Abstract
Objective
To assess the effect of measles, mumps and rubella (MMR) vaccination on disease activity in children with juvenile idiopathic arthritis (JIA).
Methods
A retrospective observational multicentre cohort study was performed in 314 patients with JIA, born between 1989 and 1996. Disease activity and medication use were compared during the period of 6 months before vaccination versus 6 months after vaccination. Disease activity was measured by joint counts, the Physician's global assessment scale and erythrocyte sedimentation rate. Next, we compared disease activity in patients vaccinated between 8 and 9 years of age with the activity in patients who had not been vaccinated at this time (who received MMR between the ages of 9 and 10 years).
Results
No increase in disease activity or medication use was seen in the 6 months after MMR vaccination (n = 207), including in patients using methotrexate (n = 49). No overt measles infections were noted. When disease activity in vaccinated patients (n = 108) was compared with activity in those not yet vaccinated (n = 86), there were no significant differences.
Conclusions
The MMR booster vaccination does not seem to aggravate disease activity in JIA. This indicates that the most patients with JIA can be vaccinated safely with the MMR vaccine. A prospective study is recommended.
Keywords: measles, mumps and rubella vaccination, juvenile idiopathic arthritis, methotrexate, disease activity parameters, flares
The pathogenesis of autoimmune disease is largely unknown.1 The association of viral infections and vaccinations with acute arthritis has led to the hypothesis that viruses such as rubella may cause chronic arthritis.2 Vaccinated healthy children were shown to experience more limb symptoms after the measles, mumps and rubella (MMR) vaccination, and a small number developed acute arthritis.3 The meningococcal C, influenza and hepatitis B vaccines seem to be safe to use in patients with juvenile idiopathic arthritis (JIA) and rheumatoid arthritis (RA).4,5,6
There are case reports of chronic arthropathy induced by rubella vaccination, but controlled studies have failed to establish this association.7,8 The MMR vaccination is included in the Dutch vaccination programme at 14 months and as a booster at 8–10 years.9 Guidelines state that live vaccines are contraindicated in patients using immunosuppressive drugs.10 Moreover, decreasing herd immunity renders patients at increased risk for naturally acquired infections, as illustrated by the recent mumps epidemic in the USA.11
To date, no evidence exists on the safety MMR vaccination in patients with JIA. The aim of the present retrospective cohort study was to investigate the effect of the MMR booster vaccination on disease course in patients with JIA.
Patients and methods
Patients
In total, 413 patients with JIA born between 1989 and 1996 were eligible (see supplementary figure 1, available online at http://ard.bmj.com/supplemental). We confirmed the diagnosis according to International League of Associations for Rheumatology (ILAR) criteria, but human leucocyte antigen B27 typing was performed only in 111.12 All patients were asked for the vaccination date, and to comment on side‐effects after vaccination, such as clinically overt measles, and on reasons for refusing vaccination. We retrieved missing vaccination dates from the National Vaccination Institute.
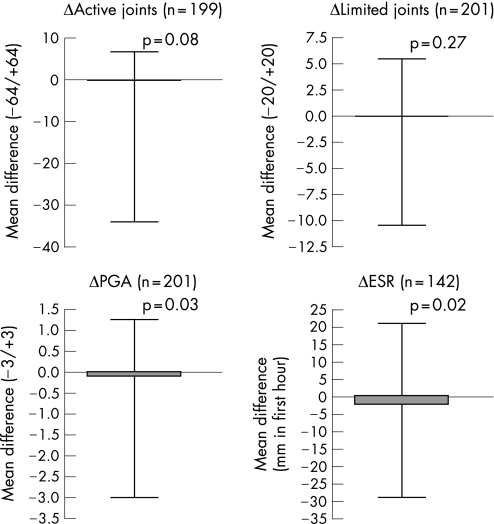
Figure 1 Differences (open triangles) in disease activity criteria before and after the MMR vaccination in the whole group. Mean values in 6 months before the MMR were subtracted from the mean values after. ESR, erythrocyte sedimentation rate; PGA, physician's global assessment.
Disease activity and medication use
Disease activity was measured as the number of joints with active arthritis, the Physician's global assessment (PGA) of disease activity on a 3‐cm visual analogue scale (VAS), and the erythrocyte sedimentation rate (ESR).13 We measured limitation of movement in the joints included in the Paediatric Escola Paulista de Medicina range of motion score.14 As expected in a retrospective study, there were many missing values for the Childhood Health Assessment Questionnaire (CHAQ) and this was therefore not analysed. Patients who did not consult their physician were assumed to have stable disease activity.
We compared the number of flares in the 6 months before and after MMR. A flare was defined as a worsening of ⩾40% in ⩾2 disease activity parameters without a simultaneous improvement of ⩾30% in ⩾2 of the remaining parameters.15 Medication use was noted for each visit.
Statistical methods
The mean of each individual disease parameter and the number of flares in the 6 months before MMR was compared with disease activity in the 6 months after MMR, using the Wilcoxon signed rank test for paired samples. We then compared disease activity in patients vaccinated between 8 and 9 years of age (group A, n = 108) with activity in children eligible to receive the MMR vaccine but who had not yet been vaccinated (group B, n = 86). Comparison of baseline characteristics between groups was calculated using the Mann–Whitney U test (two‐tailed). Influence of MMR vaccination on the risk of active disease was calculated using logistic regression. Adjustments for the covariates of JIA type and medication use were made using the propensity score.16
Results
Demographics
Of 413 eligible patients, 159 (38%) were excluded. In total, 59 of these did receive an MMR booster, but we were unable to retrieve their exact vaccination dates; 23 patients did not reply or had moved elsewhere; 14 patients had visited outpatient clinics elsewhere; 3 patients refused to participate; and 60 patients who developed JIA at any time point after the MMR booster were also excluded. Only one patient developed JIA within 1 month after vaccination.
Of 254 included patients, 47 had not received the MMR vaccination and were excluded. The reasons for not receiving vaccination were: doctor's advice (n = 27), fear of side effects (n = 4), reasons of principle (n = 3), and practical reasons (n = 5). The 207 vaccinated patients with JIA had the following subtypes: persistent oligoarthritis (n = 101), extended oligoarthritis (n = 22), RF‐negative polyarthritis (n = 55), RF‐positive polyarthritis (n = 5), systemic arthritis (n = 17), enthesitis related arthritis (n = 3) and psoriatic arthritis (n = 4) (supplementary tables 1 and 2; available online at http://ard.bmj.com/supplemental).
Table 1 Disease activity before and after the patients with juvenile idiopathic arthritis using methotrexate, who received the measles, mumps and rubella vaccination (n = 49).
| Patients using methotrexate (n = 49) | Before MMR* | After MMR* | p Value | |
|---|---|---|---|---|
| Disease activity parameters | ||||
| Active joints | 1 (0 to 24) | 1 (0 to 14) | 0.016 | |
| Limited joints | 1 (0 to 12) | 1 (0 to 3) | 0.198 | |
| PGA | 0.7 (0.0 to 2.7) | 0.3 (0.0 to 1.8) | 0.004 | |
| ESR | 12 (2 to 32) | 10 (2 to 33) | 0.016 | |
| Flares | ||||
| Per patient | 0 (0 to 3) | 0 (0 to 2) | 0.186 | |
| Patients with ⩾1 flares, n (%) | 13 (26.5) | 21 (42.9) | 0.115 | |
| Medication | ||||
| Methotrexate dose/person, mg/m2 | 11 (7 to 25) | 11 (0 to 22) | 0.666 | |
| Oral steroid dose per person, mg/kg | 0.0 (0.0 to 0.2) | 0.0 (0.0 to 0.2) | 0.333 | |
| Patients on NSAIDs, n (%) | 45 (91.8) | 44 (89.8) | 1.000 | |
| Patients on intra‐articular steroids, n (%) | 9 (18.4) | 1 (2.0) | 0.008 | |
| Patients on other DMARDs, n (%) | 6 (12.2) | 6 (12.2) | 1.000 | |
| Patients on anti‐TNFα therapy, n (%) | 1 (2.0) | 1 (2.0) | 1.000 | |
DMARDs, disease‐modifying anti‐rheumatic drugs; MMR, measles, mumps and rubella; NSAIDs, non‐steroidal anti‐inflammatory drugs; TNF, tumour necrosis factor.
*Values are calculated for the period of 6 months before and after the MMR vaccination.
Data are median and range unless otherwise indicated; median and range are given if data are skewed.
Table 2 Adjusted and non‐adjusted risk of disease activity and flares in patients with juvenile idiopathic arthritis, who received the measles, mumps and rubella vaccination.
| Disease activity | Non‐adjusted values* | Adjusted values* | ||||
|---|---|---|---|---|---|---|
| n | OR (95% CI) | p Value | n | OR (95% CI) | p Value | |
| Active joints ⩾1 | 187 | 1.6 (0.9 to 3.0) | 0.109 | 173 | 1.6 (0.8 to 3.1) | 0.157 |
| Limited joints ⩾1 | 188 | 1.7 (0.9 to 3.1) | 0.095 | 173 | 1.6 (0.8 to 3.2) | 0.220 |
| PGA >0.3 | 188 | 1.7 (0.9 to 3.2) | 0.113 | 173 | 1.7 (0.8 to 3.6) | 0.146 |
| ESR >15 | 144 | 1.4 (0.6 to 3.3) | 0.437 | 132 | 1.4 (0.5 to 3.7) | 0.558 |
| Flare ⩾1 | 194 | 1.7 (0.9 to 3.3) | 0.118 | 175 | 1.4 (0.7 to 2.9) | 0.364 |
ESR, erythrocyte sedimentation rate; PGA, physician's global assessment.
Non‐adjusted odds ratios (OR) were calculated by univariate logistic regression analysis.
Adjusted values were calculated using a multivariate regression analysis, adjusting for JIA type and medication use with the propensity score.
PGA scores of up to 0.3 (10% of the maximum scale) were considered normal.
Side effects
Nine patients had experienced joint complaints or aggravation of disease. Fever or general malaise was noted in six patients and two reported skin rash. No measles, mumps or rubella infections were reported. This was also true for patients using methotrexate.
Before versus after analysis
No worsening of mean disease activity parameters was seen during the 6 months after compared with the 6 months before the MMR (figure 1). In total, 40 flares occurred in 36 patients before the MMR versus 56 flares in 50 patients after the MMR (supplementary table 2; available online at http://ard.bmj.com/supplemental). This difference was not significant. Ten flares (4.8% of all patients) were seen in the first month after vaccination.
Medication use
No increase was observed in the number of patients using oral or intra‐articular steroids or methotrexate. The mean dose of methotrexate or oral steroids after vaccination was also unchanged (supplementary table 2; available online at http://ard.bmj.com/supplemental).
Subgroup of vaccinated patients using methotrexate
Most of the patients using methotrexate at time of vaccination (51%) had polyarticular arthritis. As expected, baseline disease activity criteria were higher in the methotrexate group (p<0.01) More patients in the methotrexate group also used non‐steroidal anti‐inflammatory drugs (NSAIDs) (p<0.001) and oral steroids (p = 0.047). No aggravation of active joint count, PGA or ESR was seen in the vaccinated patients on methotrexate (table 1). No increase in flare occurrence and medication use was detected.
Historical randomisation analysis
Except for the number of limited joints, patients in group A had higher baseline disease activity (supplementary table 3, available online at http://ard.bmj.com/supplemental). There were more polyarticular patients (35.2% versus 24.4%) and fewer patients with persistent oligoarticular propblems (42.6% versus 59.3%) in group A, and relatively more patients in this group used methotrexate (23.8% versus 12.7%) and NSAIDs (50.5% versus 36.6%). Mean methotrexate dose was higher in group A, although this was not significant. From these data, we can conclude that the patients in group A had higher disease activity than those in group B.
Adjusted for JIA type and medication, the MMR vaccination did not increase the risk for an elevated active or limited joint count, PGA, ESR (table 2), or flare (odds ratio = 1.4, 95% CI 0.7 to 2.9), although all odds ratios were >1, indicating a possible increased risk of active disease.
Discussion
This study shows that the MMR vaccination appears to be safe in JIA. We detected no changes in disease activity, flare occurrence or medication use after the MMR vaccination. No overt measles infections were noted. After adjustments for the possible confounders of JIA type and medication use, risk of active disease was not significantly increased in the vaccinated patients.
The main reason for exclusion was inability to retrieve the exact vaccination date (14.3% of patients). Patients with less severe disease are more likely to have no follow‐up data. However, we had to exclude only 3.3% of the total population for this reason. The 47 patients who did not received the MMR vaccination tended to have more severe disease or used anti‐tumour necrosis factor α therapy, so our results are not applicable to those patients with JIA. However, 49 patients using methotrexate at time of vaccination showed no aggravation of disease.
Of the 60 patients that were diagnosed after the MMR, only one developed JIA within 1 month of vaccination. A temporal relation between the MMR vaccination and JIA onset seems more likely than a causal relationship for several reasons. Firstly, cases of JIA with a disease onset >3 months after the MMR vaccination are unlikely to be related to the vaccination. Secondly, the age at onset of JIA varies between 1 and 16 years. There was no increase in the number of JIA diagnoses made shortly after vaccination. The distribution of age at onset in our population is similar to that of a cohort of patients with JIA from Sweden, where children are vaccinated at 2–12 years of age.17 Finally, a decrease in incidence of JIA was observed in the decades after introduction of the routine MMR vaccination.18
Given the retrospective nature of this study, the number of visits per patient varied. CHAQs were not routinely obtained for each visit. Therefore, we used an adjusted flare definition that does not require all six criteria. As PGA and active joints are the strongest indicators for disease activity,19 we were able to detect flares using these parameters.
As a substitute for randomisation, we developed a ”quasi‐randomised” historical cohort. Although the allocation of the MMR vaccination is random, more patients with polyarticular disease were seen in the vaccinated group and more patients with oligoarticular disease in the group that had not yet been vaccinated. This indicates that the time of vaccination receipt was independent of disease type or severity. This strengthens our analysis, as confounding by indication was highly unlikely. We were able to adjust for JIA type and medication use by propensity scoring, a method for bias reduction in a non‐randomised control group. Unfortunately, it was not possible to adjust for differences in baseline core set criteria. As a result, it is likely that there is residual bias in our dataset for which we could not correct. This residual bias can explain the raised odds ratios for disease activity after vaccination. However, we cannot fully rule out that there was a small influence of the MMR vaccination on disease activity. Even though this was one of the largest cohorts of vaccinated patients with JIA, we realise that its statistical power is limited. To further ascertain safety and efficacy of the MMR vaccination in JIA, a prospective trail is recommended.
Supplementary material can be viewed online on the ARD website at http://ard.bmj.com/supplemental.
Copyright © 2007 BMJ Publishing Group and European League Against Rheumatism
Acknowledgements
We thank Petra Oomen (National Vaccination Institute), Jan Jaap van der Net and Mirjam Visser (Wilhelmina Children's Hospital) for providing patient data. Nico Wulffraat was supported by the Dutch League against Rheumatism. Mare Pileggi was supported by the ALFA project from the Pediatric Rheumatology International Trial Organisation.
Abbreviations
CHAQ - Childhood Health Assessment Questionnaire
DMARD - disease‐modifying anti‐rheumatic drug
ESR - erythrocyte sedimentation rate
ILAR - International League of Associations for Rheumatology
JIA - juvenile idiopathic arthritis
MMR - measles, mumps and rubella
NSAID - non‐steroidal anti‐inflammatory drug
PGA - physician's global assessment
RF - rheumatoid factor
Footnotes
Competing interests: None declared.
Supplementary material can be viewed online on the ARD website at http://ard.bmj.com/supplemental.
References
- 1.Wraith D C, Goldman M, Lambert P H. Vaccination and autoimmune disease: what is the evidence? Lancet 20033621659–1666. [DOI] [PubMed] [Google Scholar]
- 2.Shoenfeld Y, Aron‐Maor A. Vaccination and autoimmunity – ‘vaccinosis': a dangerous liaison? J Autoimmun 2000141–10. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin C M, Chew G C, Silman A J. Joint and limb symptoms in children after immunisation with measles, mumps, and rubella vaccine. BMJ 19923041075–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zonneveld‐Huijssoon E, Ronaghy A, Rossum van M, Rijkers G T, Klis van der F, Sanders E.et al Safety and efficacy of meningococcal C vaccination in juvenile idiopathic arthritis. Arthritis Rheum. 2007; doi:10. 1136/ard. 2006. 063586 [DOI] [PubMed]
- 5.Kasapcopur O, Cullu F, Kamburoglu‐Goksel A, Cam H, Akdenizli E, Calykan S.et al Hepatitis B vaccination in children with juvenile idiopathic arthritis. Ann Rheum Dis 2004631128–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malleson P N, Tekano J L, Scheifele D W, Weber J M. Influenza immunization in children with chronic arthritis: a prospective study. J Rheumatol 1993201769–1773. [PubMed] [Google Scholar]
- 7.Howson C P, Katz M, Johnston R B, Jr, Fineberg H V. Chronic arthritis after rubella vaccination. Clin Infect Dis 199215307–312. [DOI] [PubMed] [Google Scholar]
- 8.Ray P, Black S, Shinefield H, Dillon A, Schwalbe J, Holmes S.et al Risk of chronic arthropathy among women after rubella vaccination. Vaccine Safety Datalink Team. JAMA 1997278551–556. [PubMed] [Google Scholar]
- 9.Inspectorate for Dutch Health Care Vaccination situation in the Netherlands per January 2002 (abstract in English). The Hague: Inspectorate for Dutch Health Care 2004
- 10.BSR Clinical Affairs Committee Vaccinations in the immunocompromised person: guidelines for the patient taking immunosuppressants, steroids and the new biologic therapies London: British Society for Rheumatology. 2006
- 11. Update: multistate outbreak of mumps – United States, January 1–May 2, 2006. MMWR Morb Mortal Wkly Rep 200655559–563. [PubMed] [Google Scholar]
- 12.Petty R E, Southwood T R, Manners P, Baum J, Glass D N, Goldenberg J.et al International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 200431390–392. [PubMed] [Google Scholar]
- 13.Giannini E H, Ruperto N, Ravelli A, Lovell D J, Felson D T, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997401202–1209. [DOI] [PubMed] [Google Scholar]
- 14.Len C, Ferraz M B, Goldenberg J, Oliveira L M, Araujo P P, Quaresma M R.et al Pediatric Escola Paulista de Medicina Range of Motion Scale: a reduced joint count scale for general use in juvenile rheumatoid arthritis. J Rheumatol 199926909–913. [PubMed] [Google Scholar]
- 15.Brunner H I, Lovell D J, Finck B K, Giannini E H. Preliminary definition of disease flare in juvenile rheumatoid arthritis. J Rheumatol 2002291058–1064. [PubMed] [Google Scholar]
- 16.D'Agostino R B., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group. Stat Med 1998172265–2281. [DOI] [PubMed] [Google Scholar]
- 17.Andersson G B, Fasth A, Andersson J, Berglund G, Ekstrom H, Eriksson M.et al Incidence and prevalence of juvenile chronic arthritis: a population survey. Ann Rheum Dis 198746277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson L S, Mason T, Nelson A M, O'Fallon W M, Gabriel S E. Juvenile rheumatoid arthritis in Rochester, Minnesota 1960–1993. Is the epidemiology changing? Arthritis Rheum 1996391385–1390. [DOI] [PubMed] [Google Scholar]
- 19.Magni‐Manzoni S, Cugno C, Pistorio A, Garay S, Tsitsami E, Gasparini C.et al Responsiveness of clinical measures to flare of disease activity in juvenile idiopathic arthritis. Clin Exp Rheumatol 200523421–425. [PubMed] [Google Scholar]