Abstract
Objective
To evaluate the clinical response after switching from one tumour necrosis factor (TNF)α antagonist to another in patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA).
Methods
In this ongoing, longitudinal, observational study, data were prospectively collected on efficacy and safety since 2000 for patients starting biological treatments. The present analysis was restricted to patients with a diagnosis of spondyloarthropathy (SpA) who switched from one TNFα antagonist to another because of inadequate efficacy or adverse events.
Results
In total, 589 anti‐TNFα‐naive patients were registered, of whom 165 had a diagnosis of SpA; 7 patients with AS and 15 with PsA received >1 TNFα antagonist. Two patients with PsA were treated with all the drugs. In all, 16 subjects switched from infliximab to etanercept, 7 from etanercept to adalimumab and 1 from etanercept to infliximab. Overall, a clinical response was seen in 75% of patients who changed from infliximab to etanercept, and in 57.1% who switched from etanercept to adalimumab.
Conclusions
The findings of this study on a selected population of patients with SpA indicate that the failure of an initial TNFα antagonist does not preclude the response to another one. Further trials are needed to confirm this preliminary observation.
Keywords: spondyloarthropathy, ankylosing spondylitis, psoriatic arthritis, TNFα antagonists, switching
The tumour necrosis factor (TNF)α antagonists infliximab, etanercept and adalimumab have been shown to be effective in controlling symptoms in ankylosing spondylitis (AS) and psoriatic arthritis (PsA), and in retarding radiological progression in PsA. These drugs are currently reserved for patients who are non‐responsive to conventional treatments. Although there have been no direct comparison studies, the various TNFα antagonists appear to have similar efficacy, with a clinical response in 50–60% of patients. However, around 30% of patients with AS or PsA treated with these drugs withdraw from treatment due to adverse events (AEs) or loss of efficacy.1 Data from clinical trials or national registers suggest that patients with rheumatoid arthritis (RA) who do not tolerate or do not respond to a TNFα antagonist may be successfully treated with another.2,3,4,5 To date, there have been few data on spondyloarthropathy (SpA). Delaunay et al reported a favourable clinical outcome in 15 patients with SpA who switched from infliximab to etanercept because of inefficacy or intolerance.6 An open‐label, prospective study has recently demonstrated the efficacy and tolerability of etanercept in 23 patients with active AS who were resistant or intolerant to infliximab.7
In this paper, we report the results of an ongoing, longitudinal, observational study, and evaluate the clinical response after switching from one TNFα antagonist to another in patients with AS and PsA within a ‘real‐life' clinical setting.
Patients and methods
In this ongoing, longitudinal, observational study, we prospectively collected data since 2000 on efficacy and safety for patients starting biological treatments in our rheumatology division.
The present analysis was restricted to patients with a diagnosis of SpA who switched from one TNFα antagonist to another, with a minimum of 6 months' follow‐up by the end of December 2006 (the first SpA patient started treatment in December 2001). Patients with AS were classified according to the modified New York Criteria,8 and patients with PsA according to the Moll and Wright criteria modified by Helliwell.9 The choice of biological agent was based on clinical considerations only; thus, these patients represented a ‘real‐life' sample of subjects treated with TNFα antagonists. Infliximab 3–5 mg/kg was administered intravenously at weeks 0, 2 and 6, then every 6–8 weeks; etanercept (25 mg twice weekly) and adalimumab (40 mg alternate weekly) were given subcutaneously.
Clinical assessment
Patients were evaluated by the same rheumatologist at baseline (before starting the TNFα antagonist), every 3 months and at the last administration of the drug. Data, including demographics, diagnosis, date of diagnosis, comorbidities, past and present treatments, TNFα antagonist prescribed and date of beginning, concomitant medications, were recorded on a standardised form. Current disease activity in patients with AS was measured by the modified Ritchie Index,10 and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).11 Clinical assessment in patients with PsA was performed according to the Psoriatic Arthritis Response Criteria (PsARC).12 Each patient completed the Health Assessment Questionnaire (HAQ). Patient and physician global assessments (on a visual analogue scale, VAS) were also performed.13 Response to TNFα antagonist was defined by a 50% response on the BASDAI (BASDAI 50)14 for patients with AS, and by PsARC for patients with PsA. Drug discontinuation was based on the rheumatologist's opinion and the reason of withdrawal recorded as lack of efficacy (patients who never reached a satisfactory response), loss of efficacy (patients who relapsed after an initial good response), AEs or other. The wash‐out period between TNFα antagonists was 6 weeks.
Statistical analysis
Qualitative differences between subgroups were analysed by the χ2 and Fisher exact tests. The Wilcoxon paired test was used to compare quantitative variables in the same group. Statistical significance was set at p<0.05.
Results
The study comprised 589 anti‐TNFα‐naive patients, of whom 165 had a diagnosis of SpA. In total, 22 patients with SpA (7 patients with AS (mean age 33.5 years, range 22–49 years), and 15 with PsA (mean age 47.9 years, range 24–57 years)) received >1 TNFα antagonist (table 1). Two patients with PsA were consecutively treated with all three TNFα antagonists.
Table 1 Clinical and therapeutic features of the patients .
| Pt | Diag | Sex | Disease duration (months) | Infliximab | Etanercept | Adalimumab | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concomitant DMARD treatment | Treatment duration (months) | Reason for disconuation | Concomitant DMARD treatment | Treatment duration (months) | Currently on treatment | Reason for disconuation | Concomitant DMARD treatment | Treatment duration (months) | Currently on treatment | Reason for disconuation | |||||||||||||||||||
| 1 | AS | F | 48 | MTX | 12 | IRR | MTX | 21 | Yes | — | — | — | — | — | |||||||||||||||
| 2 | AS | F | 300 | — | 7 | IRR | — | 31 | Yes | — | — | — | — | — | |||||||||||||||
| 3 | AS | F | 288 | — | 20 | IRR | — | 18 | Yes | — | — | — | — | — | |||||||||||||||
| 4 | AS | M | 132 | — | 11 | LoE | — | 32 | Yes | — | — | — | — | — | |||||||||||||||
| 5 | AS | F | 144 | — | 24 | LoE | SSZ | 11 | Yes | — | — | — | — | — | |||||||||||||||
| 6 | AS | M | 60 | SSZ | 12 | LaE | SSZ | 3 | No | LaE | — | — | — | — | |||||||||||||||
| 7 | PsA | F | 96 | MTX | 5 | IRR | LFN | 33 | Yes | — | — | — | — | — | |||||||||||||||
| 8 | PsA | F | 192 | — | 3 | IRR | — | 36 | Yes | — | — | — | — | — | |||||||||||||||
| 9 | PsA | M | 60 | MTX | 12 | LoE | — | 32 | Yes | — | — | — | — | — | |||||||||||||||
| 10 | PsA | M | 84 | Cs | 36 | LoE | — | 12 | Yes | — | — | — | — | — | |||||||||||||||
| 11 | PsA | F | 372 | MTX | 27 | LoE | — | 24 | Yes | — | — | — | — | — | |||||||||||||||
| 12 | PsA | M | 72 | — | 25 | LoE | — | 18 | Yes | — | — | — | — | — | |||||||||||||||
| 13 | PsA | F | 72 | MTX | 10 | AE* | — | 24 | Yes | — | — | — | — | — | |||||||||||||||
| 14 | PsA | F | 228 | MTX | 7 | Other† | — | 6 | Yes | — | — | — | — | — | |||||||||||||||
| 15 | PsA | M | 72 | MTX | 6 | IRR | Cs | 12 | No | LoE | Cs | 6 | No | LaE | |||||||||||||||
| 16 | PsA | F | 336 | — | 8 | LoE | — | 3 | No | LaE | — | 4 | No | LaE | |||||||||||||||
| 17 | PsA | F | 60 | — | — | — | — | 3 | — | IRR | — | 9 | Yes | — | |||||||||||||||
| 18 | PsA | F | 60 | — | — | — | MTX | 12 | — | LoE | MTX | 3 | No | LaE | |||||||||||||||
| 19 | PsA | F | 60 | — | — | — | MTX | 6 | — | LoE | MTX | 16 | Yes | — | |||||||||||||||
| 20 | PsA | F | 24 | — | — | — | MTX | 12 | — | LoE | MTX | 3 | Yes | — | |||||||||||||||
| 21 | PsA | F | 65 | — | — | — | — | 3 | — | AE‡ | — | 16 | Yes | — | |||||||||||||||
| Etanercept | Infliximab | ||||||||||||||||||||||||||||
| 22 | AS | M | 36 | — | 6 | LoE | MTX | 16 | Yes | — | — | — | — | — | |||||||||||||||
Concom, concomitant; Cs, ciclosporin A; Diag, diagnosis; Discon, discontinuation; IRR, infusion/injection related reaction; LaE, lack of efficacy; LFN, leflunomide; LoE, loss of efficacy; MTX, methotrexate; SSZ, salazopyrine.
*Autoimmune hepatitis; †surgery for hip replacement; ‡hypertransaminasaemia.
A clinical response was seen in 12/16 (75%) patients who changed from infliximab to etanercept, and in 4/7 (57.1%) who switched from etanercept to adalimumab (figs 1 and 2). Patients who switched because of AEs and those who changed because of inadequate efficacy (ie patients who never reached a satisfactory response, or patients who relapsed after an initial good response) presented a similar clinical response (70% and 61.5%, respectively). The mean duration of etanercept treatment was longer than the previous infliximab treatment (19.7 vs 14.1 months, respectively; NS). The mean duration of treatment was not different in patients treated with adalimumab after etanercept (7.3 vs 8.1 months, respectively; NS) (table 1).
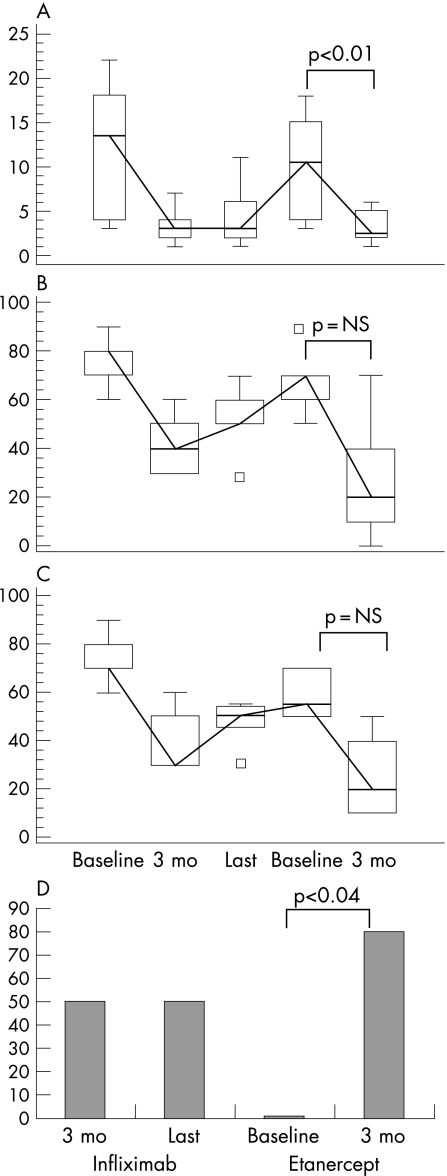
Figure 1 Clinical parameters in patients with AS (n = 6) who switched from infliximab to etanercept. Box and whiskers plot (median, quartiles, range and possible extreme values) of (A) Ritchie Index, (B) patient and (C) physician global assessment (on visual analogue scale); (D) percentage of patients who had 50% improvement on the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI 50). Values shown are the mean values at baseline (before infliximab treatment), after 3 months of infliximab treatment, at last visit while on infliximab, at baseline (before etanercept treatment) and after 3 months of etanercept treatment.
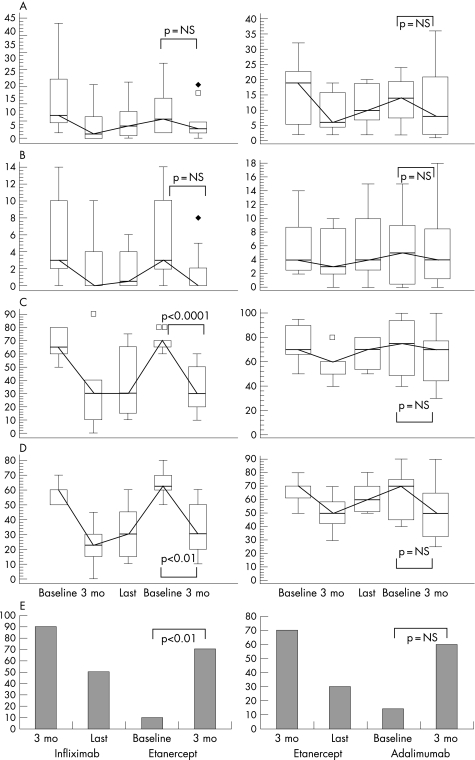
Figure 2 Clinical parameters in patients with psoriatic arthritis (PsA) who switched from infliximab to etanercept (n = 10, column 1) and from etanercept to adalimumab (n = 7, column 2). Box and whiskers plot (median, quartiles, range and possible extreme values) of (A) tender joint count, (B) swollen joint count, (C) patient and (D) physician global assessment (on visual analogue scale), and (E) percentage of patients who met Psoriatic Arthritis Response Criteria (PsARC). Values shown in column 1 are the mean values at baseline (before infliximab treatment), after 3 months of infliximab treatment, at last visit while on infliximab, at baseline (before etanercept treatment) and after 3 months of etanercept treatment. Values shown in column 2 are the mean values at baseline (before etanercept treatment), after 3 months of etanercept treatment, at last visit while on etanercept, at baseline (before adalimumab treatment) and after 3 months of adalimumab treatment.
Six patients with AS switched from infliximab to etanercept (mean age 31.8 years, range 16–49) (table 1). After 3 months of etanercept, the proportion of responders grew from 0% (baseline before etanercept) to 83.3% (p<0.04). Changes in Ritchie Index, patient and physician global assessment, and BASDAI 50 are shown in fig 1.
Ten patients with PsA switched from infliximab to etanercept (mean age 47 years, range 24–56). After 3 months of etanercept, the proportion of PsARC responders increased from 10% (baseline before etanercept) to 70% (fig 2), and HAQ score decreased significantly (p<0.01). Seven patients with PsA switched from etanercept to adalimumab (mean age 49.8 years, range 39–57). After 3 months of adalimumab, the proportion of PsARC responders increased from 14.3% (baseline before adalimumab) to 57.1% (fig 2).
One patient with AS received etanercept as first TNFα antagonist (table 1). He had a satisfactory response at 3 months, which was subsequently lost, thus he was switched to infliximab. After 3 months, a clinical response was seen.
Discussion
The findings of this longitudinal, observational study on a selected population of patients with SpA indicate that the failure of a first anti‐TNFα agent does not preclude the response to another, irrespective of the reason for switching.
The introduction of biological agents has allowed a radical change in the standard of treatment in SpA. Clinical response rates to anti‐TNFα agents range from 43% to 71% by BASDAI 50 in patients with AS and from 62% to 87% by PsARC in subjects with PsA.1 However, a significant proportion of patients withdraw from treatment because of failure or poor tolerability. Because the TNFα antagonists differ in chemical structure, mechanism of action and safety profile, there is a strong rationale for switching. Several reports support this possibility and, switching to another TNFα antagonist has now become a common practice in RA.2,3,4,5 Very few data are currently available for patients with SpA. Delaunay et al, in their retrospective evaluation, described 15 patients with various SpAs who switched from infliximab to etanercept because of AEs or inadequate efficacy. A clinical response was observed in 42.8% of the seven patients with AS and in all patients with undifferentiated SpA or PsA.6 A prospective study has recently reported the efficacy and tolerability of etanercept in 23 patients with AS who were resistant or intolerant to previous infliximab treatment. In particular, a 54‐week ASsessment in Ankylosing Spondylitis (ASAS) 20, ASAS 50, and ASAS 70 response rate was reported in 74%, 61%, and 39% of patients, respectively.7 In the present study, we evaluated the efficacy of switching between TNFα antagonists for patients with AS and PsA. Of the patients who switched from infliximab to etanercept, a clinical response was seen after 3 months in 83% of patients with AS (BASDAI 50) and in 70% of patients with PsA (PsARC). Of the patients with PsA who switched from etanercept to adalimumab, 57.1% showed a clinical response. Patients who switched because of AEs and those who changed because of inadequate efficacy presented a similar clinical response. Hence, our data concur with previous studies on RA, in which a better response to a second TNFα antagonist was observed in most patients, regardless of the reason of failure of the previous treatment.3,4,5 In our population, 81.3% of patients who had switched from infliximab to etanercept continued the treatment. Conversely, only 57.1% of patients who had changed from etanercept to adalimumab maintained the treatment. Interestingly, two of the three patients who stopped adalimumab because of inadequate response had already failed the other two TNFα antagonists. This observation seems to confirm previous data on RA patients, suggesting that the failure of two TNFα inhibitors predicts ineffectiveness to the third.15
There are some limitations to our study. First, it was a prospective, observational design without randomisation of treatment options. Second being a real‐life study, the decision to withdraw one TNFα antagonist and to switch to another depended only on the treating doctor's judgement.
In conclusion, the results of this study suggest that patients with SpA with inadequate response or AEs to one TNFα antagonist may be successfully treated with another, regardless of the reason for switching. Considering the sample size and the design of this study, larger prospective trials are warranted in order to confirm this observation.
Abbreviations
AE - adverse events
AS - ankylosing spondylitis
ASAS - ASsessment in Ankylosing Spondylitis
BASDAI - Bath Ankylosing Spondylitis Disease Activity Index
HAQ - Health Assessment Questionnaire
PsA - psoriatic arthritis
PsARC - Psoriatic Arthritis Response Criteria
RA - rheumatoid arthritis
SpA - spondyloarthropathy
TNF - tumour necrosis factor
VAS - visual analogue scale
Footnotes
Competing interests: None declared.
References
- 1.Kavanaugh A, Tutuncu Z, Catalan‐Sanchez T. Update on anti‐tumor necrosis factor therapy in the spondyloarthropathies including psoriatic arthritis. Curr Opin Rheumatol 200618347–353. [DOI] [PubMed] [Google Scholar]
- 2.van Vollenhoven R F. Switching between biological agents. Clin Exp Rheumatol 200422(Suppl)S115–S121. [PubMed] [Google Scholar]
- 3.Wick M C, Ernestam S, Lindblad S, Bratt J, Klareskog L, van Vollenhoven R F. Adalimumab (Humira®) restores clinical response in patients with secondary loss of efficacy from infliximab (Remicade®) or etanercept (Enbrel®): results from the STURE registry at Karolinska University Hospital. Scand J Rheumatol 200534353–358. [DOI] [PubMed] [Google Scholar]
- 4.Iannone F, Trotta F, Montecucco C, Giacomelli R, Galeazzi M, Matucci‐Cerinic M.et al Etanercept maintains the clinical benefit achieved by infliximab in patients with rheumatoid arthritis who discontinued infliximab because of side effects. Ann Rheum Dis 200766249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyrich K L, Lunt M, Watson K D, Deborah P, Symmons M, Silman AJ for the British Society for Rheumatology Biologics Register Outcomes after switching from one anti‐tumor necrosis factor α agent to a second anti‐tumor necrosis factor α agent in patients with rheumatoid arthritis. Results from a large UK national cohort study. Arthritis Rheum 20075613–20. [DOI] [PubMed] [Google Scholar]
- 6.Delaunay C, Farrenq V, Marini‐Portugal A, Cohen J D, Chevalier X, Claudepierre P. Infliximab to etanercept switch in patients with spondyloarthropathies and psoriatic arthritis: preliminary data. J Rheumatol 2005322183–2185. [PubMed] [Google Scholar]
- 7.Cantini F, Niccoli L, Benucci M, Chindamo D, Nannini C, Olivieri I.et al Switching from infliximab to once‐weekly administration of 50 mg etanercept in resistant or intolerant patients with ankylosing spondylitis: results of a fifty‐four‐week study. Arthritis Rheum 200655812–816. [DOI] [PubMed] [Google Scholar]
- 8.van der Linden S M, Valkenburg H A, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of New York criteria. Arthritis Rheum 198427361–368. [DOI] [PubMed] [Google Scholar]
- 9.Helliwell P, Marchesoni A, Peters M, Barker M, Wright V. A re‐evaluation of the osteoarticular manifestations of psoriasis. Br J Rheumatol 199130339–345. [DOI] [PubMed] [Google Scholar]
- 10.Gladman D D. Psoriatic arthritis. Baillieres Clin Rheumatol 19959319–329. [DOI] [PubMed] [Google Scholar]
- 11.Garrett S, Jenkinson T, Kennedy L G, Whitelock H, Gaisford P, Calin A. New approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994212286–2291. [PubMed] [Google Scholar]
- 12.Clegg D O, Reda D J, Mejias E, Cannon G W, Weisman M H, Taylor T.et al Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 1996392013–2020. [DOI] [PubMed] [Google Scholar]
- 13.Fries J F, Spitz P, Kraines R G, Holman H H. Measurement of patient outcome in arthritis. Arthritis Rheum 198023137–145. [DOI] [PubMed] [Google Scholar]
- 14.Braun J, Davis J, Dougados M, Sieper J, van der Linden S, van der Heijde D for the ASAS Working Group First update of the International ASAS Consensus Statement for the use of anti‐TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis 200665316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solau‐Gervais E, Laxenaire N, Cortet B, Dubucquoi S, Duquesnoy B, Flipo R ‐ M. Lack of efficacy of a third tumor necrosis factor α antagonist after failure of a soluble receptor and a monoclonal antibody. Rheumatology 2006451121–1124. [DOI] [PubMed] [Google Scholar]