Abstract
Objectives
To assess whether body mass index (BMI) and body fat (BF) differ between rheumatoid arthritis (RA) patients, patients with non‐inflammatory arthritis (osteoarthritis, OA) and healthy individuals, and whether disease specific measures of adiposity are required to accurately reflect BF in these groups.
Methods
641 individuals were assessed for BMI (kg/m2) and BF (bioelectrical impedance). Of them, 299 (174 RA, 43 OA and 82 healthy controls (HC)) formed the observation group and 342 (all RA) the validation group. RA disease characteristics were collected.
Results
ANOVA revealed significant differences between disease groups for BMI (p<0.05) and BF (p<0.001). ANCOVA showed that age accounted for the differences in BMI (F1,294 = 5.10, p<0.05); age (F1,293 = 22.43, p<0.001), sex (F1,293 = 380.90, p<0.001) and disease (F2, 293 = 18.7, p<0.001) accounted for the differences in BF. For a given BF, patients with RA exhibited BMI levels reduced by 1.83 kg/m2 (p<0.001) compared to HC; there were no significant differences between OA and HC. A predictive model for BF was developed (R2 = 0.769, p<0.001) and validated using limits of agreement Analysis against measured BF in the validation group (95%LIMAG = 6.17; CV = 8.94).
Conclusions
In individuals with RA, BMI cut‐off points should be reduced by 2 kg/m2 (that is, to 23 kg/m2 for overweight and 28 kg/m2 for obesity). The equation developed can be used to accurately predict BF from BMI in RA patients. These findings may be important in the context of the cardiovascular comorbidity of RA.
Keywords: rheumatoid arthritis, cardiovascular risk, body mass index, body composition, bioelectrical impedance
Excess body fat (BF) is a prominent health hazard1 significantly contributing to the development of cardiovascular disease (CVD).2 About two‐thirds of patients who have had a myocardial infarction (MI) exhibit increased body weight.3 Obesity increases the risk of coronary heart disease (CHD) through a number of different pathophysiological pathways, including insulin resistance, type 2 diabetes, hypertension and dyslipidaemia.4,5
Assessments for overweight or obesity include the calculation of body mass index6 (BMI, in kg/m2) or more accurate estimations of relative adiposity (BF percentage) through a number of techniques (for example, skinfold thickness, hydrostatic weighing and bioelectrical impedance).7 BF estimations require sophisticated equipment and trained personnel, whereas BMI is easy to obtain and is widely used in the routine clinical setting.
In the general population, BMI of <25 kg/m2, 25–30 kg/m2 and >30 kg/m2 indicate healthy, overweight, and obese individuals and associate with low, medium and high CVD risk, respectively.8,9 However, BMI is only a proxy of body fat,6 and over recent years its validity has been questioned.3,7,10,11,12,13 Overweight as defined by BMI of >25 kg/m2, has poor specificity in detecting excess body fat in healthy men and women of all ages6 as well as in patients with coronary heart disease.3 In specific subpopulations, such as people of Indian‐Asian race,10 women11,12,13 and large size athletes,7 new BMI cut‐off points have been suggested that optimally reflect BF and may better predict CVD risk.
The weakness of BMI is that it does not distinguish between lean body mass and fat mass. Consequently people of similar stature and weight, but different muscle content, will have the same BMI but different BF levels. This tends to be more evident in individuals with low BMI levels.6 Such limitations of the BMI may explain the better cardiovascular outcomes observed in overweight and mildly obese patients with established CHD compared to their normal weight counterparts, who may have proportionately more BF.3 Therefore, although it is well established that CHD risk increases with advancing BMI levels,9 global cut‐off points may be misleading for several populations.
Central obesity poses a great risk for CVD.13,15 Regional fat distribution, as measured by waist to hip ratio, has been proposed as a more accurate predictor of CHD risk than BMI.14,15 Indeed, it has been suggested that obesity should be redefined based on waist to hip ratio instead of BMI, since waist to hip ratio is significantly associated with MI risk in most ethnic groups.16 However, its predictive strength can be negatively affected by sex and overall body weight,17 in a way that pear‐shaped or obese individuals might have optimal waist to hip ratio but increased overall body weight. More research is necessary to identify the optimal definition of obesity as a predictor for CHD in the general population and specific subgroups.18
Patients with rheumatoid arthritis (RA) have an increased risk for CHD events.19 RA is a chronic inflammatory disease which affects predominantly synovial joints, causing pain, swelling, stiffness and eventually irreversible damage and deformity, all of which may lead to significant reduction in physical activity. RA associates with increased mortality particularly from CHD,19 most probably because of accelerated atherogenesis secondary to the metabolic and vascular effects of systemic inflammation.20 Nearly two‐thirds of all individuals with RA experience involuntary loss of fat‐free mass and progressively increased fat mass in the presence of stable or even slightly decreased weight, a condition referred to as rheumatoid cachexia.21 The exact mechanisms causing rheumatoid cachexia remain undetermined, but muscle loss due to systemic inflammation and reduced physical activity may both contribute.22
We hypothesised that for a given BMI, RA patients exhibit significantly higher proportions of fat mass than healthy individuals, or even than patients with movement restriction due to a non‐inflammatory arthritis, such as osteoarthritis (OA). The possible consequences of this, in the context of the increased CVD mortality in RA, are obvious. In the present study we aimed to investigate whether BMI and BF differ according to arthritic disease (OA vs RA) and within RA according to disease state (for example, active vs inactive, early vs established disease). We also developed and validated RA specific BMI cut‐off levels and algorithms to calculate BF from BMI.
Methods
Participants
Consecutive patients attending routine rheumatology or orthopaedic outpatient clinics at the Dudley Group of Hospitals NHS Trust, UK, and healthy controls (hospital and university staff) were invited to participate. The study had local research ethics committee approval by the Dudley ethics committee, and all volunteers provided informed consent. The observation group (n = 299) included 174 volunteers with RA (1987 revised American Rheumatism Association criteria23), 43 with OA of the hip25 or knee,26 and 82 healthy controls (individuals who by self report did not have any known clinical conditions and were taking no medication). The validation group (n = 342) consisted of RA patients only. Demographic and disease characteristics from all subjects appear in table 1.
Table 1 Demographic and disease characteristics of all volunteers (mean (SD)).
| Observation group | Validation group | |||||||
|---|---|---|---|---|---|---|---|---|
| Male (n = 110) | Female (n = 189) | Male | Female | |||||
| RA | OA | HC | RA | OA | HC | RA | RA | |
| Number | 56 | 15 | 39 | 118 | 28 | 43 | 99 | 243 |
| Age | 60.6 (11.8)** | 56.7 (13.3)* | 45.1 (13.3) | 59.6 (12.2)** † | 52.8 (12.5)* | 46.8 (11.5) | 62.1 (11.6) | 61.7 (11.9) |
| Height | 173.6 (7)* | 171.3 (6.7)* | 177.3 (6.7) | 159.1 (6.5)** | 161 (5) | 163.6 (6.9) | 174 (6.8) | 160.4 (6.7) |
| Weight | 83.6 (13.3) | 78.4 (14.8) | 80.9 (11.4) | 68.6 (15) | 70.8 (16.5) | 68.1 (16.3) | 82.7 (15.8) | 70.2 (14.4) |
| BMI | 27.7 (4.3)* | 26.8 (4.7) | 25.7 (3) | 26.9 (5.7) | 27.2 (5.7) | 25.4 (5.5) | 27.3 (4.4) | 27.3 (5.3) |
| BF | 28.7 (7.7)** | 24.8 (7.9)* | 19.2 (5.2) | 38.3 (7.3)** † | 35.2 (8.5) | 32.1 (8.2) | 27 (6.4) | 38.3 (7.1) |
| Trunkal fat | 30.5 (8)** | 26.6 (8.9)* | 21.4 (6) | 35.7 (8.6)** † | 31.6 (9.6) | 29.1 (8.7) | 27.4 (7.7) § | 35.4 (8.1) |
| DAS28 | 4.2 (1.2) | 4.3 (1.4) | 4.1 (1.4) | 4.3 (1.4) | ||||
| ESR (mm in 1st hour) | 23.2 (18.5) | 26 (22.1) | 25.3 (21.5) | 30 (26.3) | ||||
| CRP | 15.6 (15) | 15.8 (14.9) | 16.8 (18.6) | 17.6 (23.6) | ||||
| Disease duration | 11.4 (10.2) | 11.3 (9.9) | 12.5 (11) | 13.2 (11) | ||||
RA, rheumatoid arthritis; OA, osteoarthritis; HC, healthy controls; BMI, body mass index; BF, body fat; DAS28, disease activity score‐28; ESR, erythrocyte sedimentation rate; CRP, C‐reactive protein.
One way ANOVA: *Significant difference compared to HC (p<0.05).
**Significant difference compared to HC (p<0.001).
†Significant difference compared to OA (p<0.05).
§Significant difference compared to experimental RA group (p<0.001).
Assessments
All volunteers were subjected to the same data collection procedures overseen by the same trained investigators. Specifically, standing height was measured to the nearest 0.5 cm on a Seca 214 Road Rod portable stadiometer. Body composition was assessed by bioelectrical impedance, using a Tanita BC‐418 MA Segmental Body Composition Analyzer, which incorporates eight tactile electrodes (Tanita Corporation, Tokyo, Japan). This apparatus measures total body mass and assesses body composition in terms of percentage body fat, fat mass, fat free mass and total body water, as well as fat distribution in different body segments (abdominal and peripheral fat) and has a standard error of <3.26 After initial manual entry of their demographic details, participants stood bare footed on the analyser and held the handgrips provided until the apparatus printed the results. BMI (kg/m2) was calculated on the basis of measured height and weight. In RA patients, contemporary serological inflammation and clinical disease activity were assessed by the erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP) (using routine laboratory procedures) and the disease activity score‐28 (DAS28).27 Disease duration was recorded from review of the patients' hospital notes.
Data management and analysis
Data were inserted in a purpose designed spreadsheet (Microsoft Excel 2003) and audited for accuracy weekly. They were exported for analysis to the Statistical Package for Social Sciences version 11.0 (SPSS Inc, Chicago, IL, USA). Preliminary evaluation of the variables using a Kolmogorov‐Smirnov test of normality revealed that none of them required logarithmic transformation to reach normality. Means (SD) were calculated for all variables.
The method of analysis was to define either BMI or BF as the dependent variable and then to incorporate all other known parameters thought to influence these measures of adiposity as either factors in an ANOVA or factors with covariates in an ANCOVA. Factors included sex and disease status (RA, OA and HC) while age, disease activity and duration, and serological inflammation were entered as continuous covariates. The initial ANCOVA analysis incorporated all these factors and covariates, but only those found to be significant were subsequently retained and reported in the prediction equation model below.
Within the RA population of the observation group, correlations of disease activity (DAS28, ESR, CRP) and disease duration with BMI and BF were obtained for each sex. RA patients were also subgrouped according to their clinical disease activity (DAS remission <2.6, mild 2.7–3.2, moderate 3.3–5.1, high >5.127), serological inflammation (ESR28 and CRP29), disease duration (early <3 years, established 3–10 years, longstanding >10 years), rheumatoid factor positivity (ever), or corticosteroid administration (yes/no ever): differences between these subgroups in relation to BMI and BF were assessed using ANCOVA (table 2). The level of significance was set at p<0.05.
Table 2 BMI and BF of RA patients (observation group) according to categorisation based on their disease characteristics.
| Disease characteristics | Categories | BMI | BF | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| DAS28 (DAS28 score) | Remission (<2.6) | 27.2 (3.46) | 27.2 (5.6) | 26.5 (7.6) | 39.5 (6.7) | |
| Mild (2.7–3.2) | 28 (4.3) | 27.3 (4.6) | 28 (6) | 39.3 (6.6) | ||
| Moderate (3.3–5.1) | 27.8 (4.5) | 27 (5.3) | 27.4 (6.8) | 37.3 (7.7) | ||
| High (>5.1) | 25.3 (5.5) | 27.3 (5.5) | 26.1 (5.6) | 37.7 (7.2) | ||
| ESR (mm in 1st hour) | Normal* | 27.9 (4.4) | 26.9 (4.8) | 27.1 (7.2) | 38.3 (6.3) | |
| High | 26.4 (4.6) | 27.6 (6.1) | 26.7 (5.9) | 37.6 (8.9) | ||
| CRP (mg/l) | Low (<3) | 26.5 (2.4) | 28.3 (6.2) | 25.9 (5.4) | 38.5 (8.7) | |
| Normal (3–8) | 27.8 (4.7) | 26.5 (4.7) | 26.7 (8) | 37.6 (6.6) | ||
| High (>8) | 26.9 (4.6) | 27.6 (5.7) | 27.3 (5.8) | 38.3 (7.9) | ||
| Disease duration (years) | Early (<3) | 26.4 (5) | 26.1 (5) | 26.4 (7.9) | 37.9 (8.3) | |
| Established (3–10) | 28.8 (4.1) | 27.8 (5.7) | 27.8 (6.3) | 38.2 (7.5) | ||
| Longstanding (>10) | 26.8 (4.4) | 27.1 (5.1) | 27.7 (5.7) | 38.8 (6.7) | ||
| Rheumatoid factor | Positive | 26.6 (3.6) | 27.2 (5.7) | 25.1 (6.7) | 38.3 (7.3) | |
| Negative | 27.5 (5) | 27.1 (5.1) | 27.7 (6.4) | 37.9 (7.3) | ||
| Corticosteroid administration | Yes | 27.1 (4.4) | 27.3 (5.3) | 26.2 (6) | 38.1 (7.4) | |
| No | 24.5 (4.9) | 26.7 (5.3) | 27.8 (7.3) | 37.7 (7) | ||
DAS28, disease activity score‐28; ESR, erythrocyte sedimentation rate; CRP, C‐reactive protein.
For all differences between groups: p>0.05.
*Normal ESR: <50 years: male <15, female <20.
>50 years: male <20, female <30.
The external validity of the predictive model was tested with the limits of agreement (LIMAG) method30 against BF of the validation group. The limits of agreement were obtained as follows:
We calculated the mean (d) and the standard deviation (s) of the differences that indicate the level of bias and the random variation between the two measures of BF (that is, the predicted BF and measured BF of the validation group, respectively).
Provided the differences are normally distributed, the 95% limits of agreement are given by: d ± (1.96×s).
Bland and Altman30 argue that, provided that differences within these limits are not clinically important, the two measurement methods can be used interchangeably.
Results
Observation group
Within the RA population of the observation group, no significant correlations were found between DAS28, ESR, CRP, disease duration and BMI or BF. Similarly, when RA patients were grouped according to these variables as well as rheumatoid factor positivity and corticosteroid use, no significant differences for BMI and BF were observed (p>0.05 in all cases, see table 2).
Between the different disease groups, one way ANOVA revealed significant differences in BMI (p<0.05) and BF (p<0.001; table 1): RA males had higher BMI and BF (including trunkal fat) than HC males, and RA females had higher BF than HC females, even though their BMI did not differ significantly. ANCOVA revealed that BMI differences between the groups were mainly the result of the significant effect of the covariate age (F1,294 = 5.10, p <0.05) and not because of disease (F2,294 = 1.00, p >0.05), sex (F1,294 = 0.59, p >0.05) or their interactions.
ANCOVA also revealed that RA and OA patients exhibited lower BMI levels than their HC for a given BF. However, differences were only significant for the RA patients (RA:−1.826 kg/m2 (p<0.001); OA: −0.352 kg/m2 (p>0.05)). BMI was significantly (p<0.001) predicted by age, disease, sex and BF (R2 = 0.58).
When BF was adopted as the dependent variable, ANCOVA identified significant differences between disease groups (F2,293 = 18.70, p<0.001) and sex (F1,293 = 380.90, p<0.001) together with a significant covariate, age (F1,293 = 22.43, p<0.001). The contribution of BMI as a covariate in this analysis was also significant (F1,293 = 370.74, p<0.001). For a given BMI, RA patients exhibited significantly increased levels of BF (4.273, p<0.001) compared to healthy controls. The difference for OA patients was non‐significant (1.648, p>0.05). The variation of BF was predicted by age, gender, BMI, and disease type (R2 = 0.769, p<0.001). This was only very slightly improved (for RA) by the addition of RA disease duration (F1,293 = 0.340, p>0.05) in the equation (from 76.9% to 77.1%), so we did not include this variable in the final model. The predictive model obtained from this analysis is:
-
BF = disease status + sex − 0.719 + 0.108 × age + 1.059 × BMI
-
-
Disease status: RA = 4.273, OA = 1.648, HC = 0.
-
-
Sex: male = −11.294, female = 0.
-
-
Validation group
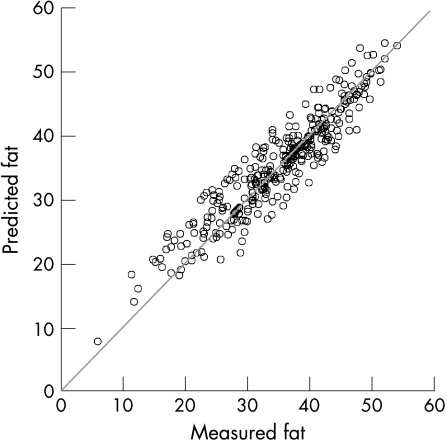
To establish external validity of our predictive model, we assessed its agreement with the measured BF in 342 patients with RA. Preliminary analyses for LIMAG revealed no heteroscedasticity, thus the LIMAG can be reported as absolute measurements.30 Our analyses suggested that the bias of our prediction is 0.4 (that is, our model overpredicts BF by 0.4) with a standard error of 3.2 (95% LIMAG = 6.17, coefficient of variation = 8.9; fig 1). The difference is statistically significant (t = 2.3, p<0.05), but the coefficient variation (CV = 8.9) is within acceptable limits.
Figure 1 Agreement between predicted and measured fat in patients with RA. Body fat was measured by bioelectrical impedance using a Tanita BC‐418 MA Segmental Body Composition Analyzer. Predicted fat was assessed using the formula: BF = 4.273 + sex − 0.719 + 0.108 × age + 1.059 × BMI. 95% limits of agreement were 6.17 with a coefficient of variation of 8.9.
RA specific BMI cut‐off levels
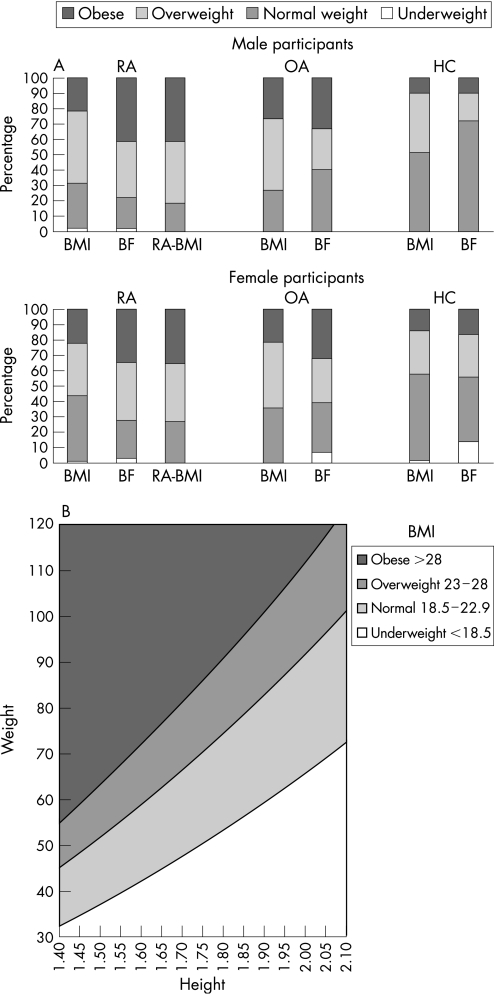
The fact that patients with RA exhibited increased BF values for a given BMI compared to HC suggested that BMI cut‐off points in the RA population would be more appropriate if they were reduced by approximately 2 kg/m2 (to 23 kg/m2 and 28 kg/m2 for overweight and obesity, respectively). We therefore compared the proportions of subjects in each group that would be correctly classified as overweight or obese using the widely accepted BMI cut‐offs of 25 kg/m2 and 30 kg/m2 vs the proposed (for RA) 23 kg/m2 and 28 kg/m2 vs the age and sex specific cut‐off points of measured BF. This analysis showed that 9% of male and 15% of female RA patients would be misclassified as of normal weight based on traditional BMI cut‐offs. Such misclassification was not a problem either for OA or HC, where if anything, BMI overestimated BF. Application of the proposed RA specific BMI cut‐offs of 23 kg/m2 and 28 kg/m2 corrected this misclassification (fig 2A). A modified, RA specific BMI chart for the classification of patients with RA into underweight, normal, overweight and obese categories was developed and is provided in figure 2B.
Figure 2 (A) Classification of male (top) and female (bottom) participants into obese, overweight, normal and underweight groups according to currently accepted BMI cut‐off points (BMI), body fat content (BF) and RA specific BMI cut‐off points (RA‐BMI). Accepting BF as the most accurate assessment of body fatness, currently accepted BMI cut‐off points misclassify a significant proportion of both males and females with RA (notice the difference in the respective bars). This misclassification is corrected when the proposed RA specific BMI cut‐off points are applied. RA, patients with rheumatoid arthritis; OA, patients with osteoarthritis; HC, healthy controls; BMI, classification according to existing body mass index (BMI) cut‐off points of 25 kg/m2 for overweight and 30 kg/m2 for obesity; BF, classification according to age and sex specific cut‐off points for body fat percentage; RA‐BMI, classification according to the proposed RA specific BMI cut‐off points of 23 kg/m2 for overweight and 28 kg/m2 for obesity. (B) BMI chart developed specifically for patients with RA. Values were calculated using the formula: BMI = weight (in kg)/height2 (in metres) for the rheumatoid arthritis specific BMI levels identified in the present study (23 kg/m2 for overweight, 28 kg/m2 for obesity). The generally accepted lower threshold for normal BMI (18.5 kg/m2) was not altered.
Discussion
The validity of BMI as an acceptable measure of overweight or obesity, and as an accurate reflection of body fat (BF) content, has been repeatedly questioned and the need for population specific BMI cut‐off points has been highlighted.7,10,11,12,13 Ideally, individualised assessment of BF should be pursued in the clinical setting, as BF percentage is a more reliable measure of fatness than BMI, at least in the general population.31 Indeed, our data indicate that only 58% of the variance in BMI can be predicted, as opposed to 77% in BF. BF in vivo can be determined via a number of methods such as underwater weighing, dual energy x ray absorptiometry, total body water, total body nitrogen, 40K whole body counting and urinary creatinine excretion.32,33,34 BF can also be estimated from the thickness of partial subcutaneous fat, near infrared rays and ultrasound.35 However, none of these methods can be practically used in the routine clinical setting as they require sophisticated apparatus and specialised personnel.33
In recent years, a bioelectrical impedance method for the estimation of BF in different populations has become popular and widely recommended, as it is reliable, objective, practical, relatively inexpensive and does not require highly trained personnel.32,33 The validity of this method has been confirmed in various studies.32,36,37,38,39 Devices with eight tactile electrodes using single frequency electrical current, similar to the one used in this study, generate highly reproducible measurements of total BF and segmental fat distribution.40 Their correlation with the “gold standards” of dual energy x ray absorptiometry and hydrostatic weighing is 0.90 and 0.80, respectively, with a standard error of around 3.0, producing a coefficient of variation of <10%.33 This suggests that bioelectrical impedance measurements (especially when using eight electrodes) are valid and suitable for body composition studies.32,39,40 Patients are usually happy to undergo such a measurement because of its simplicity and similarity to normal weighing.
In the absence of the necessary equipment or expertise, the predictive model presented here can be used to easily calculate BF of RA patients from BMI. The cross validation of this predictive model in patients with RA is reassuring. Even though there was a statistically significant difference between the measured and the predicted BF, closer examination of the means indicates that this difference is at a level of less than 0.5% of BF with a coefficient of variation of <10%. The statistical significance of such a small difference can be attributed to the very large number of the validation group and is clinically not significant. However, the parts of the equation referring to OA patients and healthy individuals need further prospective validation in sufficiently large samples of the relevant populations.
BMI remains the most commonly used indicator of body fatness in the clinical setting, and the cut‐off points of 25 kg/m2 and 30 kg/m2 (for overweight and obesity, respectively) used for the general population are also routinely applied in RA patients. This study shows that application of these BMI cut‐off points misclassified 9% of male and 15% of female RA patients in terms of actual body fatness. For a given BMI, RA patients exhibited an average 4.3% increase in BF compared to healthy controls. In contrast, for the same level of BF, RA patients had BMI values almost 2 kg/m2 lower than those of healthy controls. We propose that BMI cut‐off points in the RA population should be lowered to 23 kg/m2 (from 25 kg/m2) for overweight, and 28 kg/m2 (from 30 kg/m2) for obesity. The lowest limit for normal BMI (that is, 18.5 kg/m2) should remain unaltered, as low BMI levels have been related to increased cardiovascular risk in patients with RA.41,42 We also provide a chart for the classification of RA patients in normal, overweight and obese categories according to these BMI cut‐offs, for use in the routine clinical setting (fig 2B).
The most likely explanation for the BMI and BF differences observed in RA is rheumatoid cachexia associated with the chronic inflammatory response, given that such differences were not as prominent in OA. RA patients experience accelerated involuntary loss of fat‐free mass, predominantly in the skeletal muscle, in excess of what is normally expected as a result of the ageing process.43 Although the underlying mechanisms for rheumatoid cachexia remain unknown, possible contributing factors include the overproduction of inflammatory cytokines such as tumour necrosis factor α and interleukin 1β.43,44 Our subanalyses within the RA population revealed that neither BMI nor BF were associated with current clinical or serological disease activity, seropositivity for rheumatoid factor (which tends to associate with more severe disease) or corticosteroid administration. This is not totally surprising as disease activity may vary within small periods of time, depending on medication and the disease itself, whereas changes in body composition are longer term processes. On the other hand, disease duration appeared to be of some importance. It is possible that most alterations in body composition of RA patients occur in the first few years of the disease, as it has previously been reported,21 irrespective of disease characteristics or medical treatment.
The results of the present study are reminiscent of the observations made for Asian populations, which have significantly higher CVD risk than white people: BF in Asians has been found to be 3–5% higher than that of white people with similar BMI, whereas BMI was 3–4 kg/m2 lower than that of white people with similar BF.32 Differences in body build (trunk to leg length ratio and slenderness) and in muscularity have been suggested as possible explanations for these discrepancies. As a result, new cut‐off points for Asian populations have been set at 23 kg/m2 and 27 kg/m2 for overweight and obesity, respectively,10 and have been shown to be more sensitive in identifying Asians at increased risk for CVD.45
In our participants, lowered BMI cut‐off points would reflect an average reduction of 5–6 kg, or 8%, in the ideal weight (the weight one should have in order to be below the BMI cut‐off for overweight). Such reductions in body weight are likely to lead to physiological benefits in the cardiovascular system: in the general population, even a 5% reduction of body weight is known to favourably affect most classic CVD risk factors.46,47
The reduced BMI cut‐off points for RA suggested here may be of significance both for the management of individual patients and for further research into the cardiovascular morbidity and mortality of RA. In the clinical arena, the reduction of these thresholds would identify an additional 10–15% of people with RA as overweight or obese, and may trigger closer scrutiny for other CVD risk factors and appropriate intervention, if necessary. Moreover, obesity, defined by the BMI, is one of the WHO criteria for the metabolic syndrome.46 Aggressive identification and reduction of classic CVD risk factors in patients with RA is an obvious strategy for reducing the increased cardiovascular mortality of this disease.19 From the research perspective, the new thresholds may trigger re‐analysis of previously published cohorts or further analysis of prospective cohorts as to the importance of body fat as a predictor of CVD in RA and its association with other individual risk factors.
We conclude that, in the clinical setting, body fatness of RA patients should be evaluated based on the BMI cut‐off points of 23 kg/m2 for overweight and 28 kg/m2 for obesity. In the absence of specialised equipment, if necessary, BF of patients with RA can be estimated from BMI using the equation provided.
Acknowledgements
This study was funded by the Dudley Group of Hospitals R&D directorate cardiovascular programme grant and a Wolverhampton University equipment grant. The department of rheumatology has an infrastructure support grant from the Arthritis Research Campaign (number: 17682).
Abbreviations
ANCOVA - analysis of co‐variance
ANOVA - analysis of variance
BF - body fat
BMI - body mass index
CHD - coronary heart disease
CVD - cardiovascular disease
DAS - disease activity score
HC - healthy controls
MI - myocardial infarction
LIMAG - limits of agreement
OA - osteoarthritis
RA - rheumatoid arthritis
Footnotes
Competing interests: none.
References
- 1.Van Pelt R E, Jones P P, Davy K P, DeSouza C A, Tanaka H, Davy B M.et al Regular exercise and the age‐related decline in resting metabolic rate in women. J Clin Endocrinol Metab 1997823208–3212. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999340115–126. [DOI] [PubMed] [Google Scholar]
- 3.Romero‐Corral A, Montori V M, Somers V K, Korinek J, Thomas R J, Allison T G.et al Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006368666–678. [DOI] [PubMed] [Google Scholar]
- 4.Pi‐Sunyer F X. The obesity epidemic: pathophysiology and consequences of obesity. Obesity Res 20021097S–104. [DOI] [PubMed] [Google Scholar]
- 5.Krauss R M, Winston M, Fletcher B J, Grundy S M. Obesity: impact on cardiovascular disease. Circulation 1998981472–1476. [PubMed] [Google Scholar]
- 6.Wellens R I, Roche A F, Khamis H J, Jackson A S, Pollock M L, Siervogel R M. Relationships between the body mass index and body composition. Obes Res 1996435–44. [DOI] [PubMed] [Google Scholar]
- 7.Nevill A M, Stewart A D, Olds T, Holder R. Are adult physiques geometrically similar? The dangers of allometric scaling using body mass power laws. Am J Phys Anthropol 2004124177–182. [DOI] [PubMed] [Google Scholar]
- 8.Calle E E, Thun M J, Petrelli J M, Rodriguez C, Heath C W. Body‐mass index and mortality in a prospective cohort of US adults. N Engl J Med 19993411097–1105. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization Obesity: preventing and managing the global epidemic: report of the WHO consultation on obesity. Geneva: WHO, 3–5 June, 1997 [PubMed]
- 10.WHO Expert Consultation Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 200410157–163. [DOI] [PubMed] [Google Scholar]
- 11.Willett W C, Manson J E, Stampfer M J, Colditz G A, Rosner B, Speizer F E.et al Weight, weight change, and coronary heart disease in women. Risk within the ‘normal' weight range. JAMA 1995273461–465. [DOI] [PubMed] [Google Scholar]
- 12.Manson J E, Willett W C, Stampfer M J, Colditz G A, Hunter D J, Hankinson S E.et al Body weight and mortality among women. N Engl J Med 1995333677–685. [DOI] [PubMed] [Google Scholar]
- 13.Blew R M, Sardinha L B, Milliken L A, Teixeira P J, Going S B, Ferreira D L.et al Assessing the validity of body mass index standards in early postmenopausal women. Obesity Res 200210799–808. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh S D, Yoshinaga H. Abdominal fat distribution and coronary heart disease risk factors in men‐waist/height ratio as a simple and useful predictor. Int J Obes Relat Metab Disord 199519585–589. [PubMed] [Google Scholar]
- 15.Rimm E B, Stampfer M J, Giovannucci E, Ascherio A, Spiegelman D, Colditz G A.et al Body size and fat distribution as predictors of coronary heart disease among middle‐aged and older US men. Am J Epidemiol 19951411117–1127. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi M G, Commerford P et a l. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case‐control study. Lancet 20053661640–1649. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Engstrom G, Hedblad B, Calling S, Berglund G, Janzon L. Sex differences in the relationships between BMI, WHR and incidence of cardiovascular disease: a population‐based cohort study. Int J Obes. 2006, epub ahead of print. [DOI] [PubMed]
- 18.Bray G A. Don't throw the baby out with the bath water. Am J Clin Nutr 200479347–349. [DOI] [PubMed] [Google Scholar]
- 19.Kitas G D, Erb N. Tackling ischaemic heart disease in rheumatoid arthritis. Rheumatology 200342607–613. [DOI] [PubMed] [Google Scholar]
- 20.Stevens R J, Douglas K M, Saratzis A N, Kitas G D. Inflammation and atherosclerosis in rheumatoid arthritis. Expert Rev Mol Med 200571–24. [DOI] [PubMed] [Google Scholar]
- 21.Rall L C, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology 2004431219–1223. [DOI] [PubMed] [Google Scholar]
- 22.Metsios G S, Stavropoulos‐Kalinoglou A, Koutedakis Y, Kitas G D. Rheumatoid cachexia: causes, significance and possible interventions. Hospital Chronicles 2006120–26. [Google Scholar]
- 23.Arnett F C, Edworthy S M, Bloch D A, McShane D J, Fries J F, Cooper N S.et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 198831315–324. [DOI] [PubMed] [Google Scholar]
- 24.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K.et al The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 199134505–514. [DOI] [PubMed] [Google Scholar]
- 25.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K.et al Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986291039–1049. [DOI] [PubMed] [Google Scholar]
- 26.Tanita BC 418 MA instruction manual and technical notes. Tokyo, Japan: Tanita Corp, 2002
- 27.Prevoo M L, van't Hof M A, Kuper H H, van Leeuwen M A, van de Putte L B, van Riel P L. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 19953844–48. [DOI] [PubMed] [Google Scholar]
- 28.Brigden M L. Clinical utility of the erythrocyte sedimentation rate. Am Fam Physician 1999601443–1450. [PubMed] [Google Scholar]
- 29.Black S, Kushner I, Samols D. C‐reactive protein. J Biol Chem 200427948487–48490. [DOI] [PubMed] [Google Scholar]
- 30.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19868476307–310. [PubMed] [Google Scholar]
- 31.World Health Organization Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. World Health Organ Tech Rep Ser 20001–253. [PubMed]
- 32.Demura S, Sato S, Kitabayashi T. Percentage of total body fat as estimated by three automatic bioelectrical impedance analyzers. J Physiol Anthropol Appl Human Sci 20042393–99. [DOI] [PubMed] [Google Scholar]
- 33.Demura S, Kobayashi H, Tanaka K, Sato S, Nagasawa Y, Murase T. Comprehensive evaluation of selected methods for assessing human body composition. Appl Human Sci 19991843–51. [DOI] [PubMed] [Google Scholar]
- 34.Oppliger R A, Nielsen D H, Shetler A C, Crowley E T, Albright J P. Body composition of collegiate football players: bioelectrical impedance and skinfolds compared to hydrostatic weighing. J Orthop Sports Phys Ther. 1992, Apr 15187–192. [DOI] [PubMed] [Google Scholar]
- 35.Ellis K J. Human body composition: in vivo methods. Physiol Rev 200080649–680. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K, Kim H, Nakanishi T, Amagi H. Multifrequency impedance method for the assessment of body composition in Japanese adults. J Exercise Sports Physiol 1999637–45. [Google Scholar]
- 37.Oppliger R A, Nielsen D H, Shetler A C, Crowley E T, Albright J P. Body composition of collegiate football players: bioelectrical impedance and skinfolds compared to hydrostatic weighing. J Orthop Sports Phys Ther. 1992, Apr 15187–192. [DOI] [PubMed] [Google Scholar]
- 38.Gray D, Bray G, Gemayel N, Kaplan K. Effect of obesity on bioelectrical impedance. Am J Clin Nutr 198950255–260. [DOI] [PubMed] [Google Scholar]
- 39.Bolanowski M, Nilsson B E. Assessment of human body composition using dual‐energy x‐ray absorptiometry and bioelectrical impedance analysis. Med Sci Monit 200171029–1033. [PubMed] [Google Scholar]
- 40.Demura S, Sato S, Kitabayashi T. Estimation accuracy of percent total body fat and percent segmental fat measured by single‐frequency bioelectrical impedance analysis with 8 electrodes: the effect of difference in adiposity. J Sports Med Phys Fitness 20054568–76. [PubMed] [Google Scholar]
- 41.Escalante A, Haas R W, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med 20051651624–1629. [DOI] [PubMed] [Google Scholar]
- 42.Kremers H M, Nicola P J, Crowson C S, Ballman K V, Gabriel S E. Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum 2004503450–3457. [DOI] [PubMed] [Google Scholar]
- 43.Roubenoff R, Roubenoff R A, Cannon J G, Kehayias J J, Zhuang H, Dawson‐Hughes B.et al Rheumatoid cachexia: cytokine‐driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 1994932379–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecker S H, Solomon V, Mitch W E, Goldberg A L. Muscle protein breakdown and the critical role of the ubiquitin‐proteasome pathway in normal and disease states. J Nutr 1999129227S–237S. [DOI] [PubMed] [Google Scholar]
- 45.Deurenberg‐Yap M, Chew S K, Deurenberg P. Elevated body fat percentage and cardiovascular risks at low body mass index levels among Singaporean Chinese, Malays and Indians. Obesity Rev 20023209–215. [DOI] [PubMed] [Google Scholar]
- 46.Wilson P W F, Grundy S M. The metabolic syndrome: practical guide to origins and treatment: Part I. Circulation 20031081422–1424. [DOI] [PubMed] [Google Scholar]
- 47.Volek J S, Gomez A L, Love D M, Weyers A M, Hesslink R, Jr, Wise J A.et al Effects of an 8‐week weight‐loss program on cardiovascular disease risk factors and regional body composition. Eur J Clin Nutr 200256585–592. [DOI] [PubMed] [Google Scholar]