Abstract
Background
Acute hepatic failure secondary to paracetamol poisoning is associated with high mortality. C‐jun (NH2) terminal kinase (JNK) is a member of the mitogen‐activated protein kinase family and is a key intracellular signalling molecule involved in controlling the fate of cells.
Aim
To examine the role of JNK in paracetamol‐induced acute liver failure (ALF).
Methods
A previously developed mouse model of paracetamol poisoning was used to examine the role of JNK in paracetamol‐induced ALF.
Results
Paracetamol‐induced hepatic JNK activation both in human and murine paracetamol hepatotoxicity and in our murine model preceded the onset of hepatocyte death. JNK inhibition in vivo (using two JNK inhibitors with different mechanisms of action) markedly reduced mortality in murine paracetamol hepatotoxicity, with a significant reduction in hepatic necrosis and apoptosis. In addition, delayed administration of the JNK inhibitor was more effective than N‐acetylcysteine after paracetamol poisoning in mice. JNK inhibition was not protective in acute carbon tetrachloride‐mediated or anti‐Fas antibody‐mediated hepatic injury, suggesting specificity for the role of JNK in paracetamol hepatotoxicity. Furthermore, disruption of the JNK1 or JNK2 genes did not protect against paracetamol‐induced hepatic damage. Pharmacological JNK inhibition had no effect on paracetamol metabolism, but markedly inhibited hepatic tumour necrosis foctor α (TNF α) production after paracetamol poisoning.
Conclusions
These data demonstrated a central role for JNK in the pathogenesis of paracetamol‐induced liver failure, thereby identifying JNK as an important therapeutic target in the treatment of paracetamol hepatotoxicity.
Paracetamol (acetaminophen) poisoning is the most common cause of acute liver failure (ALF) in the UK and US.1,2 Deaths from paracetamol‐induced ALF still occur despite the availability of an effective antidote (N‐acetylcysteine (NAC) or methionine) and changes in the sale and packaging of paracetamol in the UK. The only effective treatment for severe cases of paracetamol hepatotoxicity is orthotopic liver transplantation. However, transplantation has disadvantages including limited donors, the commitment of often young recipients to lifelong immunosuppression and psychiatric or medical contraindications in approximately 50% of patients.3 Effective alternative treatments for paracetamol‐induced ALF are urgently required.
The metabolic activation of paracetamol to N‐acetyl‐p‐quinoneimine (NAPQI) and subsequent conjugation with glutathione is well characterised.4 However, the pathways linking paracetamol metabolism with hepatic necrosis remain elusive. Recent investigations have expanded our understanding of the complex interplay between the pathways of toxin metabolism, intracellular signalling and the host inflammatory response.5,6 Inflammatory cells play a permissive role in hepatic necrosis induced by paracetamol and early‐response cytokines and chemokines modulate both the injury and repair processes.7,8 The mitogen‐activated protein kinases are a family of serine/threonine kinases that are evolutionarily conserved signal‐transducing enzymes unique to eukaryotes.9,10 The c‐jun (NH2) terminal kinases (JNKs) are one subgroup of the mitogen‐activated protein kinases.11,12 The JNK signal transduction pathway is stimulated by a diverse range of extracellular and intracellular stimuli including reactive oxygen species (ROS), ultraviolet radiation and pro‐inflammatory cytokines. JNK controls many basic mammalian physiological processes and phosphorylates specific subunits (c‐Jun, JunB, JunD and activating transcription factor 2) of the activating protein 1 (AP‐1) transcription factor,13,14,15 activating genes that regulate diverse cellular functions including proliferation,16 survival17,18 and death.19,20 Recent in vitro data21,22,23,24 and in vivo studies25,26,27 suggest a role for JNK in mediating hepatic injury. Using a previously developed mouse model of paracetamol poisoning,8,28 we demonstrate a central role for JNK in the pathogenesis of paracetamol‐induced ALF, thereby identifying JNK as a potential therapeutic target.
Materials and methods
Human biopsy specimens and animal models
Archival human liver samples were obtained from the Department of Pathology, University of Edinburgh, Edinburgh, UK. All animal procedures were undertaken using C57/BL6 mice, with approved licence from the Animal Scientific Procedures Division of the Home Office (London, UK) using mice aged 8–10 weeks. Generation of JNK1−/− and JNK2−/− mice by gene‐targeting technology (on a C57/BL6 background) has been described previously.29 As control, age‐ and sex‐matched wild‐type (WT) littermates were used. After an overnight fast, mice were injected intraperitoneally (IP) with 350 mg/kg of paracetamol dissolved in sterile phosphate‐buffered saline (PBS) and warmed to 42°C as described previously,28 with carbon tetrachloride (CCl4) at a ratio of 1:3 with olive oil at a dose of 1 μl/g body weight as described previously30 or with anti‐Fas antibody (0.5 μg/g body weight; BD Pharmingen, UK). Two JNK inhibitors were used in this study: SP600125 (anthra[1,9‐cd]pyrazol‐6(2H)‐one) purchased from Calbiochem (San Diego, California, USA) and d‐JNKI1 (c‐Jun N‐terminal kinase peptide inhibitor 1, d‐stereoisomer) inhibitor purchased from Axxora (Nottingham, UK). The vehicle control for SP600125 was 40% polyethylene glycol (PEG; Sigma, UK) in PBS. The control for d‐JNKI1 was d‐TAT control peptide (Axxora) in PBS. In experiments utilising delayed JNK inhibition, pharmacological inhibitors or appropriate controls were administered IP 5 h after paracetamol injection.
Analysis of liver injury
After IP injection, groups of mice were scored as follows by a blinded observer for signs of systemic illness: clinically well (0 points), presence of piloerection (1 point), with additional hunched posture (2 points) and lack of spontaneous movement (3 points) 24 h after injection. Serum was stored at −80°C until use for determination of alanine aminotransferase (ALT) levels by an automated enzyme assay (Olympus 20700 analyser (Olympus UK Ltd, Southall, UK)). Two lobes from each liver were fixed in 4% paraformalydehyde before routine histological processing. Histological grading of hepatic necrosis was performed by two blinded observers using H&E‐stained sections as follows: <30% of the total area necrotic (1 point); 30–60% of the total area necrotic (2 points); >60% of the total area necrotic (3 points). Ten random fields were counted independently by two blinded observers to determine the number of apoptotic cells. Whole‐liver homogenates were prepared and the Cell Death Detection ELISA (Roche, Welwyn Garden City, UK; quantitative detection of histone‐associated DNA fragments in mononucleosomes and oligonucleosomes) was used as per the manufacturer's instructions.
Protein analysis
Paraffin‐wax‐embedded sections were deparaffinised and subjected to microwave antigen retrieval in citrate buffer. The primary antibodies were rabbit polyclonal anti‐phospho‐JNK (Biosource, Nivelles, Belgium) and antiparacetamol–protein adduct antibodies (HyCult Biotechnology, Uden, Netherlands). Species‐appropriate isotype control antibodies were also used for each experiment. Western blot analysis was undertaken by using the following primary antibodies: rabbit polyclonal anti‐phospho‐JNK 1&2 [pTpY183/185] (Biosource), rabbit polyclonal anti‐phospho‐p38 antibody [pTpY180/182] (Biosource, Belgium) and rabbit polyclonal anti‐β‐actin antibody (Sigma, Gillingham, UK). JNK activity was measured as described previously.31 Liver was homogenised in PBS with a protease inhibitor cocktail (Roche), and levels of immunoreactive tumour necrosis factor α (TNFα) and interferon γ (IFNγ) were measured in the supernatants by ELISA according to manufacturer's instructions (R&D systems, Abingdon, UK).
Statistical analysis
Results are presented as means (SEM). Significance of the differences between means was assessed using one‐way analysis of variance or two‐tailed Student's t test. Values of p<0.05 were considered significant. Unless stated otherwise, studies were performed on 3–6 independent occasions using 6–8 mice per group.
Results
Paracetamol induces hepatic JNK activation in human and murine liver and precedes the onset of hepatocyte death
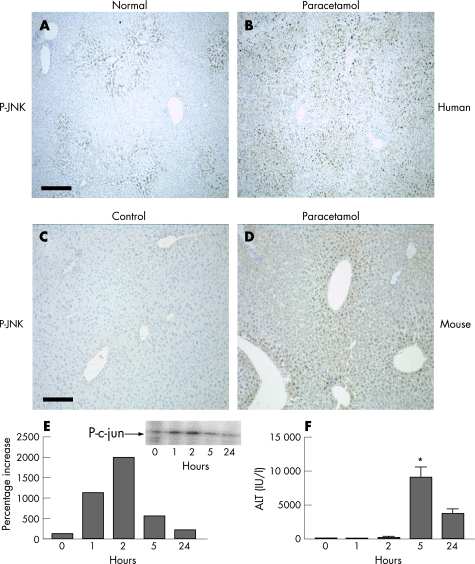
To determine whether JNK activity is increased during paracetamol‐induced liver injury, we examined phospho‐JNK expression. In a normal human liver, constitutive JNK phosphorylation occurs in areas surrounding the central veins (fig 1A). Cytoplasmic and dense nuclear staining was observed within hepatocytes. After paracetamol‐induced ALF, there was a marked increase in hepatocyte cytoplasmic and nuclear phospho‐JNK staining compared with the control normal liver (fig 1B; n = 8 cases in each group). We also examined JNK activation in a murine model of paracetamol‐induced hepatic injury. Phospho‐JNK expression was negligible in the control mouse liver (fig 1C). Similar to human cases, paracetamol‐injured mouse liver demonstrated marked upregulation of phospho‐JNK in the hepatocyte nuclei and cytoplasm in the areas surrounding the central veins (fig 1D). The time course of JNK activation after paracetamol‐induced injury was examined: hepatic JNK activity drastically increased after paracetamol administration, with peak activity 2 h after injection (fig 1E and inset). JNK activity then returned towards baseline levels by 24 h. Comparison with the time course of biochemical liver injury (determined by serum ALT) showed peak ALT 5 h after paracetamol injection (fig 1F).
Figure 1 Paracetamol induces hepatic c‐jun (NH2) terminal kinase (JNK) activation in both human and murine paracetamol‐induced liver injury, and precedes the onset of hepatocyte death. (A) Phospho‐JNK (P‐JNK) expression in normal human liver and (B) paracetamol‐induced liver injury in humans (n = 8 cases in each group). Scale bar 400 μm. (C) P‐JNK expression in mouse liver 2 h after intraperitoneal (IP) administration of control alone. (D) P‐JNK expression in mouse liver 2 h after treatment with paracetamol (350 mg/kg IP). Scale bar 200 μm. (E) Time course of hepatic JNK activation in mouse liver after paracetamol‐induced hepatic injury (350 mg/kg IP). Inset: representative phosphorimage of hepatic JNK (P‐c‐jun) activity in whole‐liver homogenates from paracetamol‐treated mice. (F) Time course of serum alanine aminotransferase (ALT) release in mice after paracetamol‐induced liver injury (350 mg/kg IP; *p<0.01).
Pharmacological inhibition of JNK activity in vivo markedly reduces murine mortality and liver injury
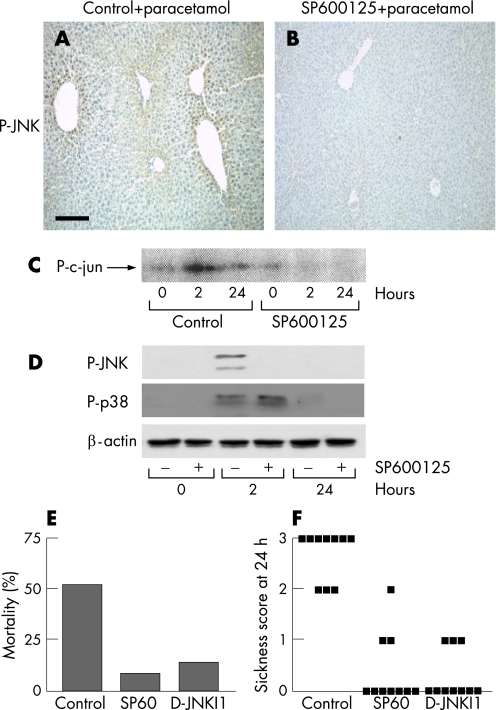
To assess the pathogenic significance of the observed increase in JNK activity, we used two pharmacological inhibitors of JNK that differ in their mechanisms of action. SP600125 is a small‐molecule reversible ATP‐competitive inhibitor,32,33 whereas d‐JNKI1 is a peptide inhibitor that inhibits the interaction of JNK with its substrates.34 Immunohistochemistry for hepatic phospho‐JNK demonstrated potent inhibition of JNK phosphorylation by SP600125 2 h after paracetamol treatment compared with control (fig 2A,B) and was confirmed by radioactive kinase assay (fig 2C) and western blotting for phospho‐JNK (fig 2D). Importantly, pretreatment with SP600125 did not inhibit phosphorylation of the other major stress‐activated protein kinase in the liver, p‐38 (fig 2D). To determine the potential clinical significance of JNK activation after paracetamol poisoning, the JNK inhibitors (or control) were injected 1 h before paracetamol administration, and mortality was assessed over 72 h. Inhibition of JNK activity with either SP600125 or D‐JNKI1 drastically reduced mortality compared with the vehicle control (fig 2E). In addition, sickness scores were assessed at 24 h, and the SP600125 and d‐JNKI1 inhibitor groups showed significantly lower sickness scores than the vehicle control group (fig 2F).
Figure 2 Pharmacological inhibition of c‐jun (NH2) terminal kinase (JNK) activity in vivo markedly reduces mortality in a mouse model of paracetamol‐induced acute liver failure. Mice were injected intraperitoneally (IP) with vehicle control or SP600125 (30 mg/kg) 1 h before IP injection with paracetamol solution (350 mg/kg). (A) Hepatic phospho‐JNK (P‐JNK) expression in the vehicle control group 2 h after paracetamol administration. (B) Hepatic P‐JNK expression in the SP600125 group 2 h after paracetamol administration. (C) Time course of hepatic JNK activity in mice injected IP with vehicle control or SP600125 (30 mg/kg) 1 h before paracetamol administration (350 mg/kg). (D) Time course of hepatic P‐JNK and phospho‐p38 in mice injected IP with vehicle control or SP600125 (30 mg/kg) 1 h before paracetamol administration (350 mg/kg). (E) Mortality at 72 h in mice injected IP with vehicle control, SP600125 (30 mg/kg) or d‐JNKI1 (c‐Jun N‐terminal kinase peptide inhibitor 1, d‐stereoisomer; 30 μg/mouse) 1 h before paracetamol administration (450 mg/kg; n = 10 mice in each group). (F) Sickness scores at 24 h in mice injected IP with vehicle control, SP600125 (30 mg/kg) or d‐JNKI1 (30 μg/mouse) 1 h before paracetamol administration (350 mg/kg; n = 10 mice in each group). Each point represents an individual mouse.
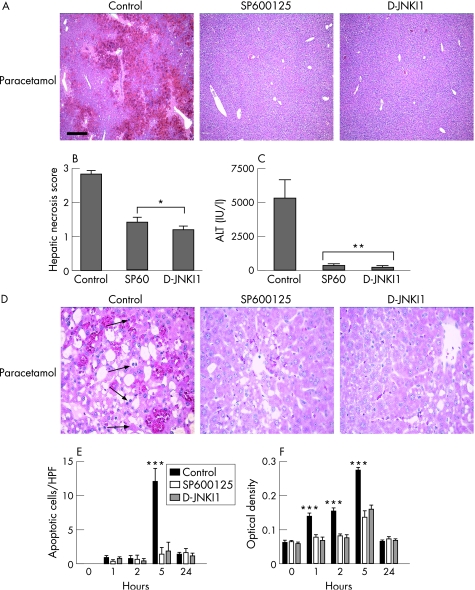
To further evaluate the striking survival benefit of JNK inhibition after paracetamol administration, liver injury was assessed after JNK inhibition. SP600125 or d‐JNKI1 dramatically reduced histological liver injury and hepatic necrosis scores compared with vehicle control (fig 3A,B). Furthermore, hepatocyte necrosis, as assessed by serum ALT release, was also reduced in the JNK inhibitor groups compared with vehicle control (fig 3C). Hepatocyte apoptosis may occur during paracetamol toxicity in the human and murine liver.35 Apoptotic cells were clearly visible in the control group 5 h after paracetamol administration; however, apoptotic cells were less abundant in the JNK inhibitor groups at this time point (fig 3D). Counting of morphologically apoptotic cells confirmed this finding, with a significant reduction in the number of apoptotic hepatocytes in the inhibitor groups compared with the control, 5 h after paracetamol administration (fig 3E). Further assessment of apoptosis by cell death ELISA using whole‐liver homogenates also demonstrated a significant reduction in apoptosis in the JNK inhibitor groups 1, 2 and 5 h after paracetamol treatment compared with the control (fig 3F, p<0.01).
Figure 3 c‐Jun (NH2) terminal kinase (JNK) inhibition in vivo reduces hepatic necrosis and apoptosis in paracetamol‐induced acute liver failure. Mice were injected intraperitoneally (IP) with vehicle control, SP600125 (30 mg/kg) or d‐JNKI1 (c‐jun N‐terminal kinase peptide inhibitor 1, d‐stereoisomer; 30 μg/mouse) 1 h before paracetamol administration (350 mg/kg; n = 6 mice in each group). (A) Liver histology at 24 h after paracetamol. Scale bar 200 μm. (B) Hepatic necrosis scores at 24 h after paracetamol administration (*p<0.001). (C) Serum alanine aminotransferase (ALT) release at 24 h after paracetamol administration (**p<0.05). (D) Liver histology at high magnification (×200) 5 h after paracetamol administration demonstrating apoptotic cells (arrowed) in the control vehicle‐treated group. (E) Time course of apoptotic cells/high power field (HPF; ***p<0.01). (F) Time course of apoptosis assessed by cell death ELISA (quantitative detection of histone‐associated DNA fragments in mononucleosomes and oligonucleosomes) from whole‐liver homogenates (***p<0.01).
Delayed administration of JNK inhibitor is more effective than NAC in limiting liver injury
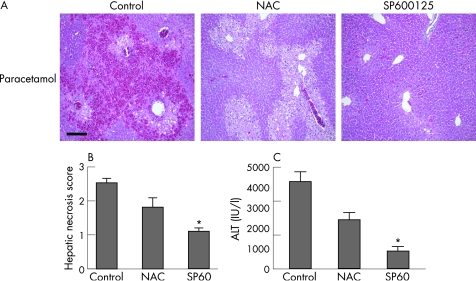
An important clinical problem in the management of paracetamol poisoning is the group of patients who present ⩾15 h after the overdose when the antidote, NAC, is much less effective. We have previously used NAC in a mouse model of paracetamol toxicity36 and demonstrated that beyond 5 h NAC is no longer effective in preventing liver injury. Therefore, we investigated whether delayed administration of the JNK inhibitor is more effective than NAC when given after a paracetamol overdose. Histological liver injury was less in the NAC and SP600125 groups (fig 4A). Furthermore, SP600125 was significantly more effective than NAC in limiting liver injury after paracetamol poisoning when given 5 h after an overdose, as assessed by hepatic necrosis scoring (fig 4B) and serum ALT release (fig 4C). However, JNK inhibition was no longer effective 8 and 24 h after paracetamol poisoning.
Figure 4 Delayed administration of c‐jun (NH2) terminal kinase (JNK) inhibitor is more effective than N‐acetylcysteine (NAC) in limiting liver injury after paracetamol poisoning in mice. Mice were treated with paracetamol (350 mg/kg intraperitoneally) and 5 h later administered control vehicle, NAC or SP600125 (30 mg/kg; n = 6 mice in each group). (A) Liver histology at 24 h after paracetamol administration. Scale bar 200 μm. (B) Hepatic necrosis scores at 24 h after paracetamol administration (*p<0.05). (C) Serum alanine aminotransferase (ALT) release 24 h after paracetamol administration (*p<0.05).
JNK inhibition is not protective in acute CCl4‐mediated or anti‐Fas antibody‐mediated hepatic injury
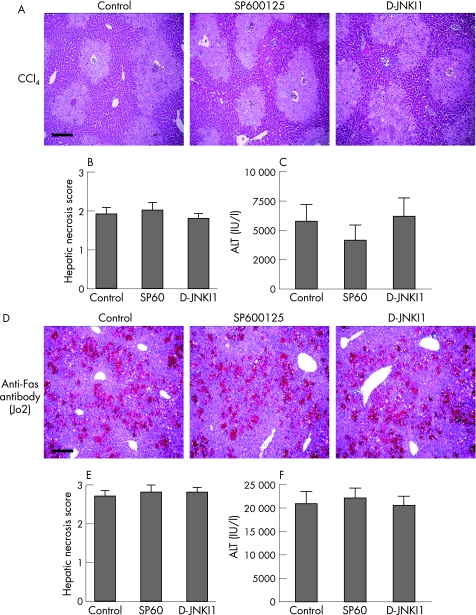
To determine whether JNK inhibition is protective in other forms of acute liver injury, we examined JNK inhibition in acute CCl4‐ and anti‐Fas antibody‐induced hepatic damage. Similar to paracetamol, CCl4 is a hepatotoxin metabolised in the liver by the cytochrome system, leading to the release of toxic free radicals and oxidant‐mediated hepatic injury. Also, CCl4 injection increases JNK activity in the whole liver in vivo.37 To assess the effect of JNK inhibition in CCl4‐mediated liver injury, mice received CCl4 (at a ratio of 1:3 with olive oil at a dose of 1 μl/g body weight) 1 h after treatment with control, SP600125 or d‐JNKI1. Similar histological liver injury was observed in all groups (fig 5A), and there was no significant difference in hepatic necrosis scores (fig 5B) or biochemical liver injury at 24 h (fig 5C). Further experiments were also undertaken using CCl4 at a ratio of 1:3 with olive oil at a dose of 0.5 μl/g body weight, with no protection provided by JNK inhibition.
Figure 5 c‐Jun (NH2) terminal kinase (JNK) inhibition is not protective in acute carbon tetrachloride (CCl4)‐ or anti‐Fas antibody‐mediated hepatic injury. CCl4 model: mice were administered CCl4 at a ratio of 1:3 with olive oil at a dose of 1 μl/g body weight 1 h after treatment with control, SP600125 (30 mg/kg) or d‐JNKI1 (c‐Jun N‐terminal kinase peptide inhibitor 1, d‐stereoisomer; 30 μg/mouse; n = 6 mice in each group). (A) Liver histology 24 h after CCl4 administration. Scale bar 200 μm. (B) Hepatic necrosis scores 24 h after CCl4 administration (p = NS). (C) Serum alanine aminotransferase (ALT) release 24 h after CCl4‐induced liver injury (p = NS). Fas model: mice were administered anti‐Fas antibody (0.5 μg/g body weight) 1 hour after treatment with control, SP600125 (30 mg/kg) or d‐JNKI1 (30 μg/mouse; n = 6 mice in each group). (D) Liver histology 6 h after anti‐Fas antibody administration. Scale bar 200 μm. (E) Hepatic necrosis scores 6 h after anti‐Fas antibody administration (p = NS). (F) ALT release 6 h after anti‐Fas antibody‐induced liver injury (p = NS).
Previous studies have suggested that the Fas death pathway may play a role in the pathogenesis of paracetamol‐induced liver injury.38,39,40 Therefore, we specifically examined the role of JNK inhibition in the anti‐Fas antibody model of hepatic injury. Administration of anti‐Fas (Jo‐2) antibody directly ligates and activates the CD95 death receptor pathway, resulting in massive hepatocyte apoptosis within hours.41,42 Mice received anti‐Fas antibody 1 h after treatment with control, SP600125 or d‐JNKI1. Similar histological liver injury was observed in all groups (fig 5D), and there was no significant difference in hepatic necrosis scores (fig 5E) or biochemical liver injury at 6 h (fig 5F). Further experiments were also undertaken using anti‐Fas antibody at a dose of 0.25 μg/g body weight, with no protection provided by JNK inhibition (data not shown).
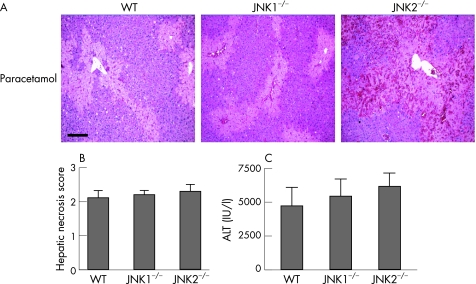
Disruption of the JNK1 or JNK2 genes does not protect against paracetamol‐induced liver injury
The JNK protein kinases are encoded by three genes.11 The liver expresses two of the three known JNK genes—JNK1 and JNK2. To discuss in further detail the role of JNK1 and JNK2 in paracetamol‐induced hepatic failure, we studied JNK1−/− and JNK2−/− mice (JNK1+2 double‐null mice are embryonic lethal). Liver histology showed no protection in the JNK1−/− and JNK2−/− mice compared with WT mice (fig 6A), and no significant difference in hepatic necrosis scores (fig 6B) or biochemical liver injury between groups (fig 6C).
Figure 6 Disruption of the JNK1 or JNK2 genes does not protect against paracetamol‐induced liver injury. Wild‐type (WT), JNK1−/− or JNK2−/− mice were administered paracetamol (350 mg/kg intraperitoneal), and livers and serum harvested at 24 h (n = 6 mice in each group). (A) Liver histology 24 h after paracetamol administration. Scale bar 200 μm. (B) Hepatic necrosis scores 24 h after paracetamol administration (p = NS). (C) Serum alanine aminotransferase (ALT) release 24 h after paracetamol‐induced liver injury (p = NS).
JNK inhibition does not affect synthesis of paracetamol–protein adducts, but markedly inhibits TNFα production in the liver after paracetamol‐induced hepatic injury
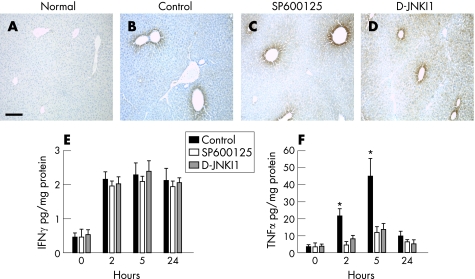
Ingestion of paracetamol results in metabolism to NAPQI, which is effectively detoxified by conjugation with glutathione. However, in paracetamol poisoning, cytosolic and mitochondrial glutathione becomes depleted, allowing covalent binding of NAPQI to hepatocellular proteins (paracetamol–protein adducts).4 Although the exact mechanisms mediating paracetamol hepatotoxicity remain elusive, recent data have highlighted the important regulatory role of the immune system and cytokine networks in determining outcomes after paracetamol‐induced liver injury.8,28,36,39,43 Therefore, we examined whether JNK inhibition interferes with the metabolism of paracetamol by measuring paracetamol–protein adducts.44 No paracetamol–protein adducts were seen in normal mouse liver (fig 7A). However, the formation of paracetamol–protein adducts (visible in hepatocytes around the central veins) was similar in all groups 5 h after paracetamol administration (vehicle control, SP600125 and d‐JNKI1 groups; fig 7B–D).
Figure 7 c‐Jun (NH2) terminal kinase (JNK) inhibition does not affect the synthesis of paracetamol–protein adducts, but markedly inhibits the production of tumour necrosis factor α (TNFα) in the liver after paracetamol‐induced hepatic injury. Mice were injected intraperitoneally with control, SP600125 (30 mg/kg) or d‐JNKI1 (c‐jun N‐terminal kinase peptide inhibitor 1, d‐stereoisomer; 30 μg/mouse) 1 h before paracetamol administration (350 mg/kg; n = 6 mice in each group). (A) Anti‐paracetamol–protein adduct staining in normal, untreated mouse liver. Scale bar 200 μm. (B–D) Formation of anti‐paracetamol–protein adduct 5 h after paracetamol administration. (E) Time course of hepatic interferon γ (IFNγ) production after paracetamol administration (p = NS). (F) Time course of hepatic TNFα production after paracetamol administration (*p<0.05).
We also explored in vivo which inflammatory cytokines may be modulated by JNK inhibition in our model of paracetamol‐induced liver injury. Previous studies have implicated interferon γ (IFNγ) in the pathogenesis of paracetamol‐induced liver injury.7,39 Hepatic IFNγ levels were increased in all groups 2 h after paracetamol administration compared with the control, and remained increased throughout 24 h. No significant difference in hepatic IFNγ levels was observed between the treatment groups at any of the time points studied (fig 7E).
TNFα is an inflammatory cytokine that may play a significant pathogenic role in paracetamol hepatotoxicity,28,45,46 although conflicting data have been reported. In vitro studies show that TNFα induces JNK activation in hepatocytes.47 In addition to the role of TNFα in apoptotic hepatocyte death, perhaps more relevant to paracetamol‐induced liver injury, the JNK/AP‐1 signalling pathway can also mediate TNFα‐induced necrotic hepatocyte death.48 JNK activation can also induce TNFα expression via AP‐1.49,50 Therefore, we examined whether the protective effect of JNK inhibition in paracetamol hepatotoxicity is mediated by modulation of TNFα expression. Hepatic TNFα levels were significantly increased in the vehicle control group 2 and 5 h after paracetamol administration compared with baseline (0 h). However, there was significantly less hepatic TNFα production in the JNK inhibitor groups 2 and 5 h after paracetamol administration compared with the vehicle control (fig 7F).
Discussion
We have shown a central role for JNK in the pathogenesis of paracetamol‐induced ALF, thereby identifying JNK as a potential therapeutic target in the treatment of paracetamol hepatotoxicity. Our data indicated that: (1) paracetamol‐induced liver injury results in hepatic JNK activation in human and murine tissue, which precedes the onset of hepatocyte death; (2) pharmacological inhibition of JNK in vivo markedly improved survival in a mouse model of paracetamol‐induced ALF and decreased both hepatic necrosis and apoptosis. The protective effect of JNK inhibition was specific for paracetamol hepatotoxicity as the inhibitors were not protective in acute CCl4‐mediated or anti‐Fas antibody‐mediated hepatic injury; (3) delayed administration of JNK inhibitor is more effective than NAC in limiting liver injury in mice; (4) disruption of either the JNK1 or JNK2 genes was not protective; and (5) inhibition of the JNK had no effect on paracetamol metabolism, but inhibited hepatic TNFα production after paracetamol‐induced ALF.
Paracetamol poisoning is the most common cause of ALF in the UK and USA, causing significant morbidity and mortality in predominantly young people. Many patients present outside the therapeutic window for the commonly used antidote, NAC. Our data demonstrated that delayed administration of the JNK inhibitor is more effective than NAC after paracetamol poisoning in mice. Although NAC can be administered up to 15 h after a paracetamol overdose in clinical practice, we found limited efficacy 5 h after paracetamol administration in the mouse model. This is in keeping with our previously published data,36 and relates to the truncated time course of liver injury in mice compared with humans. Metabolic activation of paracetamol and the histological liver injury produced in mice is similar to that observed in humans; however, the variability in terms of the clinical context in which the paracetamol overdose is taken and the genetic background of humans limits direct extrapolations of our results to the treatment of patients. Despite these limitations, our data indicate that further studies of JNK activation in cases of paracetamol poisoning in humans may lead to the development of new treatments and that pharmacological inhibition of JNK may be of particular clinical use in patients with delayed presentation after paracetamol overdose.
Our data show prolonged hepatic activation of JNK after paracetamol overdose in both the human and murine liver. Interestingly, in the normal human liver phospho‐JNK staining was observed in hepatocytes surrounding the central veins, the area in which paracetamol is preferentially activated by the hepatic cytochrome P450 system. Hepatic JNK activation may be a consequence of the oxidative stress produced during paracetamol metabolism. ROS are widely recognised to induce increased and/or prolonged JNK activation, possibly because of inactivation of cellular phosphatases.25 Although ROS alone can activate JNK, hepatic TNFα expression is increased in paracetamol‐induced liver injury.51,52,53 TNFα is a potent inducer of both JNK and ROS. Therefore, in the hepatic cytokine milieu induced by paracetamol, a positive amplification loop may exist whereby paracetamol‐induced ROS leads to JNK activation and TNFα expression via AP‐1,49,50 resulting in further prolonged massive JNK activation in the liver via TNF receptor signalling.
To investigate the potential pathogenic role of JNK activation in the development of paracetamol‐induced hepatic necrosis, we used two different JNK inhibitors with different mechanisms of action (SP600125 and d‐JNKI1). Both inhibitors have been widely studied both in vivo and in vitro. Although the specificity of pharmacological inhibitors can be questioned, we found no reduction in the hepatic activation of another stress‐activated protein kinase, p38, after administration of the JNK inhibitor and paracetamol. Furthermore, despite different mechanisms of action, both JNK inhibitors conferred significant survival benefit and profound protection against paracetamol‐induced hepatic necrosis.
Several studies have shown the mechanistic importance of the hepatic cytokine network in paracetamol‐induced liver injury. To investigate the mechanisms underlying the protective effect of JNK inhibition in paracetamol hepatotoxicity, we measured the hepatic expression of IFNγ and TNFα. Both these cytokines are induced in the liver after paracetamol poisoning.7,39,51,52,53 Furthermore, IFNγ knockout mice are resistant to paracetamol‐induced liver injury.7 However, in our model, JNK inhibition had no significant effect on hepatic IFNγ. By contrast, JNK inhibition significantly reduced hepatic TNFα expression. We and others have shown increased TNFα expression in the peripheral circulation and liver after paracetamol poisoning.28,53 In the studies reporting a protective effect of TNFα inhibition in paracetamol‐induced liver injury, the degree of protection was similar to that observed with JNK inhibition in our study.45,51,52 However, the pathogenic role of TNFα in paracetamol‐induced hepatic necrosis remains controversial. Studies using blocking antibodies and inhibitors of TNFα have produced conflicting results, with both protection and no effect being reported.51,52,53 Alternatively, some have shown attenuated liver injury in mice lacking TNF receptor 1 (TNFR1) expression, while others, using a different strain of mice, have shown increased liver injury in TNFR1 knockout mice.45,54 Our data suggest that JNK inhibition may limit liver injury via reduced TNFα expression, although in view of the reduced initial liver injury with JNK inhibition it is difficult to clarify a potential cause or an effect of the reduced TNFα expression. In a recent study, Kaplowitz et al reported that JNK inhibition limited paracetamol‐induced liver injury, possibly by interfering with translocation of members of the Bcl2 family into the mitochondrial membrane.55 This study reported that JNK inhibition protects TNFR1 knockout mice from paracetamol‐induced hepatic injury. However, TNFα expression levels were not measured in this study. These data do not invalidate our postulated mechanism as TNFα can also induce cell death via TNFR2 signalling.56,57 Further studies are required to fully understand the roles of TNFα‐induced TNFR1 and TNFR2 signalling via JNK in paracetamol‐induced liver failure.
The protective effect of JNK inhibition in paracetamol‐induced liver injury was not translated into protection in other models of liver injury. JNK inhibition was not protective after Fas ligation and CCl4 injection, which confirms previous reports.23,55 Similar to paracetamol hepatotoxicity, TNFα has been implicated in CCl4‐mediated liver injury, but again conflicting published data exist. Injection of soluble TNF receptors limits CCl4 hepatotoxicity58; however, pre‐injection of anti‐TNF antibodies has no protective effect.59 Furthermore, studies of TNF receptor 1, 2 or double‐knockout or TNFα‐knockout mice have demonstrated either no effect or protection after CCl4‐mediated liver injury.60,61,62,63 In contrast with our data on JNK inhibition in the context of paracetamol hepatotoxicity, we observed no reduction in hepatic TNFα expression after JNK inhibition and CCl4 injection (data not shown).
JNK induces the expression of TNFα in several cell types.49,50,64,65,66 Transfection of liver‐specific macrophages (Kupffer cells) with constitutively active adenovirus expressing the upstream JNK‐kinase, MKK‐7, induces TNFα production, and JNK inhibition reduces leptin‐induced TNFα production in the same cell type.65 In view of our data, it is noteworthy that a functional redundancy of JNK genes in TNFα production from macrophages has been reported.64 We speculate that pan‐inhibition of the JNK signalling pathway down regulates TNFα expression by Kupffer cells and reduces paracetamol‐induced liver injury. However, this may be difficult to study in vivo as Kupffer cell‐depleted mice are protected from paracetamol‐induced hepatic necrosis.67 Therefore, future studies with conditional cell‐specific JNK knockouts in the liver may aid further analysis in this regard.
The liver expresses two JNK genes—JNK1 and JNK2. Although complete JNK inhibition by SP600125 and d‐JNKI1 was protective, liver injury was similar in JNK1−/− and JNK2−/− mice compared with WT mice. Others have recently reported partial limitation of paracetamol‐induced liver injury in JNK2−/− mice or in mice treated with JNK2 antisense RNA, but this protection was not as effective as pan‐JNK inhibition with either pharmacological inhibitors or JNK antisense RNA.55 The context and cell‐specific effects of JNK1 or JNK2 knockout on cell death have been reported. Our data suggest redundancy between the JNK1 and JNK2 signalling pathways in the context of paracetamol‐induced liver injury. We were unable to directly address this further in our study as JNK1−/−2−/− (double null) mice are embryonic lethal, and therefore future studies with conditional knockouts in the liver may aid further analysis of the relative roles of these genes in paracetamol‐induced hepatic necrosis.
In summary, we found a massive increase in hepatic JNK activity during paracetamol‐induced hepatic failure in a murine model. Inhibition of hepatic JNK with either a pharmacological or peptide inhibitor significantly reduced liver injury and mortality without affecting paracetamol bioactivation, and confirms and expands recently published data.55 The hepatoprotective effect of JNK inhibition may be by a specific reduction in hepatic TNFα expression. From a clinical standpoint, we have shown that JNK inhibition is more efficacious in reducing liver injury at later time points when the traditional antidote NAC is no longer effective. These data demonstrate that JNK plays a crucial role in hepatocyte death after paracetamol poisoning, and suggest that JNK inhibition may find clinical applications in patients who present late after overdose or in whom timing of the overdose is unclear.
Acknowledgements
We thank Kirsten Atkinson and Anne Pryde for expert technical assistance. This work was supported by the Scottish Hospital Endowments Research Trust (SHERT; Grant Number RG56/01), the Wellcome Trust, UK and a Lothian University Hospitals NHS Trust Research and Development grant (Grant Number PG2004/26).
Abbreviations
ALF - acute liver failure
ALT - alanine aminotransferase
AP‐1 - activating protein 1
CCl4 - carbon tetrachloride
d‐JNKI1 - c‐jun N‐terminal kinase peptide inhibitor 1, d‐stereoisomer
IP - intraperitoneal
JNK - c‐jun (NH2) terminal kinase
NAC - N‐acetylcysteine
NAPQI - N‐acetyl‐p‐quinoneimine
PBS - phosphate‐buffered saline
ROS - reactive oxygen species
TNFα - tumour necrosis factor α
TNFR1 - TNF receptor 1
WT - wild type
Footnotes
Competing interests: None.
References
- 1.O'Grady J G. Acute liver failure. Postgrad Med J 200581148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson A M, Polson J, Fontana R J.et al Paracetamol‐induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005421364–1372. [DOI] [PubMed] [Google Scholar]
- 3.Simpson K J, Garden O J. The indications and implications of liver transplantation. Proc R Coll Phys Edinb 199929144–152. [Google Scholar]
- 4.Nelson S D, Bruschi S A. Mechanisms of paracetamol‐induced liver disease. In: Kaplowitz N, DeLeve L, eds. Drug‐induced liver disease. New York: Marcel Dekker, 2002287–325.
- 5.Jones B E, Czaja M J. Intracellular signaling in response to toxic liver injury. Am J Physiol 1998275874–878. [DOI] [PubMed] [Google Scholar]
- 6.Kaplowitz N. Paracetamol hepatoxicity: what do we know, what don't we know, and what do we do next? Hepatology 20044023–26. [DOI] [PubMed] [Google Scholar]
- 7.Ishida Y, Kondo T, Ohsima T.et al A pivotal involvement of IFN‐gamma in the pathogenesis of paracetamol‐induced acute liver injury. FASEB J 2002161227–1236. [DOI] [PubMed] [Google Scholar]
- 8.Hogaboam C M, Simpson K J, Chensue S W.et al Macrophage inflammatory protein‐2 gene therapy attenuates adenovirus‐ and paracetamol‐mediated hepatic injury. Gene Ther 19996573–584. [DOI] [PubMed] [Google Scholar]
- 9.Johnson G L, Lapadat R. Mitogen‐activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 20022981911–1912. [DOI] [PubMed] [Google Scholar]
- 10.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 200141037–40. [DOI] [PubMed] [Google Scholar]
- 11.Davis R J. Signal transduction by the JNK group of MAP kinases. Cell 2000103239–252. [DOI] [PubMed] [Google Scholar]
- 12.Weston C R, Davis R J. The JNK signal transduction pathway. Curr Opin Genet Dev 20021214–21. [DOI] [PubMed] [Google Scholar]
- 13.Smeal T, Binetruy B, Mercola D A.et al Oncogenic and transcriptional cooperation with Ha‐Ras requires phosphorylation of c‐Jun on serines 63 and 73. Nature 1991354494–496. [DOI] [PubMed] [Google Scholar]
- 14.Pulverer B J, Kyriakis J M, Avruch J.et al Phosphorylation of c‐jun mediated by MAP kinases. Nature 1991353670–674. [DOI] [PubMed] [Google Scholar]
- 15.Ip Y T, Davis R J. Signal transduction by the c‐Jun N‐terminal kinase (JNK)‐from inflammation to development. Curr Opin Cell Biol 199810205–219. [DOI] [PubMed] [Google Scholar]
- 16.Sabapathy K, Hochedlinger K, Nam S Y.et al Distinct roles for JNK1 and JNK2 in regulating JNK activity and c‐Jun‐dependent cell proliferation. Mol Cell 200415713–725. [DOI] [PubMed] [Google Scholar]
- 17.Xia Z, Dickens M, Raingeaud J.et al Opposing effects of ERK and JNK‐p38 MAP kinases on apoptosis. Science 19952701326–1331. [DOI] [PubMed] [Google Scholar]
- 18.Shaulian E, Schreiber M, Piu F.et al The mammalian UV response: c‐Jun induction is required for exit from the p53‐imposed checkpoint. Cell 2000103897–907. [DOI] [PubMed] [Google Scholar]
- 19.Tournier C, Hess P, Yang D D.et al Requirement of JNK for stress‐induced activation of the cytochrome c‐mediated death pathway. Science 2000288870–874. [DOI] [PubMed] [Google Scholar]
- 20.Varfolomeev E E, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell 2004116491–497. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Schattenberg J M, Rigoli R M.et al Hepatocyte resistance to oxidative stress is dependent on protein kinase C‐mediated down‐regulation of c‐Jun/AP‐1. J Biol Chem 200427931089–31097. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Lo C R, Czaja M J. NF‐kappaB inhibition sensitizes hepatocytes to TNF‐induced apoptosis through a sustained activation of JNK and c‐Jun. Hepatology 200235772–778. [DOI] [PubMed] [Google Scholar]
- 23.Schwabe R F, Uchinami H, Qian T.et al Differential requirement for c‐Jun NH2‐terminal kinase in TNFalpha‐ and Fas‐mediated apoptosis in hepatocytes. FASEB J 200418720–722. [DOI] [PubMed] [Google Scholar]
- 24.Graf D, Kurz A K, Fischer R.et al Taurolithocholic acid‐3 sulfate induces CD95 trafficking and apoptosis in a c‐Jun N‐terminal kinase‐dependent manner. Gastroenterology 20021221411–1427. [DOI] [PubMed] [Google Scholar]
- 25.Kamata H, Honda S, Maeda S.et al Reactive oxygen species promote TNFalpha‐induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005120649–661. [DOI] [PubMed] [Google Scholar]
- 26.Streetz K, Fregien B, Plumpe J.et al Dissection of the intracellular pathways in hepatocytes suggests a role for Jun kinase and IFN regulatory factor‐1 in Con A‐induced liver failure. J Immunol 2001167514–523. [DOI] [PubMed] [Google Scholar]
- 27.Uehara T, Bennett B, Sakata S T.et al JNK mediates hepatic ischemia reperfusion injury. J Hepatol 200542850–859. [DOI] [PubMed] [Google Scholar]
- 28.Hogaboam C M, Bone‐Larson C L, Steinhauser M L.et al Exaggerated hepatic injury due to paracetamol challenge in mice lacking C‐C chemokine receptor 2. Am J Pathol 20001561245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong C, Yang D D, Wysk M.et al Defective T cell differentiation in the absence of Jnk1. Science 19982822092–2095. [DOI] [PubMed] [Google Scholar]
- 30.Henderson N C, Mackinnon A C, Farnworth S L.et al Galectin‐3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci USA 20061035060–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKinnon A, Sethi T. [D‐Arg6, D‐Trp7,9, NmePhe8]‐substance P (6‐11) activates JNK and induces apoptosis in small cell lung cancer cells via an oxidant‐dependent mechanism. Methods Mol Med 200374299–307. [DOI] [PubMed] [Google Scholar]
- 32.Bennett B L, Sasaki D T, Murray B W.et al SP600125, an anthrapyrazolone inhibitor of Jun N‐terminal kinase. Proc Natl Acad Sci USA 20019813681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Z, Boyle D L, Chang L.et al c‐Jun N‐terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 200110873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borsello T, Clarke P G, Hirt L.et al A peptide inhibitor of c‐Jun N‐terminal kinase protects against excitotoxicity and cerebral ischaemia. Nat Med 200391180–1186. [DOI] [PubMed] [Google Scholar]
- 35.McGregor A H, More L J, Simpson K J.et al Liver death and regeneration in paracetamol toxicity. Hum Exp Toxicol 200322221–227. [DOI] [PubMed] [Google Scholar]
- 36.Hogaboam C M, Bone‐Larson C L, Steinhauser M L.et al Novel CXCR2‐dependent liver regenerative qualities of ELR‐containing CXC chemokines. FASEB J 1999131565–1574. [DOI] [PubMed] [Google Scholar]
- 37.Mendelson K G, Contois L R, Tevosian S G.et al Independent regulation of JNK/p38 mitogen‐activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci USA 19969312908–12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Cook J, Nickel J.et al Reduction of liver Fas expression by an antisense oligonucleotide protects mice from fulminant hepatitis. Nat Biotechnol 200018862–867. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z X, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of paracetamol hepatotoxicity. Gastroenterology 20041271760–1774. [DOI] [PubMed] [Google Scholar]
- 40.Tagami A, Ohnishi H, Hughes R D. Increased serum soluble Fas in patients with acute liver failure due to paracetamol overdose. Hepatogastroenterology 200350742–745. [PubMed] [Google Scholar]
- 41.Ogasawara J, Watanabe‐Fukunaga R, Adachi M.et al Lethal effect of the anti‐Fas antibody in mice. Nature 1993364806–809. [DOI] [PubMed] [Google Scholar]
- 42.Galle P R, Hofmann W J, Walczak H.et al Involvement of the CD95 (APO‐1/Fas) receptor and ligand in liver damage. J Exp Med 19951821223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bone‐Larson C L, Hogaboam C M, Evanhoff H.et al IFN‐gamma‐inducible protein‐10 (CXCL10) is hepatoprotective during acute liver injury through the induction of CXCR2 on hepatocytes. J Immunol 20011677077–7083. [DOI] [PubMed] [Google Scholar]
- 44.James L P, McCullough S S, Lamps L W.et al Effect of N‐acetylcysteine on paracetamol toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci 200375458–467. [DOI] [PubMed] [Google Scholar]
- 45.Ishida Y, Kondo T, Tsuneyama K.et al The pathogenic roles of tumor necrosis factor receptor p55 in paracetamol‐induced liver injury in mice. J Leukoc Biol 20047559–67. [DOI] [PubMed] [Google Scholar]
- 46.Matsumaru K, Ji C, Kaplowitz N. Mechanisms for sensitization to TNF‐induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology 2003371425–1434. [DOI] [PubMed] [Google Scholar]
- 47.Schwabe R F, Brenner D A. Mechanisms of liver injury. I. TNF‐alpha‐induced liver injury: role of IKK, JNK, and ROS pathways, Am J Physiol Gastrointest Liver Physiol 2006290583–589. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Jones B E, Bradham C.et al Increased cytochrome P‐450 2E1 expression sensitizes hepatocytes to c‐Jun‐mediated cell death from TNF‐α. Am J Physiol Gastrointest Liver Physiol 2002282257–266. [DOI] [PubMed] [Google Scholar]
- 49.Swantek J L, Cobb M H, Geppert T D. Jun N‐terminal kinase/stress‐activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF‐alpha) translation: glucocorticoids inhibit TNF‐alpha translation by blocking JNK/SAPK. Mol Cell Biol 1997176274–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kontoyiannis D, Pasparakis M, Pizarro T T.et al Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU‐rich elements: implications for joint and gut‐associated immunopathologies. Immunity 199910387–398. [DOI] [PubMed] [Google Scholar]
- 51.Blazka M E, Wilmer J L, Holladay S D.et al Role of proinflammatory cytokines in paracetamol hepatotoxicity. Toxicol Appl Pharmacol 199513343–52. [DOI] [PubMed] [Google Scholar]
- 52.Blazka M E, Elwell M R, Holladay S D.et al Histopathology of paracetamol‐induced liver changes: role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol 199624181–189. [DOI] [PubMed] [Google Scholar]
- 53.Simpson K J, Lukacs N W, McGregor A H.et al Inhibition of tumour necrosis factor alpha does not prevent experimental paracetamol‐induced hepatic necrosis. J Pathol 2000190489–494. [DOI] [PubMed] [Google Scholar]
- 54.Gardner C R, Laskin J D, Dambach D M.et al Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor‐1. Potential role of inflammatory mediators. Toxicol Appl Pharmacol 2003192119–130. [DOI] [PubMed] [Google Scholar]
- 55.Gunawan B K, Liu Z X, Han D.et al c‐Jun N‐terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 2006131165–178. [DOI] [PubMed] [Google Scholar]
- 56.Maeda S, Chang L, Li Z W.et al IKKbeta is required for prevention of apoptosis mediated by cell‐bound but not by circulating TNFalpha. Immunity 200319725–737. [DOI] [PubMed] [Google Scholar]
- 57.Jupp O J, McFarlane S M, Anderson H M.et al Type II tumour necrosis factor‐alpha receptor (TNFR2) activates c‐Jun N‐terminal kinase (JNK) but not mitogen‐activated protein kinase (MAPK) or p38 MAPK pathways. Biochem J 2001359525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czaja M J, Xu J, Alt E. Prevention of carbon tetrachloride‐induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology 19951081849–1854. [DOI] [PubMed] [Google Scholar]
- 59.Bruccoleri A, Gallucci R, Germolec D R.et al Induction of early‐immediate genes by tumor necrosis factor alpha contribute to liver repair following chemical‐induced hepatotoxicity. Hepatology 199725133–141. [DOI] [PubMed] [Google Scholar]
- 60.Simeonova P P, Gallucci R M, Hulderman T.et al The role of tumor necrosis factor‐alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol 2001177112–120. [DOI] [PubMed] [Google Scholar]
- 61.Morio L A, Chiu H, Sprowles K A.et al Distinct roles of tumor necrosis factor‐alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol 200117244–51. [DOI] [PubMed] [Google Scholar]
- 62.Sudo K, Yamada Y, Moriwaki H.et al Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine 200529236–244. [DOI] [PubMed] [Google Scholar]
- 63.Yamada Y, Fausto N. Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. Am J Pathol 19981521577–1589. [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Krishnegowda G, Gowda D C. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal‐regulated kinase, p38, c‐Jun N‐terminal kinase and NF‐kappaB pathways for the expression of proinflammatory cytokines and nitric oxide. J Biol Chem 20052808617–8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen J, Sakaida I, Uchida K.et al Leptin enhances TNF‐alpha production via p38 and JNK MAPK in LPS‐stimulated Kupffer cells. Life Sci 2005771502–1515. [DOI] [PubMed] [Google Scholar]
- 66.Ishizuka T, Terada N, Gerwins P.et al Mast cell tumor necrosis factor alpha production is regulated by MEK kinases. Proc Natl Acad Sci USA 1997946358–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michael S L, Pumford N R, Mayeux P R.et al Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 199930186–195. [DOI] [PubMed] [Google Scholar]