Abstract
Objectives
Antiphospholipid antibodies (aPLA) have been shown to enhance thrombus formation by increasing the expression of adhesive receptors such as P‐selectin on endothelial cells. The P‐selectin counter‐receptor on leucocytes is P‐selectin glycoprotein ligand‐1 (PSGL‐1). We have previously described a variable number of tandem repeats (VNTR) polymorphism in the mucin‐like region of PSGL‐1, with three alleles: allele A, 16 repeats; allele B, 15 repeats; and allele C, 14 repeats.
Methods
We compared the PSGL‐1 VNTR allele and genotype frequencies in 90 patients with antiphospholipid syndrome (APS) with thrombosis, 39 patients with persistent aPLA positivity without thrombosis, and 203 healthy controls.
Results
The frequency of the B allele was significantly higher in patients with APS with thrombosis compared with patients without thrombosis (p = 0.023). When we compared the groups by genotype frequencies, we found a markedly higher frequency of the AB genotype in patients with APS with thrombosis than in aPLA‐positive patients without thrombosis (38.9% vs 10.3%, p = 0.001) or in normal population (38.9% vs 22.2%, p<0.01).
Conclusions
We suggest that the VNTR polymorphism of PSGL‐1 is a significant determinant of thrombotic predisposition in patients with APS. Furthermore, risk appears to correlate best with the combination of alleles inherited rather than with the presence of any particular allele.
Keywords: antiphospholipid syndrome, P‐selectin, P‐selectin glycoprotein ligand‐1, PSGL‐1 VNTR polymorphism, thrombosis
The pathophysiological mechanisms causing the thrombotic complications of antiphospholipid syndrome (APS) have not been clarified. It is believed that antiphospholipid antibodies (aPLA) produce thrombosis by directly affecting the coagulation cascade or anticoagulant pathways. It has also been shown that aPLA activate endothelial cells, increasing expression of adhesive proteins such as P‐selectin and intercellular cell adhesion molecule‐1.1,2,3,4 Pierangeli et al showed that aPLA‐induced leucocyte adhesion was completely abrogated in mice deficient in both intercellular cell adhesion molecule‐1 and P‐selectin.5
P‐selectin mediates the attachment and rolling of leucocytes on activated endothelial cells, and is involved in the recruitment of leucocytes to thrombi. P‐selectin glycoprotein ligand‐1 (PSGL‐1) is the major leucocyte counter‐receptor for P‐selectin. PSGL‐1 is a transmembrane protein that exists as a disulphide‐linked homodimer. Its ligand‐binding region is separated from the plasma membrane by a mucin‐like domain. PSGL‐1 has two important properties that facilitate its interaction with P‐selectin: (1) it is located on the tips of microvilli that extend away from the cell body, allowing easier access to P‐selectin on another cell, and (2) the structure of PSGL‐1 itself allows the ligand‐binding region to extend away from the membrane.6
We have described a variable number of tandem repeats (VNTR) polymorphism in the mucin‐like region of PSGL‐1, with three alleles encoding the individual length variants: allele A, with 16 repeats; allele B, 15 repeats; and allele C, 14 repeats.7 The length of the PSGL‐1 extracellular domain has been estimated at 500 Å. The PSGL‐1 VNTR polymorphism affects the length of the mucin‐like region of PSGL‐1, and each decameric repeat is approximately 25 Å in length.7 Although the region affected by the polymorphism probably does not interact directly with P‐selectin, variation in the number of repeats may affect leucocyte tethering, either by changing the distance of the ligand‐binding domain from the plasma membrane in homozygous individuals, or by changing the relationship of P‐selectin‐binding sites on adjacent polypeptides within the dimer in heterozygous people.
In this study, we evaluated PSGL‐1 polymorphisms in aPLA‐positive patients with and without thrombosis. We found that, consistent with the role of PSGL‐1 in anchoring leucocytes and cell‐derived microparticles, the PSGL‐1 polymorphism is a significant determinant of thrombotic risk in patients with APS.
Patients and methods
The study was approved by local ethics committee. Informed consent was obtained from all participants. APS was diagnosed according to the Sapporo criteria.1
Patients with APS with thrombosis
In total, 90 patients with APS (71 with primary APS and 19 with systemic lupus erythematosus; median age 36 years, range 15 to 61; female:male ratio 70:20) with arterial and/or venous thrombosis were included. LA, lupus anticoagulant was positive in 68 patients, and anticardiolipin IgG and IgM were positive in 49 and 48 patients, respectively. In total, 26 patients had had pregnancy morbidity previously, and 21 had thrombocytopenia.
Patients positive for aPLA with no thrombosis
There were 39 patients in the group with persistent positive aPLA, who either had experienced first trimester fetal loss (26 patients) or who had thrombocytopenia (21 patients). Median age was 35 years (range 21 to 56), and female:male ratio was 37:2. LA was positive in 26 patients, and anticardiolipin IgG and IgM were positive in 28 and 22 patients, respectively. None of these patients developed thrombotic complications in the 3‐year follow‐up period. Patients with second or third trimester fetal loss or pre‐eclampsia were not included.
Normal controls
The controls comprised 203 healthy people with no history of autoimmune disease or thrombosis.
Genotyping
Genomic DNA was extracted from citrated blood samples. The oligonucleotide primers that encompass the decameric consensus repeat sequences in exon 2 of the PSGL‐1 gene were 5′‐CCT GTC CAC GGA TTC AGC‐3′ (forward) and 5′‐GGG AAT GCC CTT GTG AGT AA‐3′ (reverse). PCR products were separated on agarose gels and visualised by ethidium bromide staining. Bands of 558, 528, and 498 bp corresponded to alleles A, B and C, respectively.7
Statistical analysis
The χ2 test was used to calculate the 95% confidence intervals and p values for crude odds ratios (OR). Adjusted ORs were determined by multiple logistic regression. The statistical analysis was performed with SAS V.9.0 (SAS Institute, Cary, North Carolina, USA).
Results
Allele and genotype frequencies of PSGL‐1 VNTR polymorphism in patients and controls are shown in table 1.
Table 1 PSGL‐1 allele and genotype frequencies in patients with APS and controls .
| Thrombosis | AA, n (%) | AB, n (%) | AC, n (%) | BB, n (%) | A allele, n (%) | B allele, n (%) | C allele, n (%) |
|---|---|---|---|---|---|---|---|
| Thrombosis total (n = 90) | 52 (57.8) | 35 (38.9) | 0 | 3 (3.3) | 139/180 (77.2) | 41/180 (22.8) | 0 |
| Arterial total (n = 51) | 33 (64.7) | 17 (33.3) | 0 | 1 (2) | 83/102 (81.4) | 19/102 (18.6) | 0 |
| Venous total (n = 52) | 28 (53.8) | 21 (40.4) | 0 | 3 (5.8) | 77/104 (74) | 27/104 (26) | 0 |
| Without thrombosis (n = 39) | 32 (82) | 4 (10.3) | 1 (2.6) | 2 (5.1) | 69/78 (88.5) | 8/78 (10.2) | 1/78 (1.3) |
| Normal population (n = 203) | 139 (68.5) | 45 (22.2) | 9 (4.4) | 10 (4.9) | 332/406 (81.8) | 65/406 (16) | 9/406 (2.2) |
The difference in B allele frequency in patients with APS with thrombosis compared with patients without thrombosis was statistically significant (p = 0.02, OR = 2.2, 95% CI 1.09 to 4.5). This difference was especially significant in patients with APS with venous thrombosis (p<0.01, OR 2.5, 95% CI 1.21 to 5.26).
The AB genotype was significantly more prevalent among patients with APS with a history of thrombosis than in either patients without a thrombotic complication (p = 0.001, OR = 5.56, 95% CI 1.8 to 17) or in the normal population (p<0.01, OR = 2.23, 95% CI 1.3 to 3.8). When we compared the frequency of AB genotype in patients with APS with venous thrombosis with those with no thrombosis and with the normal population, the presence of the AB genotype was significantly higher in patients with APS with venous thrombosis (p = 0.001, OR = 5.92, 95% CI 1.8 to 19.1 vs p<0.05, OR = 2.37, 95% CI 1.2 to 4.5). The AB genotype frequency was also significantly higher in patients with APS with arterial thrombosis compared with patients without thrombosis (p = 0.01, OR 4.37, 95% CI 1.3 to 14.3). Adjusted ORs from multiple logistic regressions remained significant for the above comparisons (table 2).
Table 2 AB genotype frequencies in patients with APS with thrombosis compared with those without thrombosis and with the normal population.
| Groups | Crude OR* (95% CI) | Adjusted OR*(95% CI) | Crude OR†(95% CI) | Adjusted OR†(95% CI) |
|---|---|---|---|---|
| Thrombosis | 5.56‡ (1.8 to 17) | 4.75‡ (1.47 to 15.37) | 2.23‡ (1.3 to 3.8) | 2.50‡ (1.41 to 4.43) |
| Arterial | 4.37‡ (1.3 to 14.3) | 4.22‡ (1.19 to 14.97) | 1.75 (0.8 to 3.4) | 1.98 (0.96 to 4.12) |
| Venous | 5.92‡ (1.8 to 19.1) | 5.08‡ (1.34 to 19.32) | 2.37‡ (1.2 to 4.5) | 2.44‡ (1.24 to 4.79) |
*Comparison with the group without thrombosis, ORs adjusted for age, gender, thrombocytopenia and lupus anticoagulant positivity.
†Comparison with the normal population, ORs adjusted for age and gender only.
‡p<0.05.
Discussion
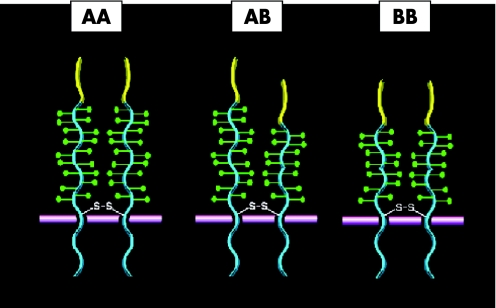
The P‐selectin/PSGL‐1 interaction is crucial in inflammation and thrombosis. P‐selectin on the activated platelets and endothelial cells binds to PSGL‐1 on the monocytes or on the leucocyte‐derived microparticles, and stimulates tissue factor expression.8 It has been shown that blockade of P‐selectin or PSGL‐1 prevents fibrin formation in animal models.9 Tissue‐factor accumulation and fibrin generation were both markedly reduced in mice deficient in P‐selectin or PSGL‐1.10 These findings show that interaction of P‐selectin with PSGL‐1 on microparticles is crucial for fibrin generation. It therefore seems reasonable to speculate that structural differences that enhance the P‐selectin/PSGL‐1 interaction may increase the likelihood of thrombosis. Because PSGL‐1 exists on the membrane as a homodimer, individuals heterozygous for the VNTR polymorphism would be expected to have at least half of the dimers composed of polypeptides of different lengths, which would probably change the relationship of the ligand‐binding regions of the two polypeptides. In homozygotes, the distance of the ligand‐binding domains from the plasma membrane would vary depending on the alleles inherited (fig 1).
Figure 1 PSGL‐1 VNTR polymorphisms.
We found that AB‐carrying patients with APS had increased risk for both arterial and venous thrombosis. The presence of BB genotype however, was not associated with thrombotic risk. We did not detect the C allele in patients with APS with thrombosis, which supports the notion that the C allele may protect against thrombosis. Known thrombophilic mutations largely produce loss of function (ie deficiencies of protein C, protein S and antithrombin). In the factor V Leiden mutation, factor V gains resistance to inactivation by activated protein C. The prothrombin gene mutation is associated with raised levels of prothrombin in the plasma. In all of these mutations, people who were homozygous for the mutation were more severely affected than heterozygotes. Interestingly, and in contrast, the risk of thrombosis associated with the PSGL‐1 VNTR polymorphism is greatest in those with the AB genotype compared with those homozygous for the genotype. As the frequency of AC and BC genotypes were very low in our study population, it is difficult to determine if these heterozygous states are also associated with an increased thrombotic risk. We know of no other prothrombotic alleles in which heterozygosity carries a higher risk than homozygousity.
The effect of PSGL‐1 VNTR polymorphisms on thrombosis has been investigated in only a few case–control studies. Lozano et al found that the alleles encoding the smaller isoforms (B and C) were associated with a lower risk of developing cerebral thrombosis.11 They also showed that neutrophils carrying the shortest C allele exhibited a significantly lower capacity to bind activated platelets. Similarly, Roldan et al suggested that the shorter alleles are protective for coronary thrombosis.12 Tregouet et al13 found that people carrying the B and C alleles had lower plasma PSGL‐1 levels, and suggested that the length of PSGL‐1 might alter the stability of PSGL‐1.
In summary, this is the first clinical study demonstrating that a genetic polymorphism of a leucocyte adhesion molecule affects the thrombotic risk of patients with APS. The findings indicate that the P‐selectin/PSGL interaction may be important in initiating thrombosis in certain pathological situations, and suggest that therapies that target this interaction might be especially efficacious in these situations.
Abbreviations
aPLA - antiphospholipid antibodies
APS - antiphospholipid syndrome
PSGL‐1 - P‐selectin glycoprotein ligand‐1
VNTR - variable number of tandem repeats
Footnotes
This work was supported by the Research Fund of the Istanbul University (Project number: T‐967/19022001), TUBA‐GEBIP (R.D.K./TUBA‐GEBIP/2004‐15), and by a grant from National Institutes of Health of the United States (Grant number RO1HL65205).
Competing interests: None declared.
References
- 1.Wilson W A, Gharavi A E, Koike T, Lockshin M D, Branch D W, Piette J C.et al International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome. Arthritis Rheum 1999421309–1311. [DOI] [PubMed] [Google Scholar]
- 2.Levine J, Branch W, Rauch J. The antiphospholipid syndrome. N Engl J Med 2002346752–763. [DOI] [PubMed] [Google Scholar]
- 3.Amengual O, Atsumi T, Kahmashta M A. Tissue factor in antiphospholipid syndrome: shifting the focus from coagulation to endothelium. Rheumatology 2003421029–1031. [DOI] [PubMed] [Google Scholar]
- 4.Blank M, Shoenfeld Y, Cabilly S, Heldman Y, Fridkin M, Katchalski‐Katzir E. Prevention of experimental antiphospholipid syndrome and endothelial cell activation by synthetic peptides. Proc Natl Acad Sci USA 1999965164–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierangeli S S, Espinola R G, Liu X, Harris E N. Thrombogenic effects of antiphospholipid antibodies are mediated by intercellular cell adhesion molecule‐1, vascular cell adhesion molecule‐1, and P‐selectin. Circ Res 200188245–250. [DOI] [PubMed] [Google Scholar]
- 6.McEver R P, Cummings R D. Perspective series: cell adhesion in vascular biology: role of PSGL‐1 bindings to selectins in leukocyte recruitment. J Clin Invest 1997100485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afshar‐Kharghan V, Diz‐Kucukkaya R, Ludwig E H, Marian A J, López J A. Human polymorphism of P‐selectin glycoprotein ligand‐1 attributable to variable numbers of tandem decameric repeats in the mucinlike region. Blood 2001973306–3307. [DOI] [PubMed] [Google Scholar]
- 8.Furie B, Furie B C. Thrombus formation in vivo. J Clin Invest 20051153355–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers D, Wrobleski S, Londy F, Fex B, Hawley A, Schaub R.et al New and effective treatment of experimentally induced venous thrombosis with anti‐inflammatory rPSGL‐Ig. Thromb Haemost 200287374–382. [PubMed] [Google Scholar]
- 10.Falati S, Liu Q, Gross P, Merrill‐Skoloff G, Chou J, Vandendries E.et al Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P‐selectin glycoprotein ligand‐1 and platelet P‐selectin. J Exp Med 20031971585–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozano M L, Gonzales‐Conejero R, Corral J, Rivera J, Iniesta J A, Martinez C.et al Polymorphisms of P‐selectin glycoprotein ligand‐1 are associated with neutrophil‐platelet adhesion and with ischaemic cerebrovascular disease. Br J Haematol 2001115969–976. [DOI] [PubMed] [Google Scholar]
- 12.Roldan V, Gonzales‐Conejero R, Marin F, Pineda J, Vicente V, Corral J. Short alleles of P‐selectin glycoprotein ligand‐1 protect against premature myocardial infarction. Am Heart J 2004148602–605. [DOI] [PubMed] [Google Scholar]
- 13.Tregouet D A, Barbaux S, Poirier O, Blankenberg S, Bickel C, Escolano S.et al SELPG gene polymorphisms in relation to plasma SELPG levels and coronary artery disease. Ann Hum Genet 200367504–511. [DOI] [PubMed] [Google Scholar]