Abstract
Objectives
Increased numbers of neutrophils expressing proteinase 3 on their membrane (mPR3) have been reported in anti‐neutrophil cytoplasm antibody (ANCA)‐associated vasculitis (AAV) and are suggested to be involved in AAV immunopathogenesis. In most studies, neutrophils were analysed for mPR3 expression without priming with TNFα, suggesting that mPR3 expression on neutrophils is dependent on other priming events, such as isolation procedures . These priming events can be variable. Therefore, we analysed mPR3 expression on neutrophils before and after priming with TNFα to assess whether standardised assessment of mPR3 expression requires priming. Using neutrophils before and after priming with TNFα, we assessed percentages of mPR3+ neutrophils in patients with AAV and in disease and healthy controls.
Methods
Neutrophils from patients with PR3‐AAV and MPO‐AAV, systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), and from healthy controls were analysed before and after priming with TNFα for mPR3 expression.
Results
42% of all individuals analysed showed minimal expression for mPR3 on all neutrophils before priming with TNFα, whereas after priming a clear mPR3+ subset was observed next to mPR3– neutrophils, corresponding to bimodal mPR3 expression. In patients with PR3‐AAV or MPO‐AAV, the percentage of mPR3+ neutrophils after priming with TNFα was significantly increased (p<0.01 and p<0.05, respectively) compared with healthy controls. Percentages of mPR3+ PMN were also increased in patients with SLE (p<0.01) but not in RA.
Conclusion
Standardised assessment of proteinase 3 on the membrane of neutrophils requires priming with TNFα. Percentages of mPR3+ PMN are increased in AAV and SLE, but not in RA.
Keywords: proteinase 3, Wegener's granulomatosis, systemic lupus erythematosus, vasculitis, chronic inflammation
Proteinase 3 (PR3) is a proteolytic enzyme that is mainly stored in the azurophilic granules and, to a lesser extent, in the specific granules and secretory vesicles of neutrophils.1,2,3 Upon stimulation with a low dose of pro‐inflammatory cytokines such as TNFα,4,5,6 by isolation procedure7 or by in vitro incubation,7,8 the enzyme proteinase 3 is translocated to the cell membrane. This process is called priming . Translocation of PR3 from the different pools to the cell membrane is sequentially regulated, starting with exocytosis of readily mobilisable secretory vesicles, followed by specific granules and, finally, by limited exocytosis of azurophilic granules.1,2,3 On the basis of membrane‐bound proteinase 3 (mPR3) expression, two subsets of neutrophils can be defined: neutrophils that hardly express proteinase 3 (mPR3– neutrophils) after priming, and neutrophils that substantially express proteinase 3 (mPR3+ neutrophils) after priming.7,9,10,11 The percentage of mPR3+ neutrophils ranges from 0% to 100% of the total population of neutrophils within individuals.7,9,10,11,12 Individuals in whom both subsets are simultaneously present are designated as bimodal for mPR3 expression. When only one subset (a population of only mPR3– or mPR3+ neutrophils) is present, individuals are designated as monomodal in mPR3 expression. In addition, the percentage of mPR3+ neutrophils is stable over time in a particular individual and is not affected by neutrophil activation, disease activity or treatment, which implies genetic control.7,9,10,12
In Wegener's granulomatosis (WG), the percentage of mPR3+ neutrophils increases.7,10,12,13 WG is a systemic autoimmune disease characterised by the presence of ANCA, which are in most cases directed against PR3.14,15,16 PR3‐ANCA can activate neutrophils in vitro, resulting in degranulation and oxidative burst.4,17,18,19 mPR3 expression is a prerequisite for neutrophil activation after stimulation with PR3‐ANCA and, as such, of major importance in immunopathogenesis.20,21
In RA, the percentage of mPR3+ neutrophils has been reported to increase as well,7 although others did not find increased numbers of mPR3+ neutrophils in chronic inflammatory disease controls such as RA or SLE.6 In these studies, neutrophils were analysed for mPR3 expression without priming with TNFα. mPR3 expression on mPR3+ neutrophils is normally not observed in whole‐blood experimental procedures, implying that neutrophils need priming to express mPR3.7,8 Isolation procedures alone can prime neutrophils, possibly owing to mechanical stress during centrifugation procedures, which results in translocation of PR3 to the plasma membrane.22 Induction of mPR3 expression on mPR3+ neutrophils during isolation procedures is not controllable and may vary between experiments. Hence, percentages of mPR3‐expressing neutrophils may not be accurately assessed when neutrophils are not additionally primed with TNFα.
In the present study, we first analysed mPR3 expression on neutrophils before and after priming with TNFα to investigate whether assessment of mPR3 expression on neutrophils requires ex vivo priming with TNFα. Furthermore, we studied expression of activation markers on mPR3– and mPR3+ neutrophils. Using neutrophils before and after priming with TNFα, we assessed whether the percentage of mPR3+ neutrophils increased in patients with ANCA‐associated vasculitis. To assess the specificity of the suggested increase in percentages of mPR3+ neutrophils for AAV, we included disease controls from patients with RA and SLE.
Materials and methods
Patients and controls
The percentage of mPR3+ neutrophils is thought to be genetically determined, and so it is not affected by age, disease activity or treatment.7,9,10,12 Therefore, we included consecutive patients with ANCA‐associated vasculitis, SLE and RA.
ANCA‐associated vasculitis: A diagnosis of WG, Churg Strauss syndrome (CSS) or microscopic polyangiitis (MPA) was established according to the Chapel Hill criteria.23 PR3–ANCA or MPO‐ANCA was determined by an indirect immunofluorescence (IIF) assay on ethanol‐fixed neutrophils and by capture ELISA with specificity for PR3 or MPO, as described in previous reports .24,25 The PR3‐AAV group consisted of 25 patients with WG. The MPO‐AAV group consisted of five patients with WG, five patients with MPA, two patients with CSS and two patients with unclassified MPO‐associated vasculitis.
Rheumatoid arthritis(RA): 25 patients fulfilling the criteria of the American College of Rheumatology for definite RA were included.26
Systemic lupus erythematosus(SLE): 25 patients fulfilling the American College of Rheumatology criteria for SLE were included.27
Healthy controls: Healthy laboratory personnel were included as controls (n = 25).
Additional information on patients and controls is given in Table 1.
Table 1 Patient characteristics.
| HC | PR3‐ANCA | MPO‐ANCA | RA | SLE | |
|---|---|---|---|---|---|
| Number | 25 | 25 | 14 | 25 | 25 |
| Gender | |||||
| Male | 15 | 20 | 5 | 7 | 4 |
| Female | 10 | 5 | 9 | 18 | 21 |
| Mean age (years) | 42±9 | 59±16 | 53±18 | 50±13 | 49±14 |
| Inactive disease | — | 24(96%) | 12(93%) | 15(60%) | 24(96%) |
| Treatment at the time of testing: | |||||
| CP, AZA, MTX or MMF | — | 12(48%) | 5(38%) | 18(72%) | 9(36%) |
| Prednisolone | — | 7(28%) | 5(38%) | 1(4%) | 10(40%) |
HC, healthy control; PR‐ANCA, PR3‐ANCA‐associated vasculitis; MPO‐ANCA, MPO‐ANCA‐associated vasculitis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; CP, cyclophosphamide; AZA, azathioprine; MTX, methotrexate; MMF, mycophenolate mofetil.
Isolation and priming of neutrophils
Neutrophils were isolated from EDTA‐anticoagulated blood by centrifugation on Polymorphprep™ (Nycomed, Oslo, Norway) and hypotonic lysis of contaminating erythrocytes with ice‐cold ammonium chloride buffer. Cells were washed with cold Hanks' balanced salt solution (HBSS) without Ca2+/Mg2+ (GIBCO/Life Technologies, Breda, The Netherlands) and resuspended in HBSS with Ca2+/Mg2+ (GIBCO/Life Technologies, Breda, The Netherlands) to obtain 1×107 cells/ml. Part of the sample was primed with 2 ng/ml of recombinant TNFα (Boehringer Mannheim, Germany) for 15 min at 37°C before analysis of membrane expression. Non‐primed neutrophils were analysed for membrane expression immediately after isolation.
Membrane expression on neutrophils
Membrane expression of proteinase 3, elastase, TNFα receptor I, TNFα receptor II and CD35 (or complement receptor 1, CR1) were measured using flow cytometry as previously described.10 All steps were performed on ice. Shortly , samples containing 106 neutrophils were fixed with 0.5% paraformaldehyde for 10 min, washed with PBS/1% BSA by centrifugation at 1200 g, 4°C for 3 min, and incubated with 0.5 mg/ml heat‐aggregated goat immunoglobulins (IgG; Sigma) for 15 min to saturate Fcγ receptors. Next, cells were stained with a saturating dose of mouse monoclonal IgG1 antibody (MAb) directed against human PR3 (PR3G‐3),28 human elastase (NP57, Dako Cytomation, Glosstrup. Denmark), TNFRI (clone:16830, R&D systems, Minneapolis, USA), TNFRII (clone: 22235, R&D systems, Minneapolis, USA), human CD35 (Ber‐Mac‐DRC, Dako Cytomation, Glosstrup, Denmark) or with an irrelevant IgG1 control antibody (MCG1; IQProducts, Groningen, The Netherlands) for 30 min. Next, non‐bound antibodies were washed off with PBS/1% BSA. This step was followed by 30 min incubation with phycoerythrin (PE)‐conjugated goat anti‐mouse antibody (Southern Biotechnology Associates, Birmingham, AL USA) in the presence of 0.5 mg/ml heat‐aggregated goat IgG and a subsequent washing step. Fluorescence intensity was analysed on an ELITE flow cytometer (Becton Dickinson Immunocytometry Systems, Mountain View, CA, USA) and calibrated using Flow‐Set™ fluorospheres (Beckman Coulter, Hialeah, FL, USA). Bimodal mPR3 expression was defined as the presence of 10%–90% mPR3+ cells.10 The percentage of mPR3+ cells within primed neutrophils was calculated by integration of the peak representing the mPR3+ cells compared with the peak of mPR3– cells, irrespective of the isotype control. The level of PR3– or CD35 expression was calculated as mean fluorescence intensity (MFI of PR3 or CD35) of specific binding corrected for non‐specific binding of the isotype control antibody (MFI NSB).10
Statistical analysis
Results are expressed as mean±SEM. Statistical analysis was performed using Mann–Whitney and GraphPad Prism, version 3.0 (GraphPad Software, San Diego, CA).
Results
Patterns of mPR3 expression before and after priming with TNFα
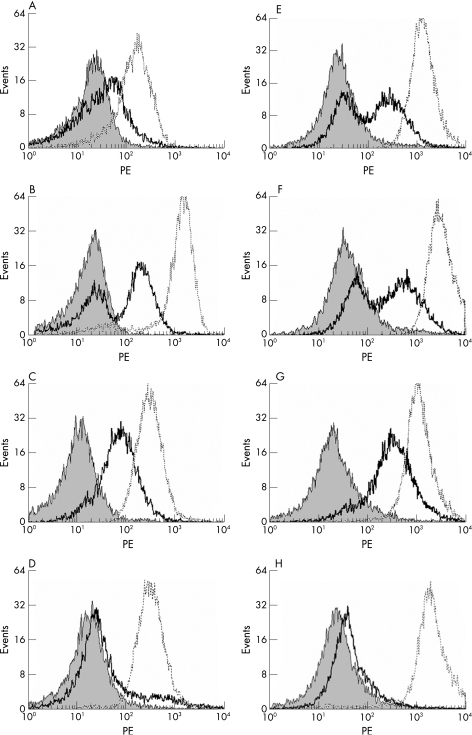
We analysed mPR3 expression on neutrophils of patients (n = 89) and healthy controls (n = 25) before and after priming with TNFα (2 ng/ml). On the basis of the percentage of mPR3‐expressing neutrophils before and after priming with TNFα, we could distinguish the following mPR3 expression patterns: (1) individuals (n = 48) who did not have a separate population of mPR3‐expressing neutrophils before priming with TNFα (monomodal low mPR3 expression), but after priming had a separate population of mPR3‐expressing neutrophils (10–90% mPR3+ cells) that could be observed (bimodal mPR3 expression) (fig 1A and E); (2) individuals (n = 38) in whom bimodal mPR3 expression could be observed before and after priming with TNFα (fig 1B and F); (3) individuals (n = 16) in whom all neutrophils showed apparent mPR3 expression before and after priming with TNFα (>90% mPR3+ cells) (fig 1C and G); and (4) individuals (n = 12) in whom all neutrophils showed a lack of mPR3 expression before and after priming with TNFα (<10% mPR3+ cells) (fig 1D and H). Thus, additional priming with TNFα was needed to translocate PR3 substantially to the membrane of neutrophils in 41% of the individuals analysed (compare fig 1A with B).
Figure 1 Patterns of mPR3 expression before and after priming with TNFα. Neutrophils were isolated and stained for mPR3 expression before (A–D) and after priming with TNFα (E–H). (A, E) Representative overlay of an individual with monomodal expression of mPR3 on neutrophils before priming (A), and (E) a bimodal expression of mPR3 observed after priming. (B, F) An individual in whom before (B) and after priming (F) bimodal mPR3 expression is observed. (C, G) An individual in whom all neutrophils before (C) and after priming (G) show apparent mPR3 expression (>90% mPR3+ cells). (D, H) An individual in whom all neutrophils show a lack of mPR3 expression before (D) and after (H) priming (>90% mPR3– cells). The grey peak represents isotype control; the black line represents mPR3 expression, and CD35 expression is represented by the dashed line.
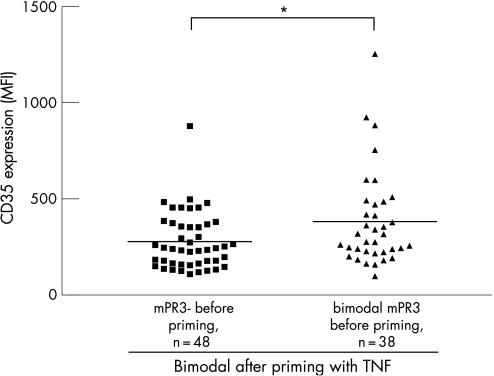
Next, we compared levels of the activation marker CD35 on neutrophils of individuals with monomodal low mPR3 expression before priming with TNFα with bimodal mPR3 expression after priming , with the expression of this marker on neutrophils from individuals already bimodal in mPR3 expression before priming. A significantly higher level of CD35 expression was present on neutrophils of the latter individuals compared with neutrophils of the former individuals before priming (382±41 MFI, n = 38, and 278±21 MFI, n = 48; p<0.05) (fig 2). CD35 expression reflects the activation state of neutrophils. The presence of a higher level of CD35 expression on neutrophils that express mPR3 without additional priming with TNFα suggests therefore that these neutrophils already have a higher level of activation compared with neutrophils that lack mPR3 expression before priming but become mPR3+ afterwards. After priming with TNFα, no significant difference in CD35 expression was present between both of these groups (data not shown).
Figure 2 CD35 expression on non‐primed neutrophils from individuals with different patterns of mPR3 expression before and after priming with TNFα. Analysis of CD35 expression showed a significantly (p<0.05) lower level of CD35 expression on neutrophils of individuals who were monomodal before priming but bimodal for mPR3 expression after priming compared with individuals already bimodal in mPR3 expression before priming. The horizontal line denotes the mean. *p<0.05.
Differential mPR3 expression is independent of other activation markers
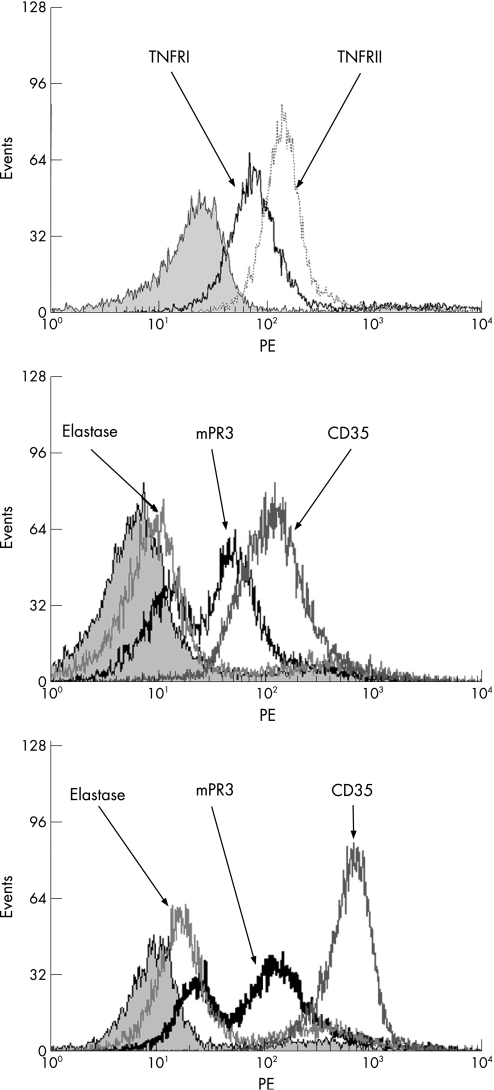
To investigate whether differential mPR3 expression in bimodal individuals results from differences in mobilisation of the secretory vesicles that transport PR3 to the membrane, we analysed CD35 levels on mPR3+ and mPR3– subsets in bimodal individuals. mPR3+ neutrophils in bimodal individuals did not have higher levels of CD35 compared with mPR3– neutrophils in these bimodal individuals, indicating that there are no differences in mobilisation of secretory vesicles between the subsets (data not shown). In addition, analysis of the expression of TNFα receptor I and TNFα receptor II on neutrophils in bimodal individuals demonstrated uniform expression of these receptors on mPR3+ and mPR3– subsets, suggesting that differential mPR3 expression after priming with TNFα is not caused by differences in expression of TNFα receptor between mPR3+ and mPR3– subsets (fig 3A). The percentage of mPR3‐expressing neutrophils in bimodal individuals were stable before and after priming with TNFα (data not shown). Membrane expression of elastase on non‐primed and primed neutrophils was weakly present and always uniform (fig 3B and 3C). Occasionally, we observed a slight upregulation of mPR3 after priming with TNFα on the mPR3– subset in bimodal individuals that was comparable with elastase expression. (fig 3B and C).
Figure 3 Monomodal expression of TNFRI, TNFRII, elastase and CD35 in bimodal mPR3 individuals. (A) Neutrophils of a bimodal mPR3 individual were isolated and stained for TNFRI (black line) and TNFRII (dashed line). Isotype control is represented by the grey peak. (B, C) Neutrophils of a bimodal mPR3 individual showing monomodal expression of elastase and CD35 before (B) and after (C) priming with TNFα.
Percentage of mPR3‐expressing neutrophils in inflammatory diseases
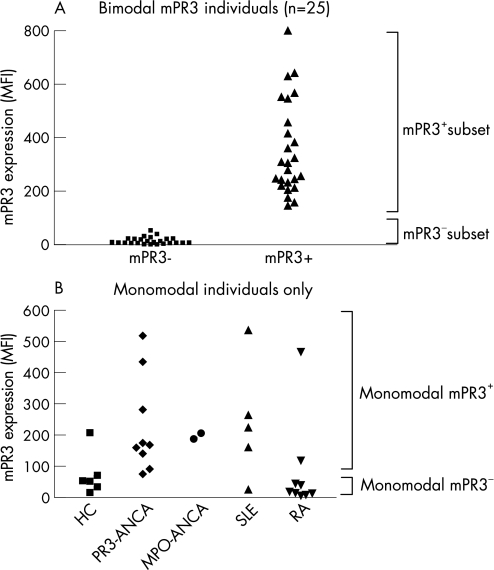
Priming with TNFα is required for standardised assessment of mPR3 expression on neutrophils, and so we analysed the percentage of neutrophils expressing mPR3 in patients with PR3‐AAV (n = 25), MPO‐AAV (n = 14), SLE (n = 25), RA (n = 25) and healthy controls (n = 25) after priming with TNFα. In this analysis, individuals who were monomodal in mPR3 expression were also included. To define monomodal mPR3– or mPR3+ individuals, we analysed mPR3 expression on the mPR3– subset and the mPR3+ subset in a random sample of 25 bimodal individuals (fig 4A). mPR3 expression on neutrophils of the mPR3– subset never reached expression levels higher than 60 MFI. Therefore an MFI of 60 was defined as the cut‐off value for mPR3 expression (fig 4A).
Figure 4 mPR3 expression on mPR3 subsets in bimodal individuals and monomodal individuals. In (A), a random sample of bimodal individuals (patients and healthy controls, n = 25) was analysed for expression of mPR3 after priming of neutrophils with TNFα. On the basis of these values, mPR3– neutrophils were defined as having an mPR3 expression ⩽60 MFI and mPR3+ neutrophils as having an mPR3 expression >60 MFI. In (B), mPR3 expression of all individuals monomodal in their mPr3 expression is depicted. Results are expressed as MFI of mPR3 from which binding of an irrelevant isotype control was withdrawn.
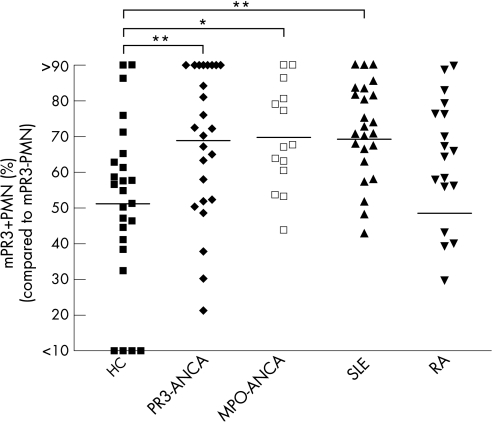
On the basis of this definition, monomodal mPR3+ individuals were defined as individuals in whom >90% of neutrophils had mPR3 expression exceeding 60 MFI, and monomodal mPR3– individuals as individuals in whom >90% of neutrophils had mPR3 expression below 60 MFI. Patients and controls who had a monomodal expression of mPR3 are depicted in figure 4b with their respective mPR3 expression and subsequent division into monomodal mPR3– and mPR3+ individuals. Including both monomodal and bimodal mPR3 individuals, we observed that patients with PR3‐AAV and MPO‐AAV had a significantly higher percentage of mPR3+ neutrophils (68.9±4.1%, n = 25, and 69.8±3.9%, n = 14, respectively ) than healthy controls (51.2±4.7%, n = 25; p<0.01 and p<0.05, respectively) (fig 5). Furthermore, patients with SLE also showed significantly higher percentages (69.3±3.6%, n = 25; p<0.01 ) of mPR3+ neutrophils compared with controls (fig 5). Percentages of mPR3+ PMN were not increased in patients with RA (48.5±5.7%, n = 25) (fig 5).
Figure 5 The percentage of mPR3+ neutrophils increased in ANCA‐associated vasculitis and SLE after priming with TNFα. Patients with PR3‐ANCA‐associated vasculitis (PR3‐ANCA, n = 25), MPO‐ANCA‐associated vasculitis (MPO‐ANCA, n = 14), SLE (SLE, n = 25), RA (RA, n = 25) and healthy controls (HC, n = 25) were analysed for their percentage of mPR3+ neutrophils after priming with TNFα. *p<0.05, **p<0.01. The horizontal line denotes the mean.
Discussion
In the present study, we first analysed whether priming of neutrophils is required for standardised assessment of the percentage of mPR3‐expressing neutrophils among individuals. In almost half of all individuals with bimodal mPR3 expression, substantial mPR3 expression on mPR3+ neutrophils was only seen after priming with TNFα. In these individuals, distinction between mPR3–and mPR3+ neutrophils could only accurately be made after ex vivo priming with TNFα. Neutrophils of these individuals had a lower expression of the activation marker CD35 than individuals already showing bimodal mPR3 expression before priming with TNFα. Therefore, neutrophils expressing mPR3 without additional priming with TNFα have a higher state of activation than neutrophils that do not express mPR3 before but do express mPR3 after priming. This suggests that neutrophils expressing mPR3 without additional priming with TNFα are already in a primed state. By contrast, neutrophils not expressing mPR3 before priming with TNFα but doing so after priming have not reached the level of activation needed for mPR3 expression on their potentially mPR3‐expressing neutrophils. Therefore, priming with TNFα is necessary to establish the percentage of mPR3+ neutrophils among individuals.
We, additionally, observed that the presence of mPR3− and mPR3+ neutrophils among individuals with bimodal mPR3 expression did not originate from differential mobilisation of secretory vesicles or differences in activation state between both subsets of neutrophils after priming with TNFα because both mPR3+ and mPR3– subsets expressed comparable levels of CD35. Elastase, another proteinase, showed uniform expression on all neutrophils as well. Analysis of the expression of TNFα receptor I and II on neutrophils demonstrated uniform expression of these receptors on the two mPR3 subsets, suggesting that differential mPR3 expression is not caused by differences in responsiveness to priming with TNFα. These observations are in line with other studies7,9,20 showing that the presence of mPR3+ and mPR3 subsets of neutrophils is not the result of differences in activation, mobilisation of vesicles or responsiveness of those subsets to priming with TNFα. By contrast, the present study suggests that mPR3+ neutrophils have to reach a certain level of activation to substantially express proteinase 3 on their membrane. So, mPR3+ neutrophils translocate proteinase 3 to the membrane when primed, and the percentage of this subset of neutrophils is stable within an individual, which is in agreement with other studies.7,9,10,12
How PR3 is attached to the membrane of neutrophils is still not clear. One may hypothesise that these binding sites of PR3, which have, as yet, not been characterised, vary among individuals. This variance may explain the presence of the mPR3–and mPR3+ subsets. By contrast, the observed subsets of neutrophils could, as well, be the result of differences in transport of PR3 from granules or vesicles to the membrane of neutrophils.
In the present study, we standardised the assessment of mPR3 expression on neutrophils in patients with AAV and disease controls by priming isolated neutrophils with TNFα. The percentage of mPR3+ neutrophils increased in patients with PR3‐AAV and MPO‐AAV and SLE compared with healthy controls, but not in RA. Also in a small subgroup (n = 3) of patients with rheumatoid vasculitis, the percentage of mPR3+ neutrophils was not higher than that in the remaining 22 RA patients without vasculitis (data not shown). This contrasts with a study by Witko‐Sarsat et al. who showed increased percentages of mPR3+ neutrophils in RA.7 In the study of Witko‐Sarsat et al., however, neutrophils were not additionally primed with TNFα, and, as we show in the present study, neutrophils need to be primed for standardised assessment of the percentage of mPR3+ neutrophils among individuals. Therefore, assessment of the percentage of mPR3+ neutrophils without additional priming, as carried out by Witko‐Sarsat et al., is less accurate and more dependent on priming resulting from isolation procedures in vitro or active disease in vivo. Muller‐Kobold et al. have already showed that neutrophils from patients with WG with active disease have an increased expression of mPR3 on mPR3+ neutrophils compared with patients with quiescent disease.29
In PR3‐AAV, autoantibodies directed against PR3 (PR3‐ANCA) activate neutrophils by binding to PR3 on the membrane of neutrophils.4,19,20,21 As a consequence, increased numbers of neutrophils able to express PR3 after priming are an obvious risk factor in this disease.10 Increased numbers of mPR3+ neutrophils were also found in MPO‐AAV and SLE, although the group of MPO‐AAV was relatively small in number and heterogeneous in clinical presentation. The clinical significance of the increased mPR3 expression on neutrophils in these disorders is not yet clear. Increased mPR3 expression might play a part in the pathophysiology of autoimmune inflammatory diseases. mPR3 on neutrophils is catalytically active against extracellular matrix proteins such as fibronectin and, surprisingly, resistant to inhibition by physiological inhibitors.30 PR3 has diverse functions, such as cleavage of major pro‐inflammatory cytokines such as TNFα, IL‐1 and IL‐18 into a bioactive form, whereas other neutrophil serine proteases such as elastase do not.31 Furthermore, PR3 has caspase‐like activities because it cleaves the cell‐cycle inhibitor p21, and, as such, induces apoptosis in endothelial cells.32 Having these functions, PR3 can be regarded as an important regulator of inflammation.31 Membrane expression of bioactive PR3 can, therefore, contribute to the inflammatory process.
In conclusion, standardised assessment of proteinase 3 on the membrane of neutrophils requires priming. Furthermore, the presence of mPR3–and mPR3+ neutrophils within individuals with bimodal mPR3 expression does not originate from differences in state of activation, mobilisation of vesicles or responsiveness to TNFα between these two subsets. The percentage of neutrophils that express proteinase 3 after priming with TNFα is increased in PR3‐AAV and MPO‐AAV and SLE, but not in RA. Whereas a pathophysiological role for (increased) mPR3 expression has been strongly suggested in PR3‐AAV, its role in other AAVs and SLE requires further study.
Footnotes
This work was supported by the Dutch Kidney Foundation (grant no. C00.1916)
Competing interest: none
References
- 1.Borregaard N, Cowland J B. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1979893503–3521. [PubMed] [Google Scholar]
- 2.Spitznagel J K, Dalldorf F G, Leffell M S, Folds J D, Welsh I R, Cooney M H, Martin L E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest 197430774–785. [PubMed] [Google Scholar]
- 3.Witko‐Sarsat V, Cramer E M, Hieblot C, Guichard J, Nusbaum P, Lopez S, Lesavre P, Halbwachs‐Mecarelli L. Presence of proteinase 3 in secretory vesicles: evidence of a novel, highly mobilizable intracellular pool distinct from azurophil granules. Blood 1999942487–2496. [PubMed] [Google Scholar]
- 4.Falk R J, Terrell R S, Charles L A, Jennette J C. Anti‐neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA 1990874115–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reumaux D, Vossebeld P J, Roos D, Verhoeven A J. Effect of tumor necrosis factor‐induced integrin activation on Fc gamma receptor II‐mediated signal transduction: relevance for activation of neutrophils by anti‐proteinase 3 or anti‐myeloperoxidase antibodies. Blood 1995863189–3195. [PubMed] [Google Scholar]
- 6.Csernok E, Ernst M, Schmitt W, Bainton D F, Gross W L. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol 199495244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witko‐Sarsat V, Lesavre P, Lopez S, Bessou G, Hieblot C, Prum B, Noel L H, Guillevin L, Ravaud P, Sermet‐Gaudelus I, Timsit J, Grunfeld J P, Halbwachs‐Mecarelli L. A large subset of neutrophils expressing membrane proteinase 3 is a risk factor for vasculitis and rheumatoid arthritis. J Am Soc Nephrol 1999101224–1233. [DOI] [PubMed] [Google Scholar]
- 8.Yang J J, Tuttle R H, Hogan S L, Taylor J G, Phillips B D, Falk R J, Jennette J C. Target antigens for anti‐neutrophil cytoplasmic autoantibodies (ANCA) are on the surface of primed and apoptotic but not unstimulated neutrophils. Clin Exp Immunol 2000121165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halbwachs‐Mecarelli L, Bessou G, Lesavre P, Lopez S, Witko‐Sarsat V. Bimodal distribution of proteinase 3 (PR3) surface expression reflects a constitutive heterogeneity in the polymorphonuclear neutrophil pool. FEBS Lett 199537429–33. [DOI] [PubMed] [Google Scholar]
- 10.Rarok A A, Stegeman C A, Limburg P C, Kallenberg C G. Neutrophil membrane expression of proteinase 3 (PR3) is related to relapse in PR3‐ANCA‐associated vasculitis. J Am Soc Nephrol 2002132232–2238. [DOI] [PubMed] [Google Scholar]
- 11.Van Rossum A P, Limburg P C, Kallenberg C G. Membrane proteinase 3 expression on resting neutrophils as a pathogenic factor in PR3‐ANCA‐associated vasculitis. Clin Exp Rheumatol 200321S64–S68. [PubMed] [Google Scholar]
- 12.Schreiber A, Busjahn A, Luft F C, Kettritz R. Membrane expression of proteinase 3 is genetically determined. J Am Soc Nephrol 20031468–75. [DOI] [PubMed] [Google Scholar]
- 13.Schreiber A, Otto B, Ju X, Zenke M, Goebel U, Luft F C, Kettritz R. Membrane proteinase 3 expression in patients with Wegener's granulomatosis and in human hematopoietic stem cell‐derived neutrophils. J Am Soc Nephrol 2005162216–2224. [DOI] [PubMed] [Google Scholar]
- 14.Fauci A S, Katz P, Haynes B F, Wolff S M. Cyclophosphamide therapy of severe systemic necrotizing vasculitis. N Engl J Med 1979301235–238. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman G S, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum 1998411521–1537. [DOI] [PubMed] [Google Scholar]
- 16.Niles J L, McCluskey R T, Ahmad M F, Arnaout M A. Wegener's granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood 1989741888–1893. [PubMed] [Google Scholar]
- 17. Falk RJ, Jennette JC: ANCA are pathogenic—oh yes they are! J Am Soc Nephrol 2002131977–1979. [DOI] [PubMed] [Google Scholar]
- 18.Kettritz R, Jennette J C, Falk R J. Crosslinking of ANCA‐antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol 19978386–394. [DOI] [PubMed] [Google Scholar]
- 19.Mulder A H, Heeringa P, Brouwer E, Limburg P C, Kallenberg C G. Activation of granulocytes by anti‐neutrophil cytoplasmic antibodies (ANCA): a Fc gamma RII‐dependent process. Clin Exp Immunol 199498270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber A, Luft F C, Kettritz R. Membrane proteinase 3 expression and ANCA‐induced neutrophil activation. Kidney Int 2004652172–2183. [DOI] [PubMed] [Google Scholar]
- 21.Van Rossum A P, Rarok A A, Huitema M G, Fassina G, Limburg P C, Kallenberg C G. Constitutive membrane expression of proteinase 3 (PR3) and neutrophil activation by anti‐PR3 antibodies. J Leukoc Biol 2004761162–1170. [DOI] [PubMed] [Google Scholar]
- 22.Fearon D T, Collins L A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol 1983130370–375. [PubMed] [Google Scholar]
- 23.Jennette J C, Falk R J, Andrassy K, Bacon P A, Churg J, Gross W L, Hagen E C, Hoffman G S, Hunder G G, Kallenberg C G.et al Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 199437187–192. [DOI] [PubMed] [Google Scholar]
- 24.Tervaert J W, Goldschmeding R, Elema J D, van der G M, Huitema M G, van der Hem G K, The T H, dem Borne A E, Kallenberg C G. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int 199037799–806. [DOI] [PubMed] [Google Scholar]
- 25.Tervaert J W, Mulder L, Stegeman C, Elema J, Huitema M, The H, Kallenberg C. Occurrence of autoantibodies to human leucocyte elastase in Wegener's granulomatosis and other inflammatory disorders. Ann Rheum Dis 199352115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S, Healey L A, Kaplan S R, Liang M H, Luthra H S.The American Rheumatism Association. 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 27.Tan E M, Cohen A S, Fries J F, Masi A T, McShane D J, Rothfield N F, Schaller J G, Talal N, Winchester R J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982251271–1277. [DOI] [PubMed] [Google Scholar]
- 28.van der Geld Y M, Limburg P C, Kallenberg C G. Characterization of monoclonal antibodies to proteinase 3 (PR3) as candidate tools for epitope mapping of human anti‐PR3 autoantibodies. Clin Exp Immunol 1999118487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller Kobold A C, Kallenberg C G, Tervaert J W. Leucocyte membrane expression of proteinase 3 correlates with disease activity in patients with Wegener's granulomatosis. Br J Rheumatol 199837901–907. [DOI] [PubMed] [Google Scholar]
- 30.Campbell E J, Campbell M A, Owen C A. Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity, and susceptibility to inhibition. J Immunol 20001653366–3374. [DOI] [PubMed] [Google Scholar]
- 31.Wiedow O, Meyer‐Hoffert U. Neutrophil serine proteases: potential key regulators of cell signalling during inflammation. J Intern Med 200557319–328. [DOI] [PubMed] [Google Scholar]
- 32.Pendergraft W F, III, Rudolph E H, Falk R J, Jahn J E, Grimmler M, Hengst L, Jennette J C, Preston G A. Proteinase 3 sidesteps caspases and cleaves p21(Waf1/Cip1/Sdi1) to induce endothelial cell apoptosis. Kidney Int 20046575–84. [DOI] [PubMed] [Google Scholar]