We read with interest the recent articles on obesity, inflammation and colorectal cancer (CRC).1,2,3 Although insulin resistance is the most widely accepted underlying mechanism explaining the association between obesity and CRC, recent evidence suggests that the effects of obesity on the immune system in general and on the gut in particular may play a role. We propose that obesity predisposes to CRC through its effects on innate immune activation (IIA) and consequent subclinical bowel inflammation. We further propose that the role of insulin resistance is either complementary or might merely represent an epiphenomenon. The justification of our argument is explained below.
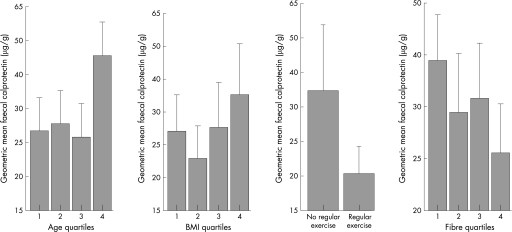
Recently, we studied the determinants of whole gut inflammation in a normal middle‐aged population by determining levels of calprotectin in faeces. Calprotectin is a calcium‐binding protein found only in neutrophils and monocytes. Levels in faeces correlate well with faecal levels of indium‐labelled white cells and inflammatory bowel disease activity. It is well established that many risk factors for CRC influence levels of IIA in a healthy individual. Lack of physical exercise, for instance, is associated with increased serum levels of C reactive protein, as are body mass index, smoking and increasing age.4 We confirmed that faecal levels of calprotectin also correlated directly with increasing age, obesity, and lack of physical activity and fibre intake, demonstrating that these same environmental risk factors are also associated with bowel inflammation5 (fig 1).
Figure 1 Relationships between age, body mass index, physical activity and fibre intake with faecal calprotectin geometric mean; bars, 1 (SE; y axis logarithmic scale). Age quartiles (years) 1 = 50–54, 2 = 55–59, 3 = 60–64 and 4 = 65–70. Body mass index (BMI) quartiles (kg/m2) 1 = 17.6–23.5, 2 = 23.6–25.6, 3 = 25.7–28.1 and 4 = 28.2–41.2. Fibre quartiles (%) 1<9.8, 2 = 10–13.9, 3 = 14–16.7 and 4 = 17–38.8.5
The link between chronic inflammation and CRC is well documented in patients with ulcerative colitis. In our late middle‐aged population, 25% of subjects had increased faecal levels of calprotectin similar to those found in patients with inflammatory bowel disease. Although these cancers follow a different histological course as compared with sporadic CRC, similar genetic mutations tend to occur with similar frequencies in both the groups, suggesting that sporadic CRC could arise or be promoted on a background of chronic subclinical inflammation.6 Many other common cancers are associated with chronic inflammation, including oesophageal, gastric, pancreatic and lung cancers. Furthermore, epidemiological studies have shown the chemoprophylactic benefits of non‐steroidal anti‐inflammatory agents in colorectal and oesophageal cancers, supporting the role of chronic inflammation in their pathogenesis.
The association between obesity, chronic subclinical bowel inflammation and ultimately CRC could result from the effects of obesity to promote generalised innate immune activation or through paracrine effects of mesenteric or serosal adipose tissue. The enlarged adipocytes of obese individuals synthesise increased amounts of various proinflammatory adipokines such as tumour necrosis factor α (TNFα) and interleukin (IL)6, as well as other adipokines known to modulate immune function such as leptin (proinflammatory) and adiponectin (anti‐inflammatory).7 Adipokines exert autocrine, paracrine or endocrine effects, influencing various metabolic and immune processes. Many of these cytokines and adipokines contribute strongly to insulin resistance, which may merely be an epiphenomenon of this activation rather than playing a direct role in the pathogenesis of CRC. Increased levels of proinflammatory cytokines and reduced levels of adiponectin have been noted in the serum of asymptomatic obese individuals, levels corresponding to the degree of obesity.7 Circulating mononuclear cells from the obese individuals have been shown to exhibit increased nuclear factor (NF)κB nuclear binding with decreased levels of NFκB inhibitor, together with increased mRNA expression of IL6, TNFα and migration inhibition factor. Furthermore, there is a good correlation between the markers of macrophage activation and plasma levels of free fatty acids, which represents an additional mechanism whereby adipose tissue could influence systemic inflammatory activity.8 Central or abdominal obesity correlates more strongly with the risk of colorectal cancer, suggesting that the paracrine effects of mesenteric or serosal fat may be important. The association between lack of physical exercise and colorectal cancer can also be explained by an activated immune state secondary to a decreased vagal tone in physically unfit individuals.9 IIA and chronic inflammation are characterised by chronic NFκB activation, which in turn promotes tumorigenesis by inhibiting apoptosis.3 These findings suggest that environmental factors could influence the development of CRC through activation of the innate immune system and its effect on promoting gut inflammation.
Proinflammatory mediators have been shown to increase gut permeability and consequently exposure to luminal antigens. For example, patients with histologically proven quiescent Crohn's disease have increased gut permeability, attributable to the heightened local expression of TNFα.10 Treatment with infliximab (anti‐TNFα agent) is associated with a decrease in gut permeability in patients with active Crohn's disease.11 Although most of the evidence emerges from studies in disease groups, similar mechanisms, albeit at a subclinical level, may operate in normal obese individuals. As well as the increased circulating levels of proinflammatory mediators and free fatty acids associated with innate immune activation, the paracrine effects of adipokines secreted by mesenteric or serosal fat could directly influence gut barrier function. Increased exposure to luminal antigens will induce a local immune response resulting in mucosal inflammation.
Epidemiological and cell culture studies have shown a correlation between CRC, hyperinsulinaemia and increased levels of insulin‐like growth factor 1 (IGF1). Although insulin has been shown to directly stimulate the growth of colon cancer lines in vitro, its role in initiating tumorigenesis is doubtful as it is known to have anti‐inflammatory properties. The role of IGF1 as a procarcinogen is much better established, acting by promoting cell growth and inhibiting apoptosis. However, as mentioned by Frezza et al,1 recent studies have not shown a significant correlation between CRC and IGF1 levels when a Bonferroni adjustment was applied. Furthermore, insulin resistance may be the result of IIA associated with obesity. This calls for a need to reconsider “insulin resistance” as the underlying mechanism.
In conclusion, IIA could be the link between environmental risk factors and CRC. Processes secondary to IIA, such as chronic gut inflammation and insulin resistance, promote tumorigenesis. We propose that gut inflammation is the dominant mechanism responsible for the increased incidence of CRC in obese indivduals, and believe that the role of insulin resistance could represent an epiphenomenon. Further research in this direction is warranted.
Footnotes
Competing interests: None declared.
References
- 1.Frezza E E, Wachtel M S, Chiriva‐Internati M. Influence of obesity on the risk of developing colon cancer. Gut 200655285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall N R. Survival in colorectal cancer: impact of body mass and exercise. Gut 2006558–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boland C R, Luciani M G, Gasche C.et al Infection, inflammation, and gastrointestinal cancer. Gut 2005541321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendall M A, Strachan D P, Butland B K.et al C‐reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J 2000211584–1590. [DOI] [PubMed] [Google Scholar]
- 5.Poullis A, Foster R, Shetty A.et al Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 200413279–284. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. At the crossroads of inflammation and cancer. Cell 2004118671–674. [DOI] [PubMed] [Google Scholar]
- 7.Kershaw E E, Flier J S. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004892548–2556. [DOI] [PubMed] [Google Scholar]
- 8.Ghanim H, Aljada A, Hofmeyer D.et al Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 20041101564–1571. [DOI] [PubMed] [Google Scholar]
- 9.Pavlov V A, Tracey K J. The cholinergic anti‐inflammatory pathway. Brain Behav Immun 200519493–499. [DOI] [PubMed] [Google Scholar]
- 10.Soderholm J D, Streutker C, Yang P C.et al Increased epithelial uptake of protein antigens in the ileum of Crohn's disease mediated by tumour necrosis factor alpha. Gut 2004531817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suenaert P, Bulteel V, Lemmens L.et al Anti‐tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol 2002972000–2004. [DOI] [PubMed] [Google Scholar]