Abstract
Objective
A study was undertaken to investigate the in vivo pathogenic role of Toll‐like receptor 4 (TLR‐4) in the antiphospholipid syndrome (APS) by studying the thrombogenic antiphospholipid (aPL) activity in lipopolysaccharide (LPS) non‐responsive (LPS–/–) mice and the association between tlr4 gene polymorphisms and APS in patients.
Methods
IgGs from two patients with APS, one with aPL negative systemic lupus erythematosus (SLE) and one with normal human serum (NHS), were evaluated for thrombosis, tissue factor (TF) activity and endothelial cell activation in LPS–/– mice displaying a tlr4 spontaneous mutation vs LPS responsive (LPS+/+) mice. Human tlr4 Asp299Gly and Thr399Ile polymorphisms were evaluated by allele‐specific PCR in 110 patients with APS with arterial/venous thrombosis and in 220 controls of the same ethnic origin.
Results
IgG‐APS produced significantly larger thrombi and more leucocytes (WBC) adhering to endothelial cells in the cremaster muscle microcirculation of LPS+/+ mice than IgG‐NHS or aPL negative SLE‐IgG. These effects were abrogated after absorption of the anti‐β2glycoprotein I activity by an affinity column. The two IgG‐APS induced significantly smaller thrombi and fewer WBC adhering to endothelial cells in LPS−/− mice than in LPS+/+ mice. IgG‐APS induced higher TF activity in carotid artery homogenates of LPS+/+ mice than in LPS−/− mice. The prevalence of Asp299Gly and Thr399Ile tlr4 polymorphisms was significantly lower than in controls.
Conclusions
These findings in LPS−/− mice and the reduction in the “protective” polymorphism in patients with APS with thrombosis suggest that TLR‐4 is involved in the interaction of aPL with endothelial cells in vivo.
Antiphospholipid (aPL) antibodies are associated with an increased risk of arterial and venous thrombosis and recurrent fetal loss. The presence of aPL antibodies in patients who experience these events defines the antiphos‐pholipid syndrome (APS).1 Although these antibodies were once believed to recognise anionic phospholipids directly, over the last decade it has become apparent that most of these antibodies instead recognise phospholipids binding proteins such as β2glycoprotein I (β2GPI).2,3,4
Previous studies have shown that plasma or serum from patients with aPL antibodies frequently contain antibodies reactive with endothelial cells (EC).5 Many of these antibodies induce EC activation as determined by measurement of the expression of endothelial cell adhesion molecules, secretion of inflammatory cytokines, upregulation of tissue factor (TF) or expression of procoagulant activity.5,6,7,8 Moreover, the ability of these antibodies to induce EC activation requires β2GPI and is mediated through pathways involving activation of nuclear factor‐κB (NF‐κB) and phosphorylation of p38MAPK.9,10,11 These observations support the hypothesis that binding of β2GPI to EC receptor, with receptor cross‐linking or clustering occurring as a result of the binding of anti‐β2GPI antibodies to receptor‐bound β2GPI, may lead to activation of the EC signalling response and cellular activation.
The nature of the endothelial receptor(s) for aPL/anti‐β2GPI antibodies is not completely known. Our group has previously shown that human β2GPI binds to EC through a cluster of lysine residues that are critical for anionic phospholipid binding and offers epitopes for anti‐β2GPI antibodies.12 Other studies have shown that annexin A2, an EC receptor for plasminogen and tissue type plasminogen activator, mediates EC activation by aPL/anti‐β2GPI antibodies.13,14 However, annexin A2 is not a transmembrane protein and it is unlikely that its interaction with β2GPI and anti‐β2GPI antibodies induces intracellular signalling.13,14 We have previously shown that the MyD88/TRAF6 signalling cascade is triggered by aPL antibodies reacting with β2GPI on the EC surface membrane, suggesting the involvement of Toll‐like receptor (TLR)‐4.15
TLR‐4 belongs to the family of TLRs,16 which are type I transmembrane receptors whose extracellular domain contains leucine‐rich repeats and whose cytoplasmic domains are analogous to that of the mammalian interleukin‐1 receptor family.17,18 Together with CD14 and the adaptor molecule MD2, TLR‐4 forms a receptor complex for lipopolysaccharide (LPS). Upon binding to this complex, LPS activates a signalling cascade which is characterised by activation of NF‐κB as well as p38MAPK, leading to subsequent induction of various genes and production of pro‐inflammatory cytokines. MyD88, a common adaptor protein for TLRs, mediates intracellular signal transduction after TLR‐4 activation.17
The C3H/HeJ mouse strain carries a missense point mutation within the tlr4 gene region encoding the cytoplasmic tail and this mutation changes a highly conserved proline to histidine, resulting in non‐responsiveness to LPS.19,20,21 Mutations in the tlr4 gene are also associated with endotoxin responsiveness in humans.22 There is evidence that the ability of certain individuals to respond properly to TLR ligands may be impaired by single nucleotide polymorphisms (SNPs) within tlr genes resulting in an altered susceptibility to infectious or inflammatory diseases. Most studies have focused on two co‐segregating SNPs—Asp299Gly and Thr399Ile—within the gene encoding tlr4. These SNPs are present in approximately 10% of white individuals.23 The prevalence of both tlr4 Asp299Gly and Thr399Ile polymorphisms has been associated with a decreased response to LPS in humans.22,24
In this study we investigated whether TLR‐4 is involved in aPL mediated thrombosis and EC activation in vivo by examining the in vivo effects of aPL antibodies in LPS non‐responsive and responsive mice. We also hypothesise that SNPs of the tlr4 gene may be less frequent in patients with APS, thus predisposing them to thrombosis and inflammatory responses. We therefore determined the prevalence of Asp299Gly and Thr399Ile polymorphisms in patients with APS and thrombosis and in control individuals.
Materials and methods
Mice
LPS responsive (LPS+/+) male mice (C3H/HeN) and LPS non‐responsive (LPS–/–) male mice (C3H/HeJ) weighing approximately 20 g were obtained from the Jackson Laboratories (Bar Harbor, Maine, USA). All animals were housed in the Animal Care (Association for Assessment and Accreditation of Laboratory Animal Care approved) facilities of the Morehouse School of Medicine. Animals were handled by trained personnel according to Institutional Animal Care and Use Committee guidelines.
Patients
One hundred and ten Caucasian patients with APS (33 men and 77 women) of mean (SD) age 40 (5) years were enrolled in the study. Patients with anti‐cardiolipin (aCL) and anti‐β2GPI antibody serum levels >40 GPL and >60 Standard G Units (SGU), respectively, were included; 62% of the patients had venous thrombosis, 30% had arterial thrombosis and 8% had both. All the patients satisfied the Sydney classification criteria for APS.1 The control group was represented by 220 age and sex matched healthy volunteers (blood donors and laboratory personnel) of the same ethnic group with no previous history of any significant clinical manifestation. Patients with APS and controls signed a consent form approved by the human studies committees of the various participating institutions.
Preparation of IgG‐APS
Total IgGs containing aPL antibodies from two consecutive patients with primary APS (IgG‐APS‐1 and IgG‐APS‐2) and from one patient with systemic lupus erythematosus (SLE) and no aPL (IgG‐SLE) were affinity purified using protein G Sepharose chromatography as previously described.25 The two patients with primary APS had thrombosis and one also had recurrent pregnancy losses. Human IgG from a healthy individual (IgG‐NHS) was purified by an identical method. High titres of aCL and anti‐β2GPI antibodies (as determined by ELISA) were found in both IgG‐APS fractions after purification, while IgG‐NHS and IgG‐SLE remained negative. Anti‐β2GPI antibodies were depleted from the IgG‐APS‐2 preparation using a β2GPI‐N‐hydroxysuccinamide activated Sepharose column as described in detail elsewhere.26 The sterile filtered IgG fractions were confirmed to be free of endotoxin contamination by the limulus amoebocyte lysate assay (E‐Toxate, Sigma, St Louis, Missouri, USA; sensitivity <0.05 IU/ml). The protein concentration was determined by the Bradford method.27 Levels of human aCL and anti‐β2GPI antibodies were measured by ELISA as previously described.25,26
Analysis of thrombus dynamics and adhesion of leucocytes to endothelium in LPS–/– mice
To investigate the role of TLR‐4 in thrombophilia and endothelium activation induced by aPL antibodies in vivo, LPS+/+ and LPS−/− mice (7–10 animals/group) were injected intraperitoneally with 500 µg IgG‐APS‐1 or IgG‐APS‐2 or with 500 µg IgG‐NHS, or with IgG‐SLE or with anti‐β2GPI depleted IgG‐APS‐2 twice (0 and 48 h later).25 Surgical procedures to study thrombus dynamics and adhesion of leucocytes to the endothelium of post‐capillary venules (number of adhering white blood cells (WBC)) in the exposed cremaster muscle (as an indication of endothelium activation) were performed 72 h after the first series of injections as described.7,25 IgG‐APS preparations injected into the mice were positive for aCL and anti‐β2GPI antibodies by ELISA (134 and 167 GPL units aCL for IgG‐APS‐1 and IgG‐APS‐2 and 208 and 250 SGU anti‐β2GPI for IgG‐APS‐1 and IgG‐APS‐2, respectively). Anti‐β2GPI depleted IgG‐APS‐2 was negative for aCL and anti‐β2GPI activity.
The mouse model of thrombosis used in this study has been described in detail.7,25 Briefly, mice were anaesthetised and the right femoral vein was exposed, resulting in a 0.5 cm segment of vein free for manipulation and observation. The vein was pinched with a pressure of 1500 g/mm2 applied to introduce a standardised injury that induced a clot. Clot formation and dissolution in the transilluminated vein were visualised with a microscope equipped with a closed circuit video system (including a colour monitor and recorder). Thrombus size (in square mm) was measured 1 min after the pinch injury by freezing the digitised image and tracing the outer margin of the thrombus. Three to five thrombi were successfully induced in each animal and mean values were computed. The number of WBC adhering (sticking) within five different post‐capillary venules in the cremaster muscle was determined and adhesion was defined as WBC that remained stationary for at least 30 s.7,25
Determination of TF activity in carotid artery homogenates in LPS–/– mice
This procedure was performed in the animals immediately after the surgical procedures and after they were killed. Pieces of approximately 5 mm of uninjured carotid arteries were dissected from both sides in each animal and collected in a TBS‐0.1% Triton X‐100 buffer containing heparin as anti‐coagulant. The samples were then homogenised. Homogenates of pooled carotid artery from four animals in each group were washed once with TBS‐0.1% Triton X‐100 containing heparin as anticoagulant and twice with TBS‐01% Triton X‐100, then resuspended in 50 μl of the same buffer and sonicated.28 TF activity was determined using a chromogenic assay that measures the conversion of factor X into factor Xa after activation by the TF‐factor VII complex (Actichrome TF, American Diagnostica, Stamford, Connecticut, USA). The amount of factor Xa generated was measured by its ability to cleave Spectroxyme Xa, a highly specific chromogenic substrate for factor Xa. The TF concentration in the lysates was measured in pM by extrapolation from a calibration curve. Results were expressed in pM/mg protein. Experiments were repeated three times for each pooled sample.
DNA extraction and tlr4 genotyping
Peripheral venous blood from patients with APS and healthy controls was collected in EDTA tubes. Genomic DNA was purified from frozen whole blood using the Wizard Genomic DNA Purification Kit (Promega, Madison, Wisconsin, USA). Genotyping was performed by PCR assays for tlr4 Asp299Gly and tlr4 Thr399Ile.29 The primer sequences for tlr4 Asp299Gly were: forward 5′ GAT TAG CAT ACT TAG ACT ACT ACC TCC ATG 3′; reverse 5′ GAT CAA CTT CTG AAA AAG CAT TCC CAC 3′; and for tlr4 Thr399Ile: forward 5′ GGT TGC TGT TCT CAA AGT GAT TTT GGG AGAA 3′, reverse 5′ ACC TGA AGA CTG GAG AGT GAG TTA AAT GCT 3′. The underlined bases in both forward primers indicate the restriction site for NcoI (tlr4 Asp299Gly) and HinfI (tlr4 Thr399Ile) restriction enzymes, respectively. Genomic DNA (20 ng) was amplified in a total volume of 50 μl containing dNTP (0.2 mM, Promega), primers (20 pmol/ml), Taq polymerase (0.25 μl, Promega) and buffer (Promega). The PCR reactions were run at 95°C for 5 min followed by 30 cycles with 95°C for 30 s, 55°C for 30 s and finally 72°C for 5 min. A 4 μl aliquot of the PCR product was digested with the appropriate restriction enzyme and electrophoresed in a 2% Nusieve GTG Agarose gel (Cambrex Bio Science Rockland Inc, Rockland Maine, USA) to identify the tlr4 alleles on the basis of the respective allele size. Both digestions give two clear bands in the heterozygote and single bands for both homozygotic mutant and wild type subjects. After digestion the wild type tlr4 allele sizes (249 bp for the 299 residue and 406 bp for the 399 residue) do not change; fragment sizes for carriers of the polymorphic allele decrease to 23 bp for the 299 residue and 377 bp for the 399 residue.
Statistical analysis
Non‐parametric statistical analyses were done for the experiments involving analysis of thrombus formation and adhesion of leucocytes to endothelium in the animals. Median (range) values are presented and Mann‐Whitney‐Wilcoxon scores (rank sums) were performed to compare the two groups of unpaired data. An independent t test was used to compare the aPL antibody levels and TF activities in different groups of mice. In the polymorphism studies the Fisher exact test was used to compare genotype frequencies between patient and control groups. p values <0.05 were considered significant. All statistical analyses were performed using SAS 9.1 software (SAS Institute, Cary, North Carolina, USA).
Results
IgG‐APS induced thrombophilia and endothelial cell activation in LPS−/− mice
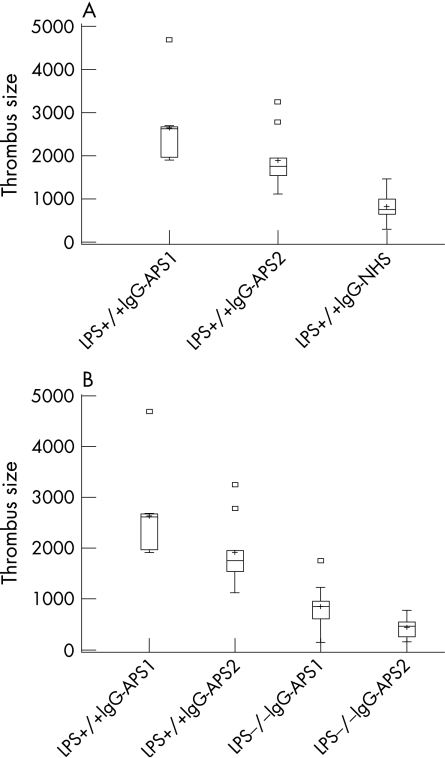
As indicated in fig 1A and table 1, thrombus size was significantly enhanced in LPS+/+ mice treated with IgG‐APS‐1 and IgG‐APS‐2 compared with LPS+/+ mice treated with IgG‐NHS (p = 0.004 for both IgG‐APS‐1 and IgG‐APS‐2, not shown in table 1). Thrombus size was not significantly different in LPS+/+ mice treated with IgG‐SLE or with anti‐β2GPI depleted IgG‐APS‐2 compared with LPS+/+ mice treated with IgG‐NHS (p = 0.8 and 0.9, respectively). The significant reduction in thrombus size after anti‐β2GPI antibody absorption from IgG‐APS‐2 in LPS+/+ mice indicates that, for the most part, the observed effects may be due to the anti‐β2GPI activity of the IgG‐APS preparation (p = 0.002). LPS−/− mice treated with IgG‐APS‐1 or IgG‐APS‐2 showed a significant reduction in thrombus size compared with LPS+/+ mice treated with the corresponding IgG‐APS (p = 0.004 and 0.005, respectively; fig 1B and table 1). Thrombus size in LPS−/− mice treated with IgG‐APS‐1 or IgG‐APS‐2 was not different from that of LPS−/− mice treated with IgG‐NHS, IgG‐SLE or anti‐β2GPI depleted IgG‐APS‐2 (p = 0.96, 0.28 and 0.4 for IgG‐APS‐1 and p = 0.2, 0.34 and 0.09 for IgG‐APS‐2) or LPS+/+ mice treated with IgG‐NHS, IgG‐SLE or anti‐β2GPI depleted IgG‐APS‐2 (p = 1.0, 0.8 and 0.9 for IgG‐APS‐1 and p = 0.5, 0.9 and 0.9 for IgG‐APS‐2).
Figure 1 Effect of anti‐phospholipid (aPL) antibodies on thrombosis in lipopolysaccharide (LPS) non‐responsive mice. LPS+/+ and LPS−/− mice in groups of 7–10 were injected with IgG from patients with antiphospholipid syndrome (IgG‐APS) or with IgG from normal human serum (IgG‐NHS) 0 and 48 h later (as described in the Methods section). The results are expressed as median (range) thrombus size in µm2. (A) The median thrombus size in IgG‐APS treated LPS+/+ mice (IgG‐APS‐1 and IgG‐APS‐2) was significantly different from the median thrombus size in IgG‐NHS treated LPS+/+ mice. (B) The median thrombus size in IgG‐APS treated LPS−/− mice were significantly different from the median thrombus size in LPS+/+ mice treated with IgG‐APS (IgG‐APS‐1 and IgG‐APS‐2). Squares indicate outliers. Plus signs indicate mean values.
Table 1 Effects of IgG‐APS on thrombus formation in LPS+/+ and LPS−/− mice.
| Treatment | Median (range) (μm2) | p Value |
|---|---|---|
| LPS+/+ mice | ||
| IgG‐NHS | 758.0 (300.0–1450.0) | |
| IgG‐APS‐1* | 2623.5 (1905.0–4689.0) | 0.004 |
| IgG‐APS‐2** | 1750.0 (1125.0–3250.0) | 0.005 |
| IgG‐SLE | 740.5 (602.0–950.0) | |
| Anti‐β2GPI depleted IgG‐APS‐2 | 825.0 (406.0–1125.0) | |
| LPS−/−mice | ||
| IgG‐NHS | 576.5 (115.0–1189.0) | |
| IgG‐APS‐1* | 845.0 (145.0–1756.0) | |
| IgG‐APS‐2** | 456.0 (149.0–778.0) | |
| IgG‐SLE | 576.5 (115–1189) | |
| Anti‐β2GPI depleted IgG‐APS‐2 | 756.0 (254–879) |
LPS, lipopolysaccharide; APS, antiphospholipid syndrome; NHS, normal healthy serum; SLE, systemic lupus erythematosus; β2GPI, β2glycoprotein I.
*p = 0.004 for comparison between groups with IgG‐APS‐1 injected into LPS+/+ vs LPS−/− mice.
**p = 0.005 for comparison between groups with IgG‐APS‐2 injected into LPS+/+ vs LPS−/− mice.
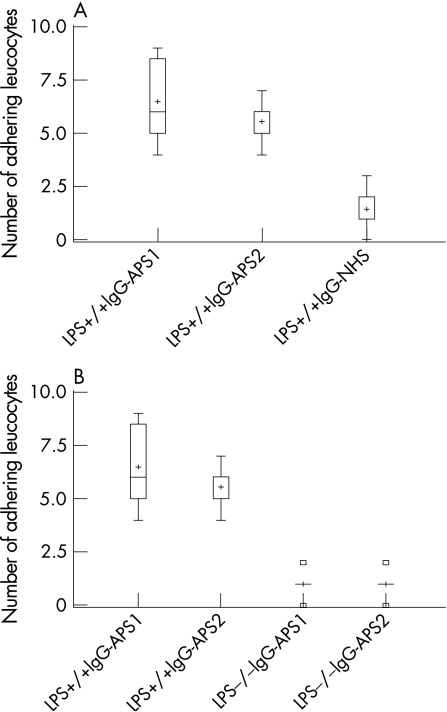
We then examined WBC adhesion to the endothelium of the cremaster muscle. As shown in fig 2 and table 2, in LPS+/+ mice IgG‐APS‐1 and IgG‐APS‐2 induced an increase in the number of WBC adhering to the cremaster endothelium compared with IgG‐NHS (p = 0.003 and 0.002, respectively; not shown in table 2). The number of WBC adhering to the endothelium of the cremaster muscle was not significantly different in LPS+/+ mice treated with IgG‐SLE or with anti‐β2GPI depleted IgG‐APS‐2 from LPS+/+ mice treated with IgG‐NHS (p = 0.1330 and 0.06, respectively; not shown in table 2). A significant reduction in the number of WBC adhering to the cremaster endothelium after anti‐β2GPI depletion again indicates that anti‐β2GPI activity of the IgG‐APS‐2 preparation was mainly responsible for the observed effects (p = 0.002 vs not depleted IgG‐APS‐2, not shown in table 2). Adhesion of WBC to the cremaster muscle endothelium induced by IgG‐APS‐1 or IgG‐APS‐2 was significantly reduced in LPS−/− compared with wild type mice (p = 0.003 and 0.004, respectively (table 2, fig 2B).
Figure 2 Effect of antiphospholipid (aPL) antibodies on adhesion of leucocytes to endothelium in lipopolysaccharide (LPS) non‐responsive mice. LPS+/+ and LPS−/− mice in groups of 7–10 were injected with IgG from patients with antiphospholipid syndrome (IgG‐APS) or with IgG from normal human serum (IgG‐NHS) 0 and 48 h later (as described in the Methods section). Adhesion of leucocytes (number of white blood cells (WBC)) to endothelium in post‐capillary venules of the cremaster muscle was measured as described in the Methods section. The results are expressed as the median (range) SD of the number of adhering WBC. (A) The median number of adhering WBC in LPS+/+ mice treated with IgG‐APS was significantly different from the median number of adhering WBC in LPS+/+ mice treated with IgG‐NHS (for IgG‐APS‐1 and IgG‐APS‐2). (B) The median number of adhering WBC in LPS−/− mice treated with IgG‐APS was significantly different from the median number of adhering WBC in LPS+/+ mice treated with IgG‐APS (for IgG‐APS‐1 and IgG‐APS‐2). Squares indicate outliers. Plus signs indicate mean values.
Table 2 Effects of IgG‐APS on adhesion of leucocytes to cremaster muscle in LPS+/+ and LPS−/− mice.
| Treatment | Median (range) number of WBC | p Value |
|---|---|---|
| LPS+/+ mice | ||
| IgG‐NHS | 1 (0–3) | |
| IgG‐APS‐1* | 6 (4–9) | 0.003 |
| IgG‐APS‐2† | 6 (4–7) | 0.004 |
| IgG‐SLE | 1 (0–1) | |
| Anti‐β2GPI depleted IgG‐APS‐2 | 1 (0–1) | |
| LPS−/− mice | ||
| IgG‐NHS | 0 (0–1) | |
| IgG‐APS‐1* | 1 (0–2) | |
| IgG‐APS‐2† | 1 (0–2) | |
| IgG‐SLE | 0 (0–0) | |
| Anti‐β2GPI depleted IgG‐APS‐2 | 1 (0–2) |
WBC, white blood cell; LPS, lipopolysaccharide; APS, antiphospholipid syndrome; NHS, normal healthy serum; SLE, systemic lupus erythematosus; β2GPI, β2glycoprotein I.
*p = 0.003 for comparison between groups with IgG‐APS‐1 injected into LPS +/+ vs LPS−/− mice.
†p = 0.004 for comparison between groups with IgG‐APS‐2 injected into LPS+/+ vs LPS−/− mice.
The adhesion of WBC in LPS−/− mice treated with IgG‐APS‐1 or IgG‐APS‐2 was not different from that of LPS−/− mice treated with IgG‐NHS or with anti‐β2GPI depleted IgG‐APS (p = 1.0, 0.7 for IgG‐APS ‐1 and p = 0.66 and 1.0 for IgG‐APS‐2) or that of LPS+/+ mice treated with IgG‐NHS (p = 0.3 for both IgG‐APS).
The titres of aCL and anti‐β2GPI antibodies in LPS+/+ mice injected with IgG‐APS‐1 or IgG‐APS‐2 were not different from those in LPS−/− mice treated with the same preparations at the time of the surgical procedures (50.8 (11.2) GPL units vs 48.2 (17.1) GPL units, p = 0.7016; 90.9 (14.2) GPL units vs 109.0 (12.8) GPL units, p = 0.89; 85.9 (12.5) SGU units vs 92.4 (24.8) SGU, p = 0.76; and 185.0 (30.3) SGU units vs 168.4 (24.1) SGU units, p = 0.80, respectively). This excludes the possibility that the protection observed against the thrombogenic and pro‐inflammatory effects of IgG‐APS observed in LPS−/− mice were related to differences in aCL/anti‐β2GPI antibody titres in the serum of the mice.
All the IgG fractions displayed endotoxin levels below 0.05 IU/ml. Although we cannot exclude small contamination in an absolute manner, the fact that NHS and SLE aPL negative IgG did not induce any thrombus formation speaks against the possibility of an effect unrelated to the aPL antibody activity.
Effect of IgG‐APS on TF activity in LPS−/− mice
TF activity was significantly increased in LPS+/+ mice treated with IgG‐APS‐1 compared with controls (p = 0.002). In LPS−/− mice injected with IgG‐APS‐1 the TF activity was abrogated by 89% (p = 0.0001, table 3).
Table 3 Effect of IgG‐APS on tissue factor (TF) activity in carotid artery homogenates of LPS−/− and LPS+/+ mice.
| Mice | Treatment | Mean (SD) TF activity (pM/mg protein) |
|---|---|---|
| LPS+/+ | IgG‐APS‐1 | 17.0 (2.0)† |
| LPS+/+ | IgG‐NHS | 1.0 (0.9) |
| LPS−/− | IgG‐APS‐1 | 2.3 (1.2)* |
LPS, lipopolysaccharide; APS, antiphospholipid syndrome; NHS, normal healthy serum.
*p = 0.0001 vs LPS+/+ mice treated with IgG‐APS‐1.
†p = 0.002 vs LPS+/+ mice treated with IgG‐NHS.
Frequency of Tlr4 polymorphisms in patients with aPL and thrombosis
Of the 110 APS patients tested, five were heterozygous for the Asp299Gly tlr4 allele and for the Thr399Ile tlr4 allele and only one was homozygous for both polymorphisms. Twenty‐five of 220 healthy subjects studied were heterozygous for the Asp299Gly and for the Thr399Ile tlr4 genes while none was homozygous. Co‐segregation of both polymorphisms was observed in all subjects. The frequency of tlr4 Asp299Gly and Thr399Ile polymorphisms in healthy subjects was 11.4% according to a previous study in Caucasian ethnic groups.23 Patients with APS had a significant reduction in this frequency (5%) compared with controls (p = 0.038, Fisher square test; table 4).
Table 4 Tlr4 genotypes in patients with antiphospholipid syndrome (APS) and in healthy controls.
| APS patients (n = 110) | Healthy controls (n = 220) | |
|---|---|---|
| tlr4 Asp299Gly polymorphism | ||
| Wild type A/A (%) | 104 (94.5) | 195 (88.6) |
| Heterozygotic mutant A/G (%) | 5 (4.54)* | 25 (11.4) |
| Homozygotic mutant G/G (%) | 1 (0.9) | 0 (0.0) |
| tlr4 Thr399Ile polymorphism | ||
| Wild type T/T (%) | 104 (94.5) | 195 (88.6) |
| Heterozygotic mutant T/i (%) | 5 (4.54)* | 25 (11.4) |
| Homozygotic mutant i/i (%) | 1 (0.9) | 0 (0) |
*p = 0.038, Fisher square test.
Discussion
This study shows for the first time that aPL mediated thrombogenic effects and the ability of aPL to activate EC in vivo are significantly reduced in mice that are non‐responsive to LPS. The TF activity in carotid artery homogenates was also significantly reduced in LPS−/− mice injected with aPL antibodies. These data are in agreement with our previous observation which showed that aPL/anti‐β2GPI antibodies induce intracellular signalling via MyD88, a pathway used by LPS when bound to TLR‐4 on EC.15
Previous studies have shown that annexin A2 mediates EC activation by aPL/anti‐β2GPI antibodies after binding to β2GPI.13,14 Interestingly, in a recent publication Cesarman‐Maus and collaborators showed that IgGs isolated from patients with APS have anti‐annexin A2 activity and that these antibodies induce TF expression in EC in vitro.30 Annexin A2 does not span the cell membrane, so this interaction may require an “adaptor” protein which is able to transduce intracellular signalling. Accordingly, there is preliminary evidence that TLR‐4 may be involved as a “co‐receptor” for annexin A2.31
This study strongly suggests that TLR‐4 associated events are at least one of the mechanism(s) involved in aPL mediated pathogenic effects in vivo. Similar to the description of the effects and intracellular events triggered by LPS ligation to TLR‐4, aPL/anti‐β2GPI antibodies activate EC through translocation of NF‐κB and phosphorylation of p38MAPK. This leads to the upregulation of pro‐inflammatory cytokines, cellular adhesion molecules and TF.5,9,10,11 However, aPL has been shown to be heterogeneous and TLRs widely expressed, so we cannot exclude the possibility that more than one mechanism/signalling pathway (or cell type other than endothelial) may be involved in causing thrombosis or in the steps upstream of clot formation.
In this study we used IgG‐APS isolated from two patients with primary APS. It is therefore unlikely that the effects observed in the animal models were due to other autoantibodies present in the preparations. In addition, we performed experiments with a whole IgG preparation from a patient with SLE and no aPL and found no thrombogenic effects or evidence of EC activation in mice.
In these experiments, enhanced thrombosis and increased adhesion of leucocytes to the endothelium were abrogated when IgG preparations were depleted of anti‐β2GPI antibodies. This decrease in the in vivo effects in mice treated with anti‐β2GPI depleted IgG‐APS was accompanied by a pronounced decrease in the titre of anti‐β2GPI antibodies in mouse serum. The effects observed in the mice are therefore most likely due to β2GPI dependent aPL antibodies. This finding is in line with previous results obtained in a different experimental model of aPL mediated thrombosis.26
The murine model of thrombosis and EC activation used in these studies is recognised as a suitable model for the study of the pathogenic role of human aPL antibodies.7,25 Furthermore, studies have shown that human aPL antibodies, such as those used in these studies, cross‐react with murine β2GPI.32
While aPL‐anti‐β2GPI antibodies are able to induce EC as well as monocyte activation per se in in vitro experimental models, there is sound evidence that complement (C′) involvement potentiates the aPL/anti‐β2GPI antibody mediated EC activation and is essential both for thrombosis and for fetal loss in vivo.26,33,34,35,36 Accordingly, we could speculate that EC membrane perturbation and C′ activation may act together in vivo, their parallel involvement being necessary since C′ blockade is able to protect from the aPL/anti‐β2GPI antibody thrombogenic effect in two different experimental models. Such a link may be related to the ability of C′ activation products (C3a, C5a and the membrane attack complex (MAC)) to specifically interact with cell membrane receptors cooperating in EC activation.37,38,39 Moreover, it has recently been reported that C5a generation can be induced by thrombin in the absence of C3.40 Hence, TLR‐4 dependent induction of endothelial TF by aPL/anti‐β2GPI antibodies (as shown in this paper) may favour the triggering of the coagulation cascade that ultimately activates C′ through some of its intermediates.
SNPs within tlr genes have been reported in humans. In particular, the Asp299Gly polymorphism has been associated with a decreased airway response to inhaled bacterial LPS and with an increased risk for Gram negative infections.23 The same polymorphisms have also been associated with the pathogenesis of different human diseases.41 Such associations have been related to an impaired inflammatory response at the cellular level by different groups,22,23,24 although not confirmed by others.42,43,44
The reduced prevalence of “protective”tlr4 polymorphisms observed in our study does not imply that IgG‐APS activates cells via TLR‐4 in humans, but it may suggest a higher susceptibility to a prothrombotic endothelium activation mediated by aPL antibodies. Alternatively, it is possible that “non‐protective” wild‐type alleles are over‐represented in patients with thrombosis, making them generally more susceptible to clotting. However, this does not seem to be the case since tlr4 polymorphisms have been associated with atherosclerotic plaque formation but not with atherothrombotic events,45 and the role of the inflammatory responses in venous thrombotic events in humans is still being debated.46 Further control of the role of tlr4 “protective” polymorphisms could be the demonstration of their increased prevalence in asymptomatic subjects with aPL or in women with recurrent fetal loss and without vascular events. However, it is very difficult to establish how long the follow‐up without thrombotic events should be before including true asymptomatic subjects with aPL. The presence or absence of “protective” tlr4 polymorphisms in patients with APS could represent a new tool to stratify patients according to their risk of developing the vascular manifestations of the syndrome.
Recurrent thrombotic events in spite of antithrombotic medications and severe side effects have raised the need for new and more efficient treatments for thrombosis in APS. Agents able to interfere with β2GPI binding to the receptor(s) and/or block cell signalling activation induced by aPL antibodies may ameliorate the procoagulant/proinflammatory phenotype and reduce the risk of thrombosis. Antagonists of the putative receptor(s) for β2GPI (ie, blocking antibodies or synthetic peptides displacing binding of β2GPI to cell membranes) might be a worthwhile approach.47,48
Acknowledgements
The authors thank Dr Anna Di Blasio (Istituto Auxologico Italiano) for her assistance in the analysis of the polymorphisms and Ms Sarah Tooms Smith, senior editor for the Sealy Center on Aging (University of Texas Medical Branch, Galveston, Texas), for her helpful comments on this manuscript.
Abbreviations
aCL - anti‐cardiolipin
aPL - antiphospholipid
APS - antiphospholipid syndrome
β2GPI - β2glycoprotein I
EC - endothelial cells
LPS - lipopolysaccharide
NF‐κB - nuclear factor‐κB
NHS - normal human serum
SLE - systemic lupus erythematosus
SNP - single nucleotide polymorphism
TF - tissue factor
TLR - Toll‐like receptor
Footnotes
This study was partially funded by a Research Center in Minority Institution grant (NIH grant #G12‐RR03034), a Minority Biomedical Research support grant from the NIH (#S02GMM08248) and Ricerca Corrente IRCCS Istituto Auxologico Italiano.
Competing interests: None.
References
- 1.Miyakis S, Lockshin M D, Atsumi T, Branch D W, Brey R L, Cervera R.et al International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 20064295–306. [DOI] [PubMed] [Google Scholar]
- 2.Galli M, Comfurius P, Maassen C, Hemker H C, de Baets M H, van Breda‐Vriesman P J.et al Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet 19903351544–1547. [DOI] [PubMed] [Google Scholar]
- 3.Matsuura E, Igarashi Y, Fujimoto M, Ichikawa K, Koike T. Anticardiolipin cofactor(s) and differential diagnosis of autoimmune disease. Lancet 1990336177–178. [DOI] [PubMed] [Google Scholar]
- 4.McNeil H P, Simpson R J, Chesterman C N, Krilis S A. Anti‐phospholipid antibodies are directed against a complex antigen that includes a lipid‐binding inhibitor of coagulation: β2glycoprotein I (apolipoprotein H). Proc Natl Acad Sci USA 1990874120–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meroni P L, Raschi E, Testoni C, Borghi M O. Endothelial cell activation by antiphospholipid antibodies. Clin Immunol 2004112169–174. [DOI] [PubMed] [Google Scholar]
- 6.Del Papa N, Guidali L, Sala A, Buccellati C, Khamashta M A, Ichikawa K.et al Endothelial cell target for antiphospholipid antibodies. Human polyclonal and monoclonal anti‐β2glycoprotein I induce endothelial cell activation. Arthritis Rheum 199740551–561. [DOI] [PubMed] [Google Scholar]
- 7.Pierangeli S S, Colden‐Stanfield M, Liu X, Barker J H, Anderson G H, Harris E N. Antiphospholipid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo. Circulation 1999991997–2000. [DOI] [PubMed] [Google Scholar]
- 8.Simantov E, LaSala J, Lo S K, Gharavi A E, Sammaritano L R, Salmon J E.et al Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest 1995962211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinola R G, Liu X, Colden‐Stanfield M, Hall J, Harris E N, Pierangeli S S. E‐selectin mediated pathogenic effects of antiphospholipid antibodies. J Thromb Haemost 20021843–848. [DOI] [PubMed] [Google Scholar]
- 10.Meroni P L, E Raschi, C Testoni, A Tincani, G Balestrieri, R Molteni, et al Statins prevent endothelial cell activation induced by anti‐phospholipid (anti‐β2 glycoprotein I) antibodies: effect on the pro‐adhesive and pro‐inflammatory phenotype. Arthritis Rheum 2001442870–2878. [DOI] [PubMed] [Google Scholar]
- 11.Vega‐Ostertag M, Casper K, Swerlick R, Ferrara D, Harris E N, Pierangeli S S. Involvement of p38 MAPK in the up‐regulation of tissue factor on endothelial cells by antiphospholipid antibodies. Arthritis Rheum 2005521545–1554. [DOI] [PubMed] [Google Scholar]
- 12.Del Papa N, Sheng Y H, Raschi E, Kandiah D A, Tincani A, Khamashta M A.et al Human β2glycoprotein I binds to endothelial cells through a cluster of lysine residues that are critical for anionic phospholipids binding and offers epitopes for anti‐β2glycoprotein I antibodies. J Immunol 19981605572–5578. [PubMed] [Google Scholar]
- 13.Ma K, Simantov R, Zhang J, Silverstein R, Hajjar K, McCrae K. High affinity binding of β2glycoprotein I to human endothelial cells is mediated by annexin II. J Biol Chem 200027515541–15548. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, McCrae K R. Annexin A2 mediates endothelial cell activation by antiphospholipid/β2glycoprotein I antibodies. Blood 20051051964–1969. [DOI] [PubMed] [Google Scholar]
- 15.Raschi E, Testoni C, Bosisio D, Borghi M O, Koike T, Mantovani A.et al Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood 20031013495–3500. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Takeda K, Kaisho T. Toll‐like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 20012675–680. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. Toll‐like receptor downstream signalling. Arthritis Res Ther 2005712–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beutler B, Du X, Poltorak A. Identification of Toll‐like receptor 4 (TLR‐4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies. J Endotoxin Res 20017277–280. [PubMed] [Google Scholar]
- 19.Akira S, Takeda K. Functions of toll‐like receptors: lessons from KO mice. CR Biol 2004327581–589. [DOI] [PubMed] [Google Scholar]
- 20.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y.et al Cutting edge: Toll‐like receptor 4 (TLR‐4)‐deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR‐4 as the LPS gene product. J Immunol 19991623749–3752. [PubMed] [Google Scholar]
- 21.Kmonickiva E, Zldek Z. Quantitative aspects of lipopolysaccharide and cytokine rrequirements to generate nitric oxide in macrophages from LPS‐hyporesponsive (Lps(d)) C3H/HeJ mice. Folia Microbiol 200449737–744. [DOI] [PubMed] [Google Scholar]
- 22.Arbour N C, Lorenz E, Schutte B C, Zabner J, Kline J N, Jones M.et al TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 200025187–191. [DOI] [PubMed] [Google Scholar]
- 23.Schröder N W J, Schumann R R. Single nucleotide polymorphisms of Toll‐like receptors and susceptibility to infectious disease. LancetInfect Dis20055156–164. [DOI] [PubMed] [Google Scholar]
- 24.Fageras‐Bottcher M, Hmani‐Aifa M, Lindstrom A, Jenmalm M C, Mai X M, Nilsson L.et al A TLR4 polymorphism is associated with asthma and reduced lipopolysaccharide‐induced interleukin‐12(p70) responses in Swedish children. J Allergy Clin Immunol 2004114561–567. [DOI] [PubMed] [Google Scholar]
- 25.Pierangeli S S, Liu X W, Anderson G H, Barker J H, Harris E N. Induction of thrombosis in a mouse model by IgG, IgM and IgA immunoglobulins from patients with the antiphospholipid syndrome. Thromb Haemost 1995741361–1367. [PubMed] [Google Scholar]
- 26.Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R.et al Thrombus formation induced by antibodies to β2‐glycoprotein I is complement‐dependent and requires a priming factor. Blood 20051062340–2346. [DOI] [PubMed] [Google Scholar]
- 27.Bradford M M. A refined and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Ann Biochem 197672248–254. [DOI] [PubMed] [Google Scholar]
- 28.Day S M, Reeve J L, Pedersen B, Farris D M, Myers D D, Im M.et al Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood 2005105192–198. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz E, Frees K L, Schwartz D A. Determination of the TLR‐4 genotype using allele‐specific PCR. Biotechniques 20013122–24. [DOI] [PubMed] [Google Scholar]
- 30.Cesarman‐Maus G, Rios‐Luna N P, Deora A B, Villa R, Craviolo M C, Alarcon‐Segovia D.et al Autoantibodies against the fibrinolytic receptorm annexin 2, in antiphospholipid syndrome. Blood 20061074375–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Lieske K, Mc Crae B, McCrae K. Activation of endothelial cells by β2glycoprotein I (β2GPI) antibodies is mediated by annexin II cross linking and may involve TLR‐4 (abstract). Blood 200410483 [Google Scholar]
- 32.Tincani A, Spatola L, Prati E, Allegri F, Ferremi P, Cattaneo R.et al The anti‐β2‐glycoprotein I activity in human anti‐phospholipid syndrome sera is due to monoreactive low‐affinity autoantibodies directed to epitopes located on native β2‐glycoprotein I and preserved during species evolution. J Immunol 19961575732–5738. [PubMed] [Google Scholar]
- 33.Salmon J E, Girardi G, Holers V M. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis 20026146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girardi G, Berman J, Redecha P, Spruce L, Thurman J, Young K.et al Complement C5a receptors and neutrophils mediate fetal injury in the antiphophospholipid syndrome. J Clin Invest 20031121644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Girardi G, Redecha P, Salmon J. Heparin prevents antiphospholipid antibody‐induced fetal loss by inhibiting complement activation. Nat Med 2004101222. [DOI] [PubMed] [Google Scholar]
- 36.Pierangeli S S, Girardi G, Vega‐Ostertag M E, Liu X, Espinola R G, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody‐mediated thrombophilia. Arthritis Rheum 2005522120–2124. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda K, Nagasawa K, Horiuchi T, Tsuru T, Nishizaka H, Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb Haemost 199777394–398. [PubMed] [Google Scholar]
- 38.Hattori E, Hamilton K K, McEver R P, Sims P J. Complement proteins C5b‐9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP‐140 to the cell surface. J Biol Chem 19892649053–9060. [PubMed] [Google Scholar]
- 39.Fischetti F, Tedesco F. Cross‐talk between the complement system and endothelial cells in physiologic conditions and in vascular diseases. Autoimmunity 200639417–428. [DOI] [PubMed] [Google Scholar]
- 40.Huber‐Lang M, Sarma J V, Zetoune F S, Rittisrch D, Neff T A, McGuire S R.et al Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med 200612682–687. [DOI] [PubMed] [Google Scholar]
- 41.Cook D N, Pisetsky D S, Schwartz D A. Toll‐like receptors in the pathogenesis of human disease. Nat Immunol 20045975–979. [DOI] [PubMed] [Google Scholar]
- 42.Erridge C, Stewart J, Poxton I R. Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the Toll‐like receptor 4 gene show no deficit in lipopolysaccharide signalling. J Exp Med 20031971787–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Aulock S, Schroder N W, Gueinzius K, Traub S, Hoffmann S, Graf K.et al Heterozygous toll‐like receptor 4 polymorphism does not influence lipopolysaccharide‐induced cytokine release in human whole blood. J Infect Dis 2003188938–943. [DOI] [PubMed] [Google Scholar]
- 44.van der Graaf C, Kullberg B J, Joosten L, Verver‐Jansen T, Jacobs L, Van der Meer J W.et al Functional consequences of the Asp299Gly Toll‐like receptor‐4 polymorphism. Cytokine 200530264–268. [DOI] [PubMed] [Google Scholar]
- 45.Pasterkamp G, van Keulen J K, de Kleijn D V P. Role of Toll‐like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Invest 200434328–334. [DOI] [PubMed] [Google Scholar]
- 46.Fox E A, Kahn S R. The relationship between inflammation and venous thrombosis. Thromb Haemost 200594362–365. [DOI] [PubMed] [Google Scholar]
- 47.Rossignol D P, Lynn M. TLR‐4 antagonists for endotoxemia and beyond. Curr Opin Invest Drugs 20056496–502. [PubMed] [Google Scholar]
- 48.Vega‐Ostertag M E, Liu X, Pierangeli S S. A peptide that mimics the Vth region of β2glycoprotein I reverses antiphospholipid‐mediated thrombosis. Lupus 200615358–365. [DOI] [PubMed] [Google Scholar]