V, Österlund and Kock Foundations, and from Reumatikerförbundet.
Competing interests: None.
References
- 1.Askling J, Fored C M, Brandt L, Baecklund E, Bertilsson L, Coster L.et al Risk and case characteristics of tuberculosis in rheumatoid arthritis associated with tumor necrosis factor antagonists in Sweden. Arthritis Rheum 2005521986–1992. [DOI] [PubMed] [Google Scholar]
- 2.Keane J, Gershon S, Wise R P, Mirabile‐Levens E, Kasznica J, Schwieterman W D.et al Tuberculosis associated with infliximab, a tumor necrosis factor alpha‐neutralizing agent. N Engl J Med 20013451098–1104. [DOI] [PubMed] [Google Scholar]
- 3.St Clair E W, van der Heijde D M, Smolen J S, Maini R N, Bathon J M, Emery P.et al Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 2004503432–3443. [DOI] [PubMed] [Google Scholar]
- 4.Bongartz T, Sutton A J, Sweeting M J, Buchan I, Matteson E L, Montori V. Anti‐TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta‐analysis of rare harmful effects in randomized controlled trials. JAMA 20062952275–2285. [DOI] [PubMed] [Google Scholar]
- 5.Listing J, Strangfeld A, Kary S, Rau R, von Hinueber U, Stoyanova‐Scholz M.et al Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 2005523403–3412. [DOI] [PubMed] [Google Scholar]
- 6.Dixon W G, Watson K, Lunt M, Hyrich K L, Silman A J, Symmons D P. Rates of serious infection, including site‐specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti‐tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006542368–2376. [DOI] [PubMed] [Google Scholar]
- 7.Askling J, Fored M, Geborek P, Jacobsson L, van Vollenhoven R, Feltelius N.et al Swedish registers to address drug safety and clinical issues in rheumatoid arthritis. Ann Rheum Dis 200665707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization International classification of diseases. Geneva: World Health Organization, 1955
- 9.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F.et al Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 200654692–701. [DOI] [PubMed] [Google Scholar]
- 10.Lunde A S, Lundeborg S, Lettenstrom G S, Thygesen L, Huebner J. The person‐number systems of Sweden, Norway, Denmark, and Israel. Vital Health Stat 2 198021–59. [PubMed] [Google Scholar]
- 11.Klareskog L, van der Heijde D, de Jager J P, Gough A, Kalden J, Malaise M.et al Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double‐blind randomised controlled trial. Lancet 2004363675–681. [DOI] [PubMed] [Google Scholar]
- 12.Maini R N, Breedveld F C, Kalden J R, Smolen J S, Furst D, Weisman M H.et al Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004501051–1065. [DOI] [PubMed] [Google Scholar]
- 13.Lipsky P E, van der Heijde D M, St Clair E W, Furst D E, Breedveld F C, Kalden J R.et al Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti‐Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 20003431594–1602. [DOI] [PubMed] [Google Scholar]
- 14.Furst D E, Schiff M H, Fleischmann R M, Strand V, Birbara C A, Compagnone D.et al Adalimumab, a fully human anti tumor necrosis factor‐alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol 2003302563–2571. [PubMed] [Google Scholar]
- 15.Weinblatt M E, Keystone E C, Furst D E, Moreland L W, Weisman M H, Birbara C A.et al Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 20034835–45. [DOI] [PubMed] [Google Scholar]
- 16.Keystone E C, Kavanaugh A F, Sharp J T, Tannenbaum H, Hua Y, Teoh L S.et al Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti‐tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo‐controlled, 52‐week trial. Arthritis Rheum 2004501400–1411. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease‐modifying antirheumatic drugs, and anti‐tumor necrosis factor therapy. Arthritis Rheum 200654628–634. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F, Michaud K, Gefeller O, Choi H K. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum 2003481530–1542. [DOI] [PubMed] [Google Scholar]