Abstract
Objectives
The study was conducted with the aim of achieving an improved understanding of the molecular mechanisms of high‐dose intravenous immunoglobulin (IVIG) in inflammatory myopathies by investigating the effects on muscle function and immunological molecules in skeletal muscle of polymyositis (PM), dermatomyositis (DM) and inclusion body myositis (IBM) patients.
Methods
Thirteen treatment‐resistant patients, 6 PM, 4 DM, 2 IBM and 1 juvenile DM, were treated with 2 g/kg of IVIG, three times at monthly intervals. Functional Index in Myositis and serum creatinine kinase (CK) levels were determined, and muscle biopsies were performed before treatment and after the third IVIG infusion. Immunological molecules were also studied in biopsies taken 24–48 h after the first infusion.
Results
Improved muscle function was observed in three patients (1 PM, 1 DM and 1 IBM) and CK levels decreased in five. T cells, macrophages, major histocompatibility complex (MHC) class I antigen on muscle fibres, intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule‐1 (VCAM‐1) expression and membranolytic attack complex (MAC) deposits on capillaries were present to an equal degree in biopsies before and after IVIG treatment. No correlation between the clinical response and molecular changes was found.
Conclusions
The clinical effects of high‐dose IVIG on muscle function in patients with refractory inflammatory active myositis did not correspond to effects on any of the investigated molecules in our study. T cells, macrophages, phenotypical changes in muscle fibres and endothelial cell activation were still present after treatment. These observations question a role for IVIG as an immune‐modulating therapy in patients with inflammatory myopathies.
The idiopathic inflammatory myopathies, polymyositis (PM), dermatomyositis (DM) and inclusion body myositis (IBM), are clinically characterised by symmetrical, mainly proximal, muscle weakness, decreased muscle endurance and skin rash (DM).1 Characteristic histopathological features are the presence of inflammatory cell infiltrates in skeletal muscle tissue, dominated by T cells and macrophages.
Microvessels are likely to play a role in disease mechanisms of myositis. This is suggested by a reduced capillary density and observation of deposits of membranolytic attack complex (MAC) in capillaries of DM patients.2,3 Furthermore, in all the three subsets of myositis, the endothelial cells of the capillaries in muscle tissue sections are morphologically changed and express activation markers, such as the intercellular adhesion molecule‐1 (ICAM‐1) and vascular cell adhesion molecule‐1 (VCAM‐1), which could promote extravasation of inflammatory cells into the muscle tissue, as well as the proinflammatory cytokine interleukin (IL)1α.4,5,6,7,8 Involvement of microvessels could also affect transportation of nutrients to the muscle tissue and thereby affect muscle function. Further support for involvement of capillaries is the observations from a controlled trial of refractory DM.9 After treatment with high‐dose intravenous immunoglobulin (IVIG), improved muscle strength was associated with decreased ICAM‐1 expression and resolution of complement deposits on capillaries in the responders who were subject to repeat muscle biopsy. This observation, together with clinical improvement and reduced endothelial cell activation in skin biopsies and reduction of IL1 release in peripheral blood mononuclear cells (PBMCs) after IVIG treatment in Kawasaki disease, indicates that IVIG could reduce endothelial cell activation and IL1 release in vivo, and thereby diminish tissue inflammation.10 This could be a mode of action of high‐dose IVIG in all subgroups of myositis patients, although the beneficial effects of IVIG at the clinical and biochemical level have been limited in myositis subgroups other than in adult DM.11,12,13,14 In an open study, PM patients showed clinical and creatinine kinase (CK) level improvement.13 As IBM patients have signs of endothelial cell activation in microvessels and IBM patients had a partial response in a previous trial, we also decided to include IBM patients in this trial.12,15
The objective of this study was to achieve an improved understanding of the molecular effects of IVIG in vivo by studying the number of capillaries and immunological molecules expressed in repeat biopsies from patients with inflammatory myopathies of various subgroups treated with high‐dose IVIG. We also wanted to relate the molecular effects of IVIG to clinical response.
Patients and methods
Patients
Thirteen treatment‐resistant myositis patients, six PM, four DM, two IBM, and one juvenile onset DM (10 women and 3 men) were included in the study. Demographic data on the patients are shown in table 1. All patients were regular patients at the Rheumatology Unit, Karolinska University Hospital, Solna, and fulfilled the diagnostic criteria for definite or probable PM/DM or sporadic IBM.16,17,18 Six patients had endomysial infiltrates (3 PM, 1 DM, 2 IBM), perivascular infiltrates (3 PM, 2 DM, 1 IBM) and non‐necrotic fibres invaded by mononuclear inflammatory cells (4 PM, 2 IBM). Perifascicular atrophy was observed in four patients (3 DM, 1 IBM). The median age was 61 years (range 17–73) at the time of the study. The median disease duration from diagnosis until IVIG treatment was 3 years (2 months to 13 years). All patients had previously been treated with high‐dose corticosteroids and other immunosuppressives, with no or limited improvement in muscle weakness or CK levels, and with sustained inflammation in the muscle tissue (table 1). All patients gave their informed consent to participate, and the local ethics committee Nord, Stockholm, approved the study.
Table 1 Demographic data of the patients.
| Patient/sex/age at treatment | Myositis diagnosis | Disease duration before IVIG treatment (years) | Anti‐Jo‐1 autoantibodies | Medication prior to IVIG treatment | Concomitant medication* | Biopsy site B.T./after first infusion/after third infusion |
|---|---|---|---|---|---|---|
| 1/F/63 | Prob PM | 6 | – | AZA, MTX, CyA | Pred. 7.5 mg/day (7) | Vas/vas/vas |
| 2/F/54 | Prob PM | 6 | – | AZA, MTX, CyX, CyA | No | Tib/vas/tib |
| 3/F/61 | Def PM | 6 | – | AZA, MTX, CyA, CyX | Pred. 5 mg/day (6), CyA 200 mg/day (4.5) | Vas/vas/vas |
| 4/F/55 | Def PM | 2 | – | AZA, MTX, CyA, CyX | Pred. 10 mg/day (2) | Tib/vas/NA |
| 5/M/52 | Prob PM | 3 | + | AZA, MTX | Pred. 17.5 mg/day(2), MTX 10 mg/week (6), AZA 200 mg/day (1.5) | Vas/vas/NA |
| 6/F/64 | Def PM | 5 | + | AZA, CyA | Pred 17.5 mg/day (2), AZA 100 mg/day (1) | Vas/vas/vas |
| 7/F/71 | Prob DM | 2 months | – | AZA, MTX | AZA 50 mg/day (1) | Vas/vas/vas |
| 8/F/63 | Def DM | 1 | – | AZA | Pred 10 mg/day (10) | Tib/tib/NA |
| 9/M/67 | Def DM | 3 | – | AZA, MTX, CyA | MTX 15 mg/week (2), CyA 200 mg/day (5) | Vas/vas/NA |
| 10/F/52 | Def DM | 13 | – | AZA, MTX, CyA | Pred 30 mg/day (13) | Vas/NA/vas |
| 11/M/17 | JDM | 4 | – | AZA, AMA | No | Vas/NA/vas |
| 12/F/56 | Def IBM | 2 | – | AZA, MTX, CyA | MTX 7.5 mg/week (2) | Tib/NA/tib |
| 13/F/73 | Def IBM | 6 months | – | AZA, Pred. | AZA 125 mg/day (1) | Tib/vas/vas† |
AMA, anti‐malaria medication; AZA, azathioprine; def, definite; B.T., before treatment; CyA, cyclosporin; CyX, cyclophosphamide; DM, dermatomyositis; F, female; IBM, inclusion body myositis; JDM, juvenile onset dermatomyositis; M, male; MTX, metotrexate; NA, not available; Pred, prednisolone; PM, polymyositis; Prob, probable; tib, tibialis; vas, vastus.
*Stability before IVIG treatment in months.
†The repeat biopsies were taken 10 and 20 months (with continuous monthly infusions), respectively, after the baseline biopsy.
Clinical and laboratory data
The patients were given infusions during 2–5 days with 2 g/kg of IVIG, three times, at monthly intervals. Concomitant medication was kept stable. Clinical and laboratory assessments were carried out before the first IVIG infusion and 2 weeks after the third IVIG infusion. These included muscle function measured by the Functional Index in Myositis (FI) with a maximum possible score of 64, on the right and left side of the body, and serum CK levels.19 The same physiotherapist performed all muscle function tests without knowledge of the laboratory or biopsy data. Nine of the 13 patients completed the FI test after the third IVIG infusion. Four patients were unable to complete the test for different reasons: one patient died after the third IVIG infusion due to a tumour, independent of the IVIG treatment, the second patient had back pain, the third patient could not perform the test due to severe weakness, while the fourth patient declined to perform the test without giving any explanation why. The serum CK levels were analysed as routine tests at the Department of Clinical Chemistry, Karolinska University Hospital, Solna, Stockholm.
Muscle biopsy data
Muscle tissue samples used for this study were obtained under local anaesthesia by an open or semi‐open technique.20 In all cases, a baseline muscle biopsy was performed within 24 h before IVIG treatment was initiated. Our intention was to carry out two repeat biopsies, one 24–48 h after the first infusion and a second biopsy 1–2 weeks after the third infusion, but this was not always feasible (table 1). The repeat biopsies were performed on the side contralateral to the previous biopsies when possible. The biopsy specimens were mounted in an embedding medium, frozen in isopentane pre‐cooled by dry ice and stored at −80°C. For each biopsy, 7 μm thick serial cryostat sections were mounted on gelatin‐coated glass slides (Cel‐line Associates, Newfield, NJ, USA). The sections were stored at −80°C until stained.
Staining procedure
Histopathology
The first and the last section of each series were stained with H&E to confirm that the histopathology of the biopsy sample was unchanged in the consecutive series of sections collected for histochemical and immunohistochemical stainings. A neuropathologist performed the histopathological evaluation on coded slides for the presence of muscle fibre degeneration, regeneration or atrophy, central nuclei and inflammatory cell infiltrates.
Immunohistochemistry
A standard immunohistochemistry protocol was applied to stain for T cells and activated macrophages, major histocompatibility complex (MHC) class I and class II antigen, the adhesion molecules ICAM‐1 and VCAM‐1 and endothelial cells (table 2).21 Staining for anti‐IL1α was performed according to a immunohistochemistry protocol for intracellular staining using saponin as detergent.15 A modified protocol for MAC involved Tween in washing buffers, primary antibody diluted in Antibody Diluent (Dako S2022), and streptavidin‐conjugated horseradish peroxidase (Dako K5001) as signal amplifier.22 To standardise the staining procedure, all biopsies from the same patient were always stained on the same occasion. We also used the same positive control for every molecule stained, as well as a negative istotype control for every molecule and biopsy section stained.
Table 2 Antibodies used for immunohistochemistry.
| Antibodies/markers | Clone no. | Isotype | Supplier |
|---|---|---|---|
| Anti‐CD3/T cells | SK7 | Mouse IgG1 | Becton Dickinson, San Jose, CA, USA |
| Anti‐CD4 | SK3 | Mouse IgG2 | Becton Dickinson, San Jose, CA, USA |
| Anti‐CD8 | SK1 | Mouse IgG3 | Becton Dickinson, San Jose, CA, USA |
| Anti‐CD163/activated macrophages | Ber‐MAC3 | Mouse IgG1 | Dako A/S, Glostrup, Denmark |
| Anti‐HLA‐ABC/MHC class I | W6/32 | Mouse IgG2a | Dako A/S, Glostrup, Denmark |
| Anti‐HLA‐DR/MHC class II | L243 | Mouse IgG2a | Becton Dickinson, San Jose, CA, USA |
| Anti‐CD54/ICAM‐1 | 84410 | Mouse IgG1 | Serotec, Oxford, UK |
| Anti‐CD106/VCAM‐1 | 51‐10C9 | Mouse IgG1 | Pharmingen, San Diego, CA, USA |
| Anti‐CD31/endothelial cells | EN4 | Mouse IgG1 | Sanbio, Uden, The Netherlands |
| Anti‐human C5b‐9/MAC | aE11 | Mouse IgG2a | Dako A/S, Glostrup, Denmark |
| anti‐IL1α | 1277‐89‐7 | Mouse IgG1 | AMS Biotechnology Europe Ltd, Abingdon, UK |
| Isotype‐matched irrelevant | DAK‐G01 | Mouse IgG1 | Dako A/S, Glostrup, Denmark |
| Isotype‐matched irrelevant | DAK‐G05 | Mouse IgG2a | Dako A/S, Glostrup, Denmark |
| Secondary antibody (biotinylated) | Lot Q0618 | Horse anti‐mouse IgG | Vector Laboratories, Inc., Burlingame, CA, USA |
| Secondary antibody (biotinylated) for MAC staining | AB2, ChemMate Detection Kit, code No. K5001 | DakoCytomation A/S, Glostrup, Denmark |
CD, cluster of differentiation; EN4, human endothelial 4; HLA‐ABC, human leucocyte antigen; ICAM‐1, intercellular adhesion molecule‐1; IL, interleukin; MAC, membranolytic attack complex; MHC, major histocompatibility complex; VCAM‐1, vascular cell adhesion molecule‐1.
Evaluation of staining
Tissue sections were analysed using Reichert‐Jung Polyvar 2, Leica DMRXA2 and Leitz DMRBE (Leica Microsystems, Wetzlar, Germany). Quantification was performed both by conventional evaluation and by a computerised image analysis system (LEICA Qwin, Leica Microsystems Imaging solutions Ltd, Cambridge, UK). All sections were analysed coded.
Conventional microscopic measurement
All markers were assessed qualitatively. Quantification was performed by manual counting either by two independent investigators (when the results differed, a third assessment was performed and a mean value was used), or in combination with image analysis (250×). The number of scattered and infiltrating cells positive for cluster of differentiation (CD)3 or CD163 and the number of capillaries, larger blood vessels (arterioles and venules) and fibres positively stained for ICAM‐1, VCAM‐1 and IL1α was estimated from the whole tissue sections. These were related to the total tissue area and are presented as number of stained cells, capillaries or larger blood vessels per mm2 of muscle tissue.
Computerised image analysis
Computerised image analysis was conducted to quantify CD163, MHC class I antigen, ICAM‐1 and IL1α, as well as the total tissue area (median 3.38, range 0.56–12.7 mm2) of the sections. The results are presented as percentage positively stained area of total tissue area. For standardised/optimal settings of each analytical condition, the following procedures were undertaken: a standard setting procedure of the microscope and computer system was applied, and, as an internal standard, one muscle biopsy section from a myositis patient was used.
Intraindividual variations of muscle histopathology and molecular expression, and definition of changes
In five patients, two tissue samples were taken from the same muscle on one occasion. These were used for determination of intraindividual variations (table 3). With regard to intraindividual sample variations, an individual response was defined as increased when the expression was doubled, and as decreased when it was less than half compared with before treatment. Otherwise it was concluded to be unchanged. MHC class I antigen expression on muscle fibres required a difference of at least three grading scores, in a six‐grade scoring system, to be defined as improved or worsened.
Table 3 Intraindividual variations of molecular expression in muscle tissue analysed by conventional and computerised image analysis.
| Indiv. | T.S. | CD3 | CD163 | MHC‐I* | MHC‐II* | MAC | ICAM‐1 | VCAM‐1 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cap† | Fibres‡ | |||||||||||
| Cells/mm2 | Cells/mm2 | % pos area | Manual | % pos area | Manual | Manual | Cap/mm2 | % pos area | Cap/mm2 | |||
| 1 | 1 | 7.02 | 116 | 0.21 | + | 3.01 | – | + | + | 3.60 | 0.20 | 0.77 |
| 2 | 14.4 | NA | NA | – | NA | – | + | + | 6.76 | 0.06 | 0.54 | |
| 2 | 1 | 38.8 | 230 | 2.96 | ++++ | 27.8 | + | ++ | +++ | 20.8 | 0.43 | 5.68 |
| 2 | 47.6 | 202 | 3.35 | ++++ | 22.9 | + | + | ++ | 19.3 | 0.24 | 6.02 | |
| 3 | 1 | 8.75 | 51.9 | 0.17 | + | 1.61 | – | ++ | – | 20.6 | 0.32 | 1.34 |
| 2 | 12.5 | NA | NA | + | NA | – | ++ | – | NA | 0.30 | 0.92 | |
| 4 | 1 | 55.9 | 537 | 1.10 | + | 4.49 | – | + | ++ | 7.62 | 0.17 | 10.8 |
| 2 | 48.6 | 429 | 1.35 | + | 10.3 | NA | NA | NA | NA | NA | 3.42 | |
| 5 | 1 | 6.6 | 80.2 | 0.36 | ++++ | 9.20 | – | + | – | 20.3 | 0.34 | 1.73 |
| 2 | 0.11 | 43.6 | 0.22 | ++ | 2.38 | – | + | – | 8.56 | 0.13 | 0.31 | |
Cap, capillaries; CD, cluster of differentiation; ICAM‐1, intercellular adhesion molecule‐1; Indiv., individuals; MAC, membranolytic attack complex; MHC, major histocompatibility complex; NA, not assessed; T.S., tissue samples; VCAM‐1, vascular cell adhesion molecule‐1. *The MHC class I and class II antigen expression on muscle fibres was manually estimated and graded as follows: –, no positively stained fibres; +, 1–20% positively stained fibres; ++, 21–40% positively stained fibres; +++, 41–60% positively stained fibres; ++++, 61–80% positively stained fibres; and +++++, 81–100% positively stained fibres.
†The following grading was used for MAC expression in the capillaries throughout the section: –, no positively expressed capillaries; +, 1–3; ++, 4–10; +++, ⩾11 per 200× field positively expressed capillaries.
‡The following grading was used for MAC expression in necrotic fibres and the surface of morphologically normal fibres throughout the section: –, no positively stained fibres; +, 1 necrotic fibre or a few normal fibres positively stained; ++, 1–3 necrotic fibres and a few/many normal fibres or only a lot of normal fibres positively stained; +++, 1–3 necrotic fibres and many normal fibres positively stained or ⩾3 necrotic fibres and a few/many normal fibres positively stained.
Statistical analysis
Data were analysed using GraphPad Prism 4.0 statistical software (GraphPad Software Inc., San Diego, CA, USA). Friedman's test (multiple analysis of variance) with Dunn's multiple comparison post‐test was used to compare expression of the different molecules at the first biopsy and the two repeat biopsies. Non‐parametric Wilcoxon's signed rank test was used for pairwise comparison. Spearman's rank correlation was used to test for correlations. p Values ⩽0.05 were considered statistically significant.
Results
Clinical data
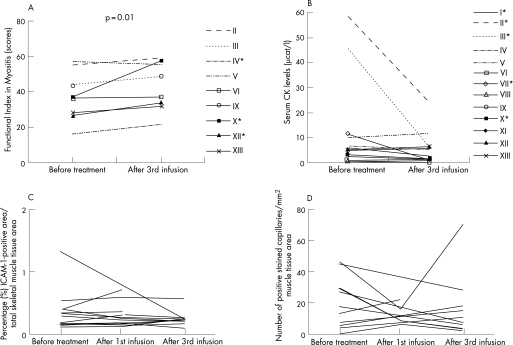
Clinical improvement, assessed by the FI, was small, although statistically significant, after the third high‐dose IVIG infusion compared with before treatment (fig 1A, table 4). Three patients improved >20% on the FI score. In none of the patients did the muscle function return to maximal score. A dramatic recovery of skin rash was noticed in three of four DM patients. CK levels decreased by >50% in five patients out of nine with elevated levels, but median CK levels were not significantly changed (fig 1B, table 4). No serious adverse events occurred; a few patients reported headaches and nausea.
Figure 1 (A) Muscle function measured by Functional Index in Myositis (FI) before intravenous immunoglobulin (IVIG) treatment and after the third infusion. *The patients improved >20% on the FI score. (B) Serum creatinine kinase (CK) levels before IVIG treatment and after the third infusion. *CK levels decreased by >50%, (C and D) Intercellular adhesion molecule‐1 (ICAM‐1) expression before treatment and in the repeat biopsies of all patients: percentage positive area of total ICAM‐1 expression in the muscle tissue section, measured by computerised image analysis (C), and ICAM‐1 expression in capillaries/mm2 tissue area calculated by manual assessment (D).
Table 4 Clinical and laboratory data and molecular expression in the muscle tissue before and after the first and third intravenous immunoglobulin infusion.
| Markers | Before treatment (n = 13) | 24–48 h after first infusion (n = 10) | 1–2 weeks after third infusion (n = 9) |
|---|---|---|---|
| FI scores† | 37.0 (16.5–57.5) | NA | 49.0 (22.0–59.0)* |
| CK levels‡ | 5.30 (0.30–58.4) μcat/l | NA | 2.80 (0.5–24.0) μcat/l |
| CD 3 | 9.01 (1.35–190) cells/mm2 | 9.68 (1.49–143) cells/mm2 | 10.4 (1.25–104) cells/mm2 |
| CD163 | 0.29 (0.01–3.16)% | 0.61 (0.03–1.78)% | 0.26 (0.10–0.99)% |
| CD163 | 88.9 (2.30–392) cells/mm2 | 98.9 (14.1–483) cells/mm2 | 82.4 (24.0–174) cells/mm2 |
| MHC‐I§ | 3.77 (1.01–37.3)% | 8.8 (0.54–46.3)% | 3.01 (1.17–46.7)% |
| ICAM‐1§ | 0.30 (0.13–1.33)% | 0.26 (0.14–0.71)% | 0.25 (0.11–0.58)% |
| ICAM‐1 | 7.47 (0.00–46.5) cap/mm2 | 11.8 (5.87–36.1) cap/mm2 | 10.9 (2.49–70.1) cap/mm2 |
| VCAM‐1 | 3.96 (0.00–6.31) cap/mm2 | 1.17 (0.19–11.1) cap/mm2 | 0.92 (0.31–5.74) cap/mm2 |
| IL1α§ | 0.09 (0.02–0.40)% | NA | 0.11 (0.01–0.39)% |
| IL1α | 16.1 (2.50–153) cells/mm2 | NA | 21.4 (1.88–36.3) cells/mm2 |
| IL1α | 26.7 (5.27–57.2) cap/mm2 | NA | 25.3 (7.89–80.5) cap/mm2 |
| IL1α | 4.16 (0.00–10.5) l.v./mm2 | NA | 4.87 (0.00–14.6) l.v./mm2 |
Cap, capillaries; CD, cluster of differentiation; FI, Functional Index in Myositis; ICAM‐1, intercellular adhesion molecule‐1; IL, interleukin; l.v., large vessels; MHC, major histocompatibility complex; NA, not assessed; VCAM‐1, vascular cell adhesion molecule‐1.
Results are shown as median (range).
*p Value = 0.012.
†Maximum FI scores = 64, FI scores from the right side and the left side correlated very well (r = 0.968, p<0.0001), thus the mean value was assessed for the analysis.
‡CK reference levels <2.5 μcat/l.
§Percentage MHC‐I, ICAM‐1 and IL1α include expression in inflammatory cells, capillaries, blood vessels and fibres.
Histopathology
No changes in histopathological signs such as degree of muscle fibre degeneration, regeneration or athrophy, centralised nuclei or inflammatory cell infiltrates were observed after the IVIG infusions when compared with before treatment. In five cases, two samples were taken from the same biopsy site. The histopathological features did not differ significantly between these two tissue samples (data not shown).
Inflammatory cells, MHC class I and class II antigens and adhesion molecules
T cells and activated macrophages were present in all patients before IVIG treatment as scattered cells or as infiltrates (table 4). The median expression of CD3‐ and of CD163‐positive cells did not differ significantly in the repeat biopsies. On an individual response level, the CD163 expression after the third infusion remained unchanged in six patients, while it increased in two and decreased in two. When comparing the CD163 results from the computerised image analysis with the manual readings before treatment and after the first and third infusion, the results correlated very well (r = 0.955, p<0.0001; r = 0.900, p = 0.0002). The CD3 expression remained unchanged in four patients, increased in four and decreased in three patients.
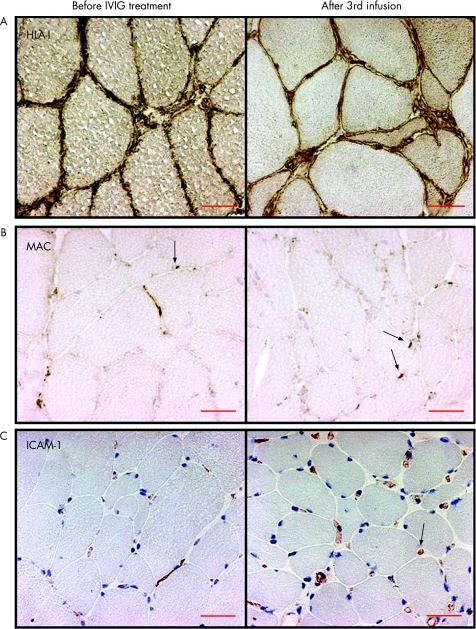
Before treatment, a distinct MHC class I and MHC class II antigen staining was present on muscle fibre membranes and in the sarcoplasm of normal, as well as on atrophic, regenerating or degenerating fibres in 12 of 13 (fig 2A, table 5) and 3 of 13 patients, respectively. A perifascicular MHC class I antigen staining pattern was observed in five patients. After the third infusion, the MHC class I antigen‐positive fibres remained unchanged in eight patients and increased in one of nine available specimens. The total MHC class I expression assessed by computerised image analysis was not significantly changed post‐infusions (table 4). MHC class II antigen was expressed on muscle fibres in three patients and remained unchanged after IVIG treatment in these, and was positive in one other patient.
Figure 2 (A) Major histocompatibility complex class I antigen expression on the muscle fibre membrane and sarcoplasma of the muscle fibres (brown staining) before intravenous immunoglobulin (IVIG) treatment remained unchanged in the repeat biopsies (patient 12, inclusion body myositis). (B) Membranolytic attack complex (MAC) deposits in the capillaries (arrows, brown staining) before IVIG treatment remained unchanged in the repeat biopsies (patient 6, polymyositis). (C) Intercellular adhesion molecule‐1 (ICAM‐1) expression in capillaries (arrow, red staining) and inflammatory cells remained unchanged in the repeat biopsies (patient 9, dermatomyositis). Scale bar = 50 μm (original magnification ×250).
Table 5 Manual evaluation of major histocompatibility complex class I antigen expression on the surface of muscle fibres, and membranolytic attack complex deposits in capillaries and in necrotic muscle fibres, and on the surface of non‐necrotic fibres.
| Patients/diagnosis | MHC class I antigen | MAC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fibres | Capillaries | Fibres | |||||||
| B.T. | After 24–48 h | After third inf | B.T. | After 24–48 h | After third inf | B.T. | After 24–48 h | After third inf | |
| 1/PM | + | – | + | + | + | ++ | + | – | – |
| 2/PM | ++++ | + | ++ | + | + | + | ++ | ++ | + |
| 3/PM | + | + | + | ++ | ++ | + | +++ | +++ | + |
| 4/PM | +++++ | +++++ | NA | ++ | +++ | NA | + | + | NA |
| 5/PM | ++++ | ++++ | NA | ++ | + | NA | +++ | + | NA |
| 6/PM | – | + | + | ++ | ++ | +++ | – | – | + |
| 7/DM | + | + | + | + | + | + | +++ | ++ | + |
| 8/DM | +++++ | ++++ | NA | +++ | ++ | NA | – | + | NA |
| 9/DM | +++ | +++++ | NA | ++ | ++ | NA | ++ | + | NA |
| 10/DM | + | NA | + | + | NA | ++ | – | NA | ++ |
| 11/JDM | + | NA | + | + | NA | + | – | NA | – |
| 12/IBM | +++++ | NA | +++++ | + | NA | + | ++ | NA | ++ |
| 13/IBM | + | +++ | +++++ | ++ | +++ | +++ | + | – | – |
B.T., before treatment; DM, dermatomyositis; IBM, inclusion body myositis; Inf, infusion; JDM, juvenile onset dermatomyositis; NA, not available/assessable; PM, polymyositis.
Grading explanations are described in table 3. Individual changes after the third infusion: in major histocompatibility complex‐I, ⇔ 1–3, 6–7, 10–12 ⇑ 13; in membranolytic attack complex fibres, ⇔ 1–2, 6, 11–13 ⇓ 3, 7 ⇑ 10; in membranolytic attack complex capillaries, ⇔ all patients.
Before treatment, ICAM‐1 was expressed in endothelial cells of capillaries in all patients, in larger blood vessels (8 patients), as well as in scattered mononuclear cells, cellular infiltrates or in degenerating fibres (3 patients). After the infusions, the median expression of total ICAM‐1 measured by computerised image analysis remained unchanged (figs 1C and 2C, table 4). The median number of ICAM‐1‐positive capillaries and larger blood vessels (data not shown) remained unchanged as well. Before treatment, VCAM‐1 was expressed in endothelial cells of capillaries (11 patients), in scattered (12 patients) or infiltrating (2 patients) mononuclear cells, larger blood vessels (12 patients) or degenerating fibres (2 patients). The median number of VCAM‐1‐positive capillaries was unchanged after treatment.
Membranolytic attack complex
Normally MAC is expressed in arterioles and venules. Therefore, the grading of MAC was based on expression on capillaries, in the sarcoplasma of necrotic muscle fibres and on the surface of normal, non‐necrotic fibres. Before treatment, MAC deposits were observed in capillaries of all patients and in the sarcoplasma of spread necrotic fibres or the surface of non‐necrotic fibres of nine patients. After the third infusion, the number of fibres with MAC deposits decreased in two patients, increased in one and remained unchanged in six patients. The MAC deposits in the capillaries did not change in any patient (fig 2B, table 5).
Interleukin 1α
IL1α was expressed in endothelial cells of capillaries, large vessels, in mononuclear cells of all patients and on the surface of few degenerating fibres of one patient before treatment (table 4). After the third infusion, no statistically significant changes occurred in any of the profiles analysed—that is, the number of IL1α‐positive capillaries, large vessels and mononuclear cells, or percentage total stained area.
Relationship between molecular findings and to muscle function
A significant correlation was determined after the third infusion between the changes of CD163 and ICAM‐1 (r = 0.867, p = 0.0045) and VCAM‐1 (r = 0.703, p = 0.043) expression in capillaries. No correlation could be found between the status before treatment or changes after treatment regarding muscle function and CK levels or the expression of investigated molecules in the muscle tissue.
Discussion
In this open study, we found a limited, though statistically significant, improvement of muscle function after three monthly high‐dose IVIG infusions in patients diagnosed with PM, DM or IBM. We also observed improved skin rash in three DM patients and decreased CK levels in five patients. However, despite these clinical signs of improvement and reduced CK levels, we were not able to confirm any significant improvement of inflammation in muscle tissue when assessed as numbers of T cells or macrophages, as expression of MHC class I and class II antigen on muscle fibres, or as endothelial cell activation, which questions the immune‐modulating effects of high‐dose IVIG in these conditions.
Although the clinical effects of IVIG treatment have been demonstrated convincingly in several disorders, the mode of action in vivo is not known.23,24,25,26,27 The most impressive clinical effects have been observed in vasculitis disorders, which were associated with reduced endothelial cell activation.10,24 Another effect of IVIG was demonstrated in in vitro experiments through up‐regulation of the inhibitory cytokine IL1 receptor α in PBMCs.28 Both these mechanisms could be relevant in myositis patients who respond to IVIG treatment, as previous observations suggest involvement of the endothelial cells of microvessels and increased expression of IL1α in capillaries. However, this could not be confirmed in our study. Although a decreased expression of endothelial activation was recorded in occasional patients as reduced ICAM‐1, VCAM‐1 and IL1α, the clinical significance of these changes is uncertain as they did not correspond to clinical improvement. This is in agreement with a previous study in DM patients, in whom serum levels of ICAM‐1 decreased in both responders and non‐responders to IVIG treatment, also questioning the clinical significance of this change.29 Furthermore, another marker of endothelial cell activation, MAC deposits, was unchanged. However, as the MAC results after 3 months of IVIG treatment were only available for three DM patients, due to lack of repeat biopsies, these data are non‐conclusive. At 24–48 h after the first IVIG infusion we could not find any specific pattern of effect on the molecules investigated, although IVIG has been demonstrated to reduce cytokine production already after 24 h of culture with PBMCs.30
Limitations of our study were the open design, which necessitates caution when interpreting the results, and our study was initiated before the clinical outcome measures were proposed by the IMACS group.31 The improved muscle performance on the group level was limited, although statistically significant, and was comparable with the effects previously reported in a placebo‐controlled trial.9 When using a predefined level of improvement of 20% for the muscle function test, only three cases were responders. This makes the clinical effects of high‐dose IVIG in myositis uncertain.
The absence of reductions of infiltrating inflammatory cells or immunological molecules expressed in muscle tissue after IVIG treatment also questions the effects of this therapy in most myositis patients regardless of subtype. Our results from muscle tissue are in disagreement with the controlled trial of refractory DM from which we have adopted our IVIG treatment protocol.9 In that study, the clinical effects were similar to ours, but were associated with decreased inflammatory infiltrates, MHC class I, ICAM‐1 and MAC deposits, and an increased number of capillaries. However, the repeat biopsy data in that study were limited to five patients.
Despite our open trial design, we believe our biopsy data are valid. Several methodological precautions were undertaken for assessment of the biopsies. The sections were evaluated coded, in a randomised order and without clinical information, by two different observers or by a combination of manual and computerised image analysis of whole tissue sections. The image system is a standardised method that is validated as sensitive and reproducible and in congruence with conventional manual evaluation, used not only by us, but also by others.32,33,34 In addition, to standardise our assessments, one biopsy from a patient was used as an internal control for the analytical conditions. To control for variations between tissue samples, we analysed two biopsy samples from five patients, and the intra‐individual variations were taken into consideration when we defined changes in cellular or molecular expression. Moreover, the high degree of correlation between the changes of CD163 and ICAM‐1 and VCAM‐1 expression in capillaries after IVIG treatment supports the validity of the data.
Contrary to muscle function data, convincing clinical effects of IVIG were recorded on the skin changes, which resolved dramatically in three of the DM patients. This effect was not associated with beneficial clinical effects on muscle function. Another contradictory finding was a decreased molecular expression seen in a few patients without clinical improvement regardless of the subset of myositis. Likewise, the lack of effect of IVIG on cellular infiltrates or expression of inflammatory molecules in muscle tissue was similar in the patients regardless of the subset of myositis.
Our findings suggest that the effects of IVIG treatment on the immunological molecules investigated vary between patients and could be due to the heterogeneity of the disease mechanisms in myositis. It is still possible that IVIG could reduce endothelial cell activation and inflammation in vivo in some patients with myositis, but the clinical relevance of this is uncertain as these changes were not consistently associated with a clinical improvement. We could not discriminate those results to any clinical subgroup of myositis or correlate the molecular changes to those of the clinical variables. Although high‐dose IVIG may still be of clinical benefit in patients with DM, by resolving the skin rash, its clinical role in PM and IBM is still uncertain.
Acknowledgements
We thank Dr Leonid Padyukov for his statistical advice. This study was supported by grants from Baxter, Swedish Rheumatism Association, King Gustaf Vth 80‐year foundation, the Professor Nanna Svartz Foundation, and The Swedish Research Council 2002‐74X‐14045‐02A.
Abbreviations
CD - cluster of differentiation
CK - creatinine kinase
DM - dermatomyositis
FI - Functional Index in Myositis
IBM - inclusion body myositis
ICAM intercellular adhesion molecule -
IL - interleukin
IVIG - intravenous immunoglobulin
MAC - membranolytic attack complex
MHC - major histocompatibility complex
PBMC - peripheral blood mononuclear cell
PM - polymyositis
VCAM - vascular cell adhesion molecule
Footnotes
Competing interests: None.
References
- 1.Plotz P H, Rider L G, Targoff I N, Raben N, O'Hanlon T P, Miller F W. NIH conference. Myositis: immunologic contributions to understanding cause, pathogenesis, and therapy, Ann Intern Med 1995122715–724. [DOI] [PubMed] [Google Scholar]
- 2.Kissel J T, Mendell J R, Rammohan K W. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med 1986314329–334. [DOI] [PubMed] [Google Scholar]
- 3.Emslie‐Smith A M, Engel A G. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol 199027343–356. [DOI] [PubMed] [Google Scholar]
- 4.Nyberg P, Wikman A L, Nennesmo I, Lundberg I. Increased expression of interleukin 1alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol 200027940–948. [PubMed] [Google Scholar]
- 5.De Bleecker J L, Engel A G. Expression of cell adhesion molecules in inflammatory myopathies and Duchenne dystrophy. J Neuropathol Exp Neurol 199453369–376. [DOI] [PubMed] [Google Scholar]
- 6.Tews D S, Goebel H H. Expression of cell adhesion molecules in inflammatory myopathies. J Neuroimmunol 199559185–194. [DOI] [PubMed] [Google Scholar]
- 7.Bartoccioni E, Gallucci S, Scuderi F, Ricci E, Servidei S, Broccolini A.et al MHC class I, MHC class II and intercellular adhesion molecule‐1 (ICAM‐1) expression in inflammatory myopathies. Clin Exp Immunol 199495166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundberg I, Kratz A K, Alexanderson H, Patarroyo M. Decreased expression of interleukin‐1alpha, interleukin‐1beta, and cell adhesion molecules in muscle tissue following corticosteroid treatment in patients with polymyositis and dermatomyositis. Arthritis Rheum 200043336–348. [DOI] [PubMed] [Google Scholar]
- 9.Dalakas M C, Illa I, Dambrosia J M, Soueidan S A, Stein D P, Otero C.et al A controlled trial of high‐dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 19933291993–2000. [DOI] [PubMed] [Google Scholar]
- 10.Leung D Y, Cotran R S, Kurt‐Jones E, Burns J C, Newburger J W, Pober J S. Endothelial cell activation and high interleukin‐1 secretion in the pathogenesis of acute Kawasaki disease. Lancet 198921298–1302. [DOI] [PubMed] [Google Scholar]
- 11.Dalakas M C, Koffman B, Fujii M, Spector S, Sivakumar K, Cupler E. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM. Neurology 200156323–327. [DOI] [PubMed] [Google Scholar]
- 12.Dalakas M C, Sonies B, Dambrosia J, Sekul E, Cupler E, Sivakumar K. Treatment of inclusion‐body myositis with IVIg: a double‐blind, placebo‐controlled study. Neurology 199748712–716. [DOI] [PubMed] [Google Scholar]
- 13.Cherin P, Herson S, Wechsler B, Piette J C, Bletry O, Ziza J M.et al Intravenous immunoglobulin for polymyositis and dermatomyositis. Lancet 1990336116. [DOI] [PubMed] [Google Scholar]
- 14.Lang B A, Laxer R M, Murphy G, Silverman E D, Roifman C M. Treatment of dermatomyositis with intravenous gammaglobulin. Am J Med 199191169–172. [DOI] [PubMed] [Google Scholar]
- 15.Lundberg I, Ulfgren A K, Nyberg P, Andersson U, Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum 199740865–874. [DOI] [PubMed] [Google Scholar]
- 16.Bohan A, Peter J B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975292344–347. [DOI] [PubMed] [Google Scholar]
- 17.Bohan A, Peter J B. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975292403–407. [DOI] [PubMed] [Google Scholar]
- 18.Griggs R C, Askanas V, DiMauro S, Engel A, Karpati G, Mendell J R.et al Inclusion body myositis and myopathies. Ann Neurol 199538705–713. [DOI] [PubMed] [Google Scholar]
- 19.Josefson A, Romanus E, Carlsson J. A functional index in myositis. J Rheumatol 1996231380–1384. [PubMed] [Google Scholar]
- 20.Henriksson K G. “Semi‐open” muscle biopsy technique. A simple outpatient procedure. Acta Neurol Scand 197959317–323. [PubMed] [Google Scholar]
- 21.Frostegård J, Ulfgren A K, Nyberg P, Hedin U, Swedenborg J, Andersson U.et al Cytokine expression in advanced human atherosclerotic plaques: dominance of pro‐inflammatory (Th1) and macrophage‐stimulating cytokines. Atherosclerosis 199914533–43. [DOI] [PubMed] [Google Scholar]
- 22.Lindvall B, Bengtsson A, Ernerudh J, Eriksson P. Subclinical myositis is common in primary Sjogren's syndrome and is not related to muscle pain. J Rheumatol 200229717–725. [PubMed] [Google Scholar]
- 23.Evoli A, Palmisani M T, Bartoccioni E, Padua L, Tonali P. High‐dose intravenous immunoglobulin in myasthenia gravis. Ital J Neurol Sci 199314233–237. [DOI] [PubMed] [Google Scholar]
- 24.Leung D Y. The immunologic effects of IVIG in Kawasaki disease. Int Rev Immunol 19895197–202. [DOI] [PubMed] [Google Scholar]
- 25.Dalakas M C. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA 20042912367–2375. [DOI] [PubMed] [Google Scholar]
- 26.Krause I, Shoenfeld Y. Intravenous immunoglobulin treatment for fibrosis, atherosclerosis, and malignant conditions. Methods Mol Med 2005109403–408. [DOI] [PubMed] [Google Scholar]
- 27.Jin F, Balthasar J P. Mechanisms of intravenous immunoglobulin action in immune thrombocytopenic purpura. Hum Immunol 200566403–410. [DOI] [PubMed] [Google Scholar]
- 28.Andersson U, Björk L, Skansen‐Saphir U, Andersson J. Pooled human IgG modulates cytokine production in lymphocytes and monocytes. Immunol Rev 199413921–42. [DOI] [PubMed] [Google Scholar]
- 29.Gottfried I, Seeber A, Anegg B, Rieger A, Stingl G, Volc‐Platzer B. High dose intravenous immunoglobulin (IVIG) in dermatomyositis: clinical responses and effect on sIL‐2R levels. Eur J Dermatol 20001029–35. [PubMed] [Google Scholar]
- 30.Andersson U G, Björk L, Skansen‐Saphir U, Andersson J P. Down‐regulation of cytokine production and interleukin‐2 receptor expression by pooled human IgG. Immunology 199379211–216. [PMC free article] [PubMed] [Google Scholar]
- 31.Rider L G, Giannini E H, Brunner H I, Ruperto N, James‐Newton L, Reed A M.et al International consensus on preliminary definitions of improvement in adult and juvenile myositis. Arthritis Rheum 2004502281–2290. [DOI] [PubMed] [Google Scholar]
- 32.Malm C, Nyberg P, Engström M, Sjödin B, Lenkei R, Ekblom B.et al Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 2000529243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunnane G, Björk L, Ulfgren A K, Lindblad S, FitzGerald O, Bresnihan B.et al Quantitative analysis of synovial membrane inflammation: a comparison between automated and conventional microscopic measurements. Ann Rheum Dis 199958493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Björk L, Fehniger T E, Andersson U, Andersson J. Computerized assessment of production of multiple human cytokines at the single‐cell level using image analysis. J Leukoc Biol 199659287–295. [DOI] [PubMed] [Google Scholar]