The porphyrias are a group of disorders caused by defects in haem biosynthesis (fig 1). Of the seven main types of porphyria recognised, two are characterised by associated liver disease (table 1). In porphyria cutanea tarda it is the liver disease which leads to the onset of the porphyria, characterised by blistering, hirsutes and skin fragility of sun‐exposed skin. A number of different liver diseases may precipitate porphyria cutanea tarda including haemochromatosis, alcoholic liver disease and hepatitis C. In contrast, in erythropoietic protoporphyria (EPP) it is the porphyria itself which leads to liver disease, due to progressive deposition and accumulation of insoluble protoporphyrin IX in hepatocytes and bile canaliculi.
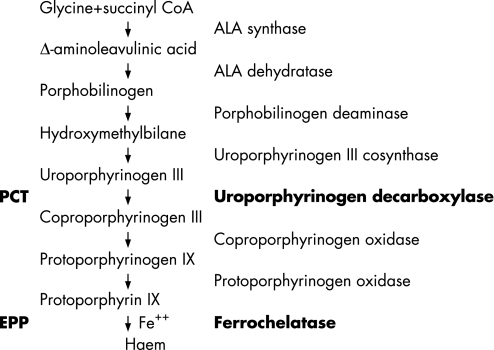
Figure 1 The haem biosynthetic pathway showing the enzyme deficiency associated with porphyria cutanea tarda (PCT) and erythropoietic protoporphyria (EPP). The final step in this pathway involves the incorporation of iron into the middle of the ring structure of protoporphyrin IX to form haem.
Table 1 Liver disease and the porphyrias: names and patterns of inheritance for the seven main clinical variants of porphyria, highlighting those characterised by concomitant liver disease.
| Disorder | Liver disease | Inheritance |
|---|---|---|
| ALA dehydratase porphyria | No | Autosomal recessive |
| Acute intermittent porphyria | No | Autosomal dominant |
| Congenital erythropoietic porphyria | No | Autosomal recessive |
| Porphyria cutanea tarda | Yes | Complex |
| Hereditary coproporphyria | No | Autosomal dominant |
| Variegate porphyria | No | Autosomal dominant, incomplete penetrance |
| Erythropoietic protoporphyria | Yes | Autosomal recessive (very rare) and autosomal dominant incomplete penetrance |
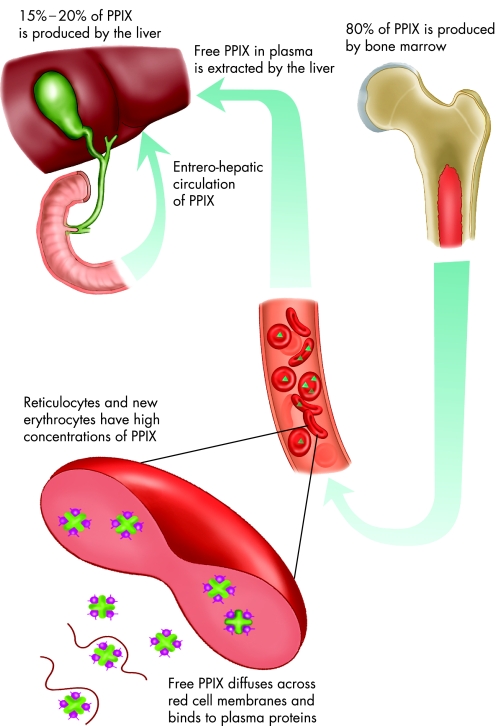
EPP is an inborn error of haem biosynthesis caused by mutations in the gene encoding the mitochondrial enzyme ferrochelatase (FECH), the final enzyme in the haem biosynthetic pathway (fig 1).1,2,3,4,5 It was first described by Magnus et al in 1962.6 Ferrochelatase catalyses the insertion of ferrous iron into protoporphyrin to form haem, and when defective or deficient, accumulation of protoporphyrin ensues. Ferrochelatase is active in cells that produce haem including erythroid precursors in the bone marrow7 and hepatocytes.8 However, the majority of protoporphyrin (80% or more) originates from bone marrow with most of the remainder generated by the liver (fig 2).7,9
Figure 2 The fate of protoporphyrin IX in erythropoietic protoporphyria.
Protoporphyrin accumulates in the maturing red blood cells during haematopoiesis. When red cells enter the circulation, free protoporphyrin diffuses across the red cell membrane and binds to plasma proteins. The liver extracts protoporphyrin from the plasma, most of which is excreted unchanged into the bile, with the remainder metabolised (by liver ferrochelatase) to haem. Some protoporphyrin is subsequently reabsorbed in an enterohepatic circulation.10
Protoporphyrin‐induced hepatotoxicity is a rare complication occurring in 1–5% of patients, for whom liver transplantation is often required. Since the first liver transplant for EPP in 1980,11 more than 40 further liver transplants have been carried out as treatment for advanced liver disease in this condition. However, liver transplantation fails to correct the underlying metabolic deficiency and protoporphyrin damage to the transplanted liver is likely.
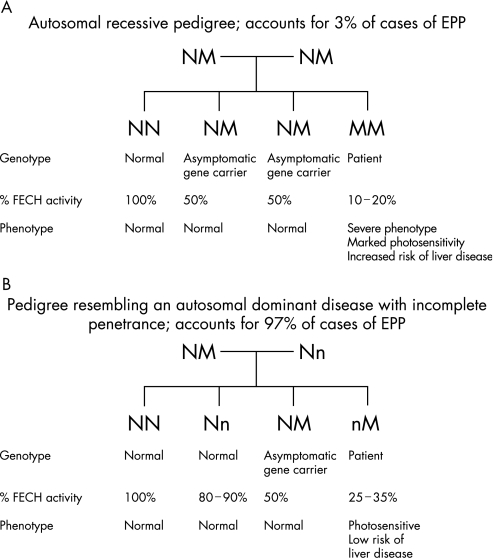
EPP is an inherited disorder with both recessive12,13,14,15,16 and dominant patterns of inheritance (fig 3A, B). In most patients with EPP, disease‐causing mutations are present on one allele in association with co‐inheritance of a low‐expression allele.17 This has been demonstrated by case‐control association in 39 families with EPP.18 Using haplotype segregation analysis, a polymorphism was identified in intron 3 (IVS3‐48C) that increases the use of an aberrant splice site.19 The aberrantly spliced mRNA has been shown to be subject to more rapid degradation resulting in a decreased steady‐state level of mRNA, leading to a further reduction in FECH enzyme activity and disease expression.19 The low‐expression variant IVS3‐48C has a prevalence in the white population of France of about 10%.18 Co‐inheritance of a FECH mutation and the low‐expression allele accounts for nearly all cases of expressed EPP,20,21,22,23 with estimated true autosomal recessive inheritance accounting for about 3% of cases.23 Very rarely, alternative mechanisms may reduce FECH activity below the critical threshold for symptomatic disease, including deletion of an FECH gene secondary to leukaemia24 or a dominant negative effect from the mutant FECH allele.25
Figure 3 Genetics of erythropoietic protoporphyria (EPP) and relationship between genotype and ferrochelatase activity (expressed as a percentage of normal). (A) Autosomal recessive inheritance. (B) Autosomal dominant inheritance due to co‐inheritance of low‐expression and mutant alleles. FECH, ferrochelatase; N, normal ferrochelatase gene; M, mutant allele; n, low‐expression allele.
The FECH gene was cloned and sequenced in 199026 and subsequently localised to the long arm of chromosome 18 (18q22.31).27 It spans 45 kb and contains 11 exons which code for an enzyme with 423 amino acid residues.28 The enzyme functions as a dimer, which may have reduced stability and catalytic activity in the presence of a mutated subunit.29 Allelic heterogeneity of the molecular defects in the FECH gene has been demonstrated.30 Analysis of the genetic mutations in EPP reveals three main categories:
Nucleotide substitutions: missense and nonsense mutations caused by single nucleotide substitutions in the coding region; nonsense mutations are null.
Splice site mutations: these may produce truncated proteins but this has never been directly shown for EPP. Furthermore, these mutations do not always produce stable mRNA transcripts; they may be null or may preserve some activity.
Frameshift mutations resulting in premature stop codons are always null, the mechanism being accelerated RNA decay.
Minder et al described a significant genotype‐phenotype correlation between so‐called “null allele” mutations and protoporphyrin‐related liver disease in EPP.31 This supported an earlier report which showed major structural alteration in the FECH protein in all of eight cases undergoing liver transplantation for EPP‐associated liver failure.32,33 However, as more data accumulate, it is increasingly clear that the FECH gene mutations by themselves do not account for the severe liver disease phenotype, as the same mutations have now been reported both in asymptomatic family members and in patients from families in which liver disease had not occurred.33,34 There is currently no way reliably to identify patients at risk, and no intervention that is uniformly effective in restoring normal liver function once hepatic failure ensues.
Liver disease in EPP
No study to date has specifically set out to document the natural history of liver disease or to identify risk factors in its causation in a large unselected cohort of unrelated patients with EPP. The largest studies reported so far have all been from single centres and many are subject to case selection bias resulting from local interest and expertise in the management of EPP‐related liver disease. Despite these shortcomings, these studies (summarised in table 2) provide a useful starting point for further analysis of this topic.
Table 2 Patient series with erythropoietic protoporphyria (EPP) worldwide with details of liver disease.
| First author | Year | Country | No of patients | % with hepatobiliary disease | Comment |
|---|---|---|---|---|---|
| Schmidt35 | 1974 | Denmark | 29 | 3% | Biochemistry not performed in all cases; 1 patient with porphyrin deposits in liver biopsy |
| DeLeo36 | 1976 | USA | 32 | 4% | No severe liver disease |
| Went,16 Baart de la Faille37 | 1984 | Netherlands | 200 | 6% | 9 with gallstones, 3 young patients with cirrhosis |
| Murphy38 | 1985 | UK | 382 | >1% | 3 patients with acute hepatic failure |
| Gross,41 Lehmann,40 Frank39 | 1998 | Germany | 140 | ∼25% | Severe liver disease in 10% of patients |
| Minder,31 Rüfenacht30 | 2002 | Switzerland and France | 55 | 16% | 9 patients with liver disease |
| Chen33 | 2002 | USA | 34 | 41% | Highly selected series. Included 10 patients who underwent liver transplant |
| Wiman20 | 2003 | Sweden | 9 | 10% | 1 patient with severe liver disease requiring liver transplantation |
Pathogenesis of liver disease in EPP
Irrespective of its origin, excess protoporphyrin is excreted by the liver into bile and enters an enterohepatic circulation. Protoporphyrin is a hydrophobic compound which is not filtered by the kidneys. When in excess, protoporphyrin becomes insoluble in bile and exerts cholestatic effects leading to architectural changes in the hepatobiliary system ranging from mild inflammation to fibrosis and cirrhosis.42 Even in early EPP, ultrastructural damage has been described in hepatocyte nuclei, endoplasmic reticulum, plasma membranes and bile canaliculi, associated with protoporphyrin crystals.43 Significant intracellular precipitates of protoporphyrin, demonstrated in liver biopsy samples by fluorescent birefringence, are invariably present in protoporphyric liver disease.44
Exposure of cultured hepatocytes to protoporphyrin inhibits cell metabolism and increases cellular fragility.45 However, it remains unclear what effect protoporphyrin has on hepatocytes in vivo and how this relates to the development of liver disease. Bloomer et al32 found that liver FECH activity in EPP‐related end‐stage liver disease was reduced more than could be explained by the decrease in ferrochelatase protein, and concluded that the liver probably contributes to the overproduction of protoporphyrin that results in its own damage. In the absence of a clear explanation for occasional severe liver disease complicating EPP in humans, Nordmann46 speculated that patients may vary in their susceptibility to protoporphyrin‐induced liver damage. This is probably so, but host factors other than deficiency of FECH activity relevant to the onset and progression of liver disease are currently unknown. Recent murine studies (highlighted later in this review) have revealed that other genetic factors are relevant to this process. It is likely that quantitative trait loci analyses will shed more light on this important topic, with the growing recognition of the importance of such factors for many inherited diseases.
Hepatobiliary disease in humans with EPP may be described under the following headings:
Cholelithiasis;
Mild liver disease;
Deteriorating liver disease; and
Terminal phase of EPP‐associated liver disease.
Cholelithiasis
Protoporphyrin in bile may crystallise out forming stones. The original case of EPP described by Magnus in 1961 underwent a cholecystectomy at the age of 29 years and a solitary gallstone was identified.6 Gallstones have subsequently been reported in EPP in many patients, including 2 patients in a series of 29 from Denmark,35 4 patients in a series of 32 reported from the USA,36 and 9 patients from a series of 200 reported from The Netherlands.37 Three of the patients in the series from the USA required cholecystectomy, and gallstones analysed from 2 of these cases revealed high levels of protoporphyrin.36 Todd highlighted the fact that, when gallstones occur in children, EPP should be included in the differential diagnosis.47
Mild liver disease
There is wide variation in the severity of liver disease in EPP. Minor abnormalities in biochemical parameters of liver function are relatively common and include raised aspartate transaminase levels and approximately twofold increases in alkaline phosphatase and γ‐glutamyl transferase.44 A study of 32 patients with EPP included a single patient with abnormal liver function.36 Analysis of liver biopsies from this case and 4 patients with normal liver function revealed protoporphyrin deposition without evidence of fibrosis or infiltrates in all 5 samples.36 In contrast, liver biopsies from 7 cases of EPP without overt liver disease from The Netherlands showed protoporphyrin deposition and mild fibrosis in 3 cases; the remainder were normal.48 In a separate study Cripps et al also reported protoporphyrin deposition in liver biopsy specimens from 5 patients with EPP and normal liver function tests; portal and periportal fibrosis was identified in 2 of these 5 samples.49 An ultrastructural study of liver biopsy specimens obtained from 11 patients with EPP, 4 of whom had overt liver disease and 7 of whom did not, revealed significant pathological changes in all samples compared with normal controls.50 It was concluded that liver damage is an early and consistent feature of EPP.50 Finally, a study with histopathology and ultrastructural studies of liver biopsy samples obtained from 4 patients with EPP (1 with severe liver disease, 1 with mild liver disease and 2 without evidence of liver disease and with normal histopathology) showed characteristic crystal‐containing vacuoles on electron microscopy in all 4 cases.51 It therefore appears that protoporphyrin deposition in hepatocytes is invariable, whereas histological evidence of damage is less common; electron microscopy will, however, show ultrastructural evidence of damage in most, if not all, patients with EPP.
Deteriorating liver disease
Patients with EPP who manifest significant liver disease will progress to decompensated cirrhosis which, in the absence of liver transplantation, is fatal. Various treatments have been attempted to preserve liver function and break the cycle of rapid deterioration that occurs in this situation in order to avoid terminal liver failure, or at least to buy time until a donor liver becomes available. The different forms of treatment have been directed at specific pathogenetic mechanisms as follows:
To increase the excretion of protoporphyrin into bile by the oral administration of the bile salts chenodeoxycholic acid50,52 or ursodeoxycholic acid.41
To reduce protoporphyrin production by suppressing erythropoiesis using iron,53,54 red cell transfusions55 or infusion of haematin,56,65 all of which are intended to reduce the drive for haem synthesis.
To reduce the pool of circulating plasma protoporphyrin by plasmapheresis,57,58 haemodialysis,59 and exchange transfusions.55,60
To reduce protoporphyrin levels by interrupting the enterohepatic circulation with administration of cholestyramine59,61 and activated charcoal.54,62
To reverse oxidative stress in EPP by intravenous vitamin E therapy.63
One or more of these treatments are sometimes combined,58,59 and this is currently the practice before liver transplantation in order to optimise the environment into which the new liver is transplanted.64,65 However, none of these treatments is effective in all cases, each has potential problems and none has been applied in sufficient numbers of patients to allow a rigorous evaluation of efficiency.
Treatment with bile acid appears to have only a modest effect on EPP‐associated liver disease. Administration of chenodeoxycholic acid resulted in no distinct improvement in ultrastructural assessment of organelle damage in EPP‐associated liver disease in three patients after 1 year of treatment,50 and its therapeutic efficacy in another study was doubtful.52 In spite of these reports, chenodeoxycholic acid continues to be used with other treatments for the treatment of acute liver decompensation before transplant surgery.64 Doss and Frank reported a patient who showed biochemical and clinical improvement from EPP‐induced decompensated liver cirrhosis following treatment with cholic acid.66
The role of iron treatment in EPP is unclear, with reports of significant efficacy53,54 but also reports of increased protoporphyrin levels in some patients.67,68 Furthermore, use of erythropoietin following orthotic liver transplantation in one patient was implicated in causing a great overproduction in protoporphyrin IX, prompting the authors to conclude that treatment with erythropoietin is risky and probably contraindicated in EPP.69 Transfusion therapy is probably the most widely reported and effective treatment for deteriorating liver function in EPP,55,70,71,72,73,74 but in one case it was implicated as the trigger for worsening liver function.75 Various hypertransfusion protocols for decompensating EPP have been used, ranging from 1 unit of blood per month for 5 months to a maximum of 1 unit every 2–7 days repeated 3–10 times.55,70,71,72,73,74 However, care is needed as transfused cells exposed to plasma protoporphyrin are more fragile than endogenous protoporphyrin‐loaded erythrocytes.75,76,77 The risk of haemolysis can be reduced by plasmapheresis conducted before transfusion and, in the context of liver transplantation, immediately before surgery.65 Exchange transfusion is seldom used and is reserved for severe or rapidly deteriorating cases. Intravenous haematin has been shown to reduce protoporphyrin levels56,66,78,79 and, more recently, haem‐albumin has been used successfully in combination with plasmapheresis before liver transplantation.65 Haemodialysis has only been used as a treatment in EPP‐related liver failure and was unsuccessful.59
The efficacy of oral cholestyramine was reported in two well documented cases.80,81 The therapeutic use of this agent in EPP‐related liver disease is seldom reported and, when used, it is usually in combination with other treatments.59,64 Some patients, however, fail to respond to this treatment.66 Cholestyramine was the main treatment used in a 36‐year‐old patient with EPP who developed liver disease but remained in good health until rapid deterioration in liver function 6 years later requiring liver transplantation.82 Activated charcoal is another treatment aimed at preventing reabsorption of protoporphyrin from the gut, and has the merit of being cheap and safe, albeit unpalatable.54,62 Long‐term treatment (27 months) with this agent has been reported to be beneficial in reducing protoporphyrin levels and restoring liver function.62 Finally, intravenous vitamin E was reported to be effective at reversing severe EPP‐related liver disease in a single case report.63
Terminal phase of EPP‐associated liver disease
Deteriorating liver disease in EPP is characterised by chole‐stasis83 followed by jaundice and generalised upper abdominal pain.66 The spleen becomes enlarged and haemolysis may ensue.75 Rapidly worsening photosensitivity due to a further reduction in biliary free protoporphyrin excretion heralds the onset of fulminant disease which is seldom reversible and, in the absence of liver transplantation, usually leads to death. Acute liver failure may rarely be the presenting feature for EPP.85 Additionally, EPP‐related liver failure may sometimes be further complicated by the development of acute pancreatitis.86 In 1986 Bonkovsky and Schned87 summarised 21 fatal cases of EPP‐related liver failure reported in the literature, and Todd identified a further 8 cases in his comprehensive review in 1994.3 The majority of these fatal cases were over the age of 30, but two teenagers and an 11‐year‐old child were also included.3 In the last 10 years liver transplantation has increasingly been available as a treatment option but, despite this, patients have continued to die from EPP‐related liver failure.30,59,88
The cycle of deterioration which characterises fulminant hepatic failure in EPP has been recorded in detail in a number of individual case reports, but the initiating event (or events) remains unclear. What is known is that cholestasis induced by protoporphyrin leads to further accumulation of protoporphyrin,45 initiating a vicious cycle of worsening cholestasis and reduced protoporphyrin excretion.45 Haemolysis leads to increased erythropoiesis, hence increased de novo porphyrin formation by the bone marrow.75 Once this cycle is established, liver decompensation is rapid and liver failure ensues (figs 4 and 5).
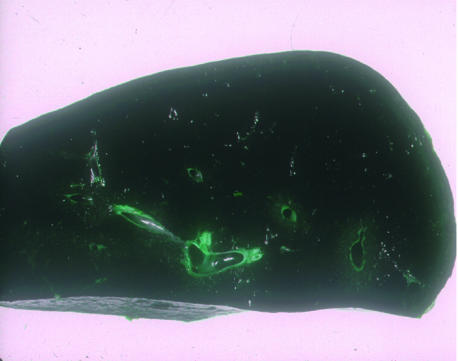
Figure 4 Liver disease in erythropoietic protoporphyria. An explanted liver showing black colour due to diffuse deposition of protoporphyrin pigment.
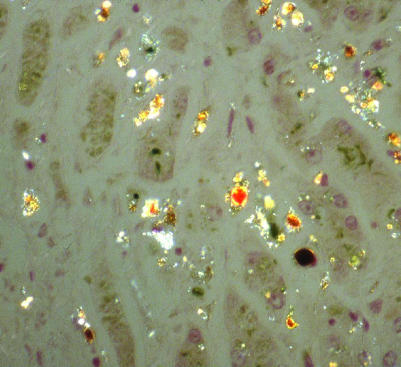
Figure 5 Magnification ×20 of liver histology from fig 4 showing the birefringence of pigment deposits due to the presence of protoporphyrin crystals.
Liver transplantation in EPP
The first liver transplant for EPP‐related liver disease was carried out in 1980.11 Since then, more than 40 further liver transplants have been reported (table 3). Published reports with clinical details of liver transplantation for EPP‐related liver disease include 41 patients (23 male) of age range 13–59 years (mean 39.2 years). The most recent figures from the European Liver Transplant Registry indicate that 19 liver transplants were performed in Europe for EPP between 1985 and 2003, 13 of whom have survived. The reasons for the deaths were gastrointestinal haemorrhage (n = 1), primary graft non‐function (n = 1), infection (n = 2) and unknown causes (n = 2) (V Karam, personal communication, July 2005).
Table 3 Published clinical reports of liver transplants for erythropoietic protoporphyria (EPP)‐related liver failure.
| First author | Year | Sex | Age | Complications | Outcome |
|---|---|---|---|---|---|
| Wells11 | 1980 | M | 19 | Death at 4 weeks after transplantation | Death attributed to disseminated candidiasis |
| Polson91 | 1988 | M | 13 | Initially none | First liver rejected. Death from complications after second liver transplant |
| Samuel9 | 1988 | F | 24 | Transplanted liver functioned poorly initially | Alive at 40 months. Good outcome |
| Bloomer92 | 1989 | F | 38 | None | Patient well 13 months after transplant but with mild hepatic fibrosis in transplant |
| M | 51 | Severe skin necrosis and photo‐injury to intestines | Death secondary to multiple intestinal perforations | ||
| Wagner93 | 1989 | M | 49 | Sepsis | Died 2 months after transplant |
| Herbert82 | 1991 | F | 42 | Skin burns and neuropathy | Slow recovery. Alive at 36 months |
| Shehade95 | 1991 | F | 40 | Skin burns and severe neuropathy | Well 3 years after transplant. Mild neuropathy persisted |
| Steinmüller94 | 1992 | M | 51 | Successful transplant | Well 1 year after transplant |
| Mion64 | 1992 | M | 38 | Postoperative seizures | Medium term survival (at least) |
| Ozawa96 | 1992 | M | 15 | Surgery successful | No follow‐up published |
| Schleiffenbaum97 | 1992 | F | 40 | Surgery successful | Patient well 5 months after transplant |
| Key75 (Bloomer32) | 1992 | M | 51 | Skin burns, small GI bleed, severe haemolytic anaemia | Sepsis and death 73 days after surgery |
| 1992 | M | 13 | Severe motor neuropathy, severe haemolytic anaemia | Good recovery following surgery | |
| Sarkany13 | 1993 | F | 17 | None | Well 14 months after surgery |
| Rank98 | 1993 | F | 36 | Postoperative bleeding | Multiorgan failure; death 5 weeks after transplantation |
| M | 51 | Severe postoperative neuropathy and skin burns | Death within weeks of transplant due to complications including sepsis | ||
| M | 13 | Massive haemolysis during surgery. Severe neuropathy | Patient at home and able to care for himself 1 year after transplant | ||
| M | 27 | Severe postoperative neuropathy | Gradual recovery of muscle strength | ||
| F | 18 | Minor skin burns | Good recovery after transplant | ||
| Meerman58,104 | 1994 | F | 34 | Repeat laparotomy for postoperative bleed | Liver fibrosis present 2 years after surgery; remains well 7 years after transplant |
| F | 39 | None | Portal fibrosis in transplant at 6 and 12 months after transplant. Liver fibrosis progressing at 7 years after transplant | ||
| de Torres89 | 1996 | F | 41 | None | Good outcome initially, but protoporphyrin damage to transplanted liver |
| Lock99 | 1996 | F | 58 | Severe polyneuropathy | Neuropathy persists 1 year after transplant, general condition markedly improved |
| Harper,83 Thunell100 | 1998 | M | 50 | Phototoxic abdominal burns, intestinal bleeding after surgery | Death from multiorgan failure 1 month after transplant |
| Gross41 | 1998 | M | 39 | None | Excellent health 5 years after surgery |
| M | 51 | Intrahepatic cholestasis | |||
| F | 59 | None | Marked improvement 1 year after surgery | ||
| F | 59 | Accumulation of protoporphyrin in transplant | Post‐transplant health good | ||
| F | 17 | None | Good | ||
| F | 58 | None | Good | ||
| M | 38 | None | Good | ||
| Rüfenacht30 | 1998 | F | 49 | Cholestasis corrected, liver function normalised | Follow‐up 4 years after transplant |
| F | 35 | Cholestasis corrected, liver function normalised | Follow‐up 2 years after transplant | ||
| Nguyen101 | 1999 | M | 54 | Polyneuropathy | Most of neuropathy had recovered 12 months after surgery |
| Reicheld,65 Do102 | 1999 2002 | M | 55 | Mild rejection episode at 3 months | Use of plasmapheresis and IV haem‐albumin to protect transplanted liver |
| Jimenez‐Saenz103 | 1999 | M | 59 | None | Good health 30 months after transplant |
| Leone105 | 2000 | M | 35 | None | Excellent health 4 years after transplant |
| 2000 | M | 50 | Poor health after transplant | Died 1 month after transplant | |
| Dellon90 | 2002 | M | 54 | Neuropathy | Transplanted liver affected by protoporphyrin. |
| Schoenfeld34 | 2003 | M | 29 | None | 3 year postoperative follow‐up |
McGuire et al84 have recently reported the outcome of 20 cases of EPP in the USA who underwent liver transplantation. Paediatric and adult survival rates were 100% and 85% respectively at 1 year, 75% and 69% at 5 years and 50% and 47% at 10 years. Recurrent EPP was noted in 11 of the 17 patients (65%) who survived more than 2 months after transplantation. Of the remaining 6 patients without evidence of recurrent EPP, serial monitoring of liver function has shown no evidence of cholestasis. The earliest interval at which recurrent disease was noted on liver biopsy was 8 months. Three patients were re‐transplanted for recurrent EPP‐associated liver disease at 1.8, 12.6 and 14.5 years. Three additional patients in this series died 61–73 months after liver transplantation, documented by extensive protoporphyrin deposits and bridging fibrosis or cirrhosis on liver biopsy. The high rate of recurrent EPP‐associated liver disease prompted the authors to recommend that bone marrow transplantation (soon after successful liver transplantation) should be considered in transplant recipients in order to correct the underlying defect and prevent this.
Liver transplantation restores normal liver function and thus the ability to excrete protoporphyrin via the biliary system. However, it does not correct the FECH enzyme deficiency in the bone marrow, which continues to be the source of significant overproduction of protoporphyrin. Transplanted patients therefore usually continue to have the symptoms of EPP and are at risk of developing EPP‐related liver disease in the transplanted liver;41,89,90,104 some patients have subsequently required a second transplant.84
A number of early patients with EPP who received a liver transplant developed life‐threatening phototoxic abdominal burns and wound dehiscence with severe haemolytic anaemia, since it had not been foreseen that prolonged visceral exposure to operating lamps would result in tissue phototoxicity analogous to that displayed in the skin under normal circumstances.82,106 Additional and unexpected complications included acute neuropathy82,101,106 resulting from high circulating protoporphyrin levels (neuropathy is not generally a feature of EPP as it is of other forms of porphyria), and acute protoporphyrin‐mediated damage to the transplanted liver resulting in delayed return of function as a result of these high circulating levels at the time of grafting.41,89,90 This led to recommendations for optimising the environment for the transplanted liver,58 which included the use of filtered theatre lights and short‐term measures aimed at keeping the level of protoporphyrin as low as possible in the immediate postoperative period.90,102 The introduction of such measures has reduced perioperative complications including haemolysis.76 Furthermore, long‐term use of plasmapheresis and intravenous haem‐albumin has been advocated as a worthwhile therapeutic measure to prolong survival of the transplanted liver in the face of chronically raised protoporphyrin levels.105 There are increasing reports of medium‐term30,103,105 and long‐term104 survival following EPP‐related liver transplantation. However, long‐term follow‐up of two patients with EPP who underwent liver transplantation for acute liver failure revealed protoporphyrin deposits and onset of fibrosis in the transplanted livers 8 months and 6 months after transplantation.104 Despite this, both patients remained in good health 7 years after surgery.104
Animal models for study of liver disease in EPP
Animal models have been used to investigate a number of therapeutic issues relating to EPP‐associated liver disease. A rat liver model showed that administration of bile acids with protoporphyrin increased biliary protoporphyrin excretion, primarily by increasing the biliary protoporphyrin concentration.107 Furthermore, chenodeoxycholic acid was found to be more effective in this regard than ursodeoxycholic acid.108 Subsequent studies with the same model showed that high levels of protoporphyrin led to the formation of biliary thrombi in the presence of bile acids, but without significantly affecting the degree of cholestasis.109
The recent resurgence in animal studies in EPP has been prompted by the availability of new models of the disease and also, perhaps, by the realisation that genetic studies on patients had failed to provide answers to the key prognostic and therapeutic problems of the condition. The first mouse model of EPP was autosomal recessive, with homozygotes displaying haemolytic anaemia, photosensitivity, cholestasis and severe hepatic dysfunction.110 Because of the severe phenotype, this model was recognised to have limited relevance to EPP in humans,110 except in those with severe disease of autosomal recessive origin.14 Pawliuk et al111 demonstrated long‐term cure of photosensitivity in murine EPP by preselective gene therapy. Ex vivo haematopoietic stem cells transduced with retrovirus expressing human FECH resulted in complete and long‐term correction of skin photosensitivity in the transplanted mice. However, the liver damage that is invariably present in this murine model of EPP failed to improve with this treatment.111 The exon 10‐deleted mouse model for EPP demonstrates FECH activity near the threshold for phenotypic expression, which is more relevant to EPP in humans and has the potential to give insight into the contribution of genetic or environmental factors in modulating the EPP phenotype.25
No human gene therapy studies for EPP have been published to date, but increasing success in mouse models makes the realisation of this more likely.112 A recent murine study described mouse recipients of bone marrow from EPP‐affected mice who then developed raised erythrocyte and plasma protoporphyrin levels but with minimal skin photosensitivity and no evidence of liver damage.113 Further murine studies with the BALB/c Fech(m1Pas) mouse model of EPP reproduces the hepatic injury seen sporadically in human EPP. Davies et al114 compared this model with griseofulvin‐induced hepatic protoporphyria, a model of acquired ferrochelatase injury resulting in excess protoporphyrin production. They found that the two models were associated with contrasting liver profiles for genes controlling haem synthesis and catabolism.114 They speculated that these gene expression profiles could be used to provide candidates for human polymorphisms that explain the sporadic expression of hepatic disease in human EPP.114 In a separate study, a mouse model has also been used to show that the genetic background modulates both anaemia and liver injury in EPP.115
Recommendations for management of liver disease in EPP
The evidence upon which recommendations for management of EPP‐related liver disease are based is inconsistent. Of fundamental importance to this debate is the incidence of liver disease in EPP. It is clear that the incidence of serious liver disease in published series of patients shows wide variation, consistent with the fluctuation expected from small sampling of this type (table 2). When the data from these series are combined (with the exclusion of one highly selected series by Chen et al33), about 30 patients out of 847 (∼3.5%) had severe liver disease. This incidence is higher than the frequently quoted figure of ∼1%,46 but a little lower than more contemporary estimates of ∼5%.116
It is perhaps not surprising that, in the face of conflicting evidence, physicians caring for patients with EPP have been unable to agree on the type and level of surveillance needed to identify liver disease. Mathews‐Roth116 recommended liver biopsy in any patient with EPP whose liver function tests were even minimally abnormal, or those in whom erythrocyte and plasma porphyrins become markedly raised (red cell porphyrin >2000 μg/dl; plasma porphyrin >50 μg/ml). Mooyaart et al48 measured liver biochemistry annually up to the age of 20 years and then every 2 years thereafter, and planned to carry out liver biopsies no more frequently than once every 5 years. Sarkany and Norris60 proposed more frequent measurement of liver function tests (every 6 months) on the basis of the speed at which liver involvement can sometimes progress. Bloomer and Bonkovsky118 advised “close follow up” and a liver biopsy for any patient with an unexplained abnormality in liver function and red cell porphyrin >1500 μg/100 ml (∼26.7 μmol/l). Thunell et al100 advised annual routine visits for biochemical assessment of liver function with referral to a hepatologist in the face of abnormal liver function, and more frequent monitoring for patients with increasing erythrocyte protoporphyrin levels above 30 μmol/l. There is currently no way of stating which of these approaches is correct, as the sensitivity of biochemical tests of liver function as a tool for diagnosing the onset of significant liver disease has never been determined. In the absence of this knowledge, histological assessment of the liver remains the gold standard with which other less invasive tests should be compared.
As highlighted earlier in this review, published genotyping data have failed to identify with absolute certainty those patients at risk of developing significant liver disease. This may be reassuring to clinicians operating in healthcare systems such as the USA and much of the developing world where ferrochelatase gene mutational analysis is not widely available. However, there appears to be an increased risk of severe liver disease in patients with null mutations20,30,31,32,33,34 and in those with recessive disease.12,13,14,18,23,117 It is therefore wise to regard any subject with recessive disease or who carries a null mutation as having a higher risk of liver disease. By extension, families in which one or more members have manifested EPP‐related liver disease should be regarded as being potentially at higher risk,30,119 although this higher risk is not always manifest in practice.32,33 At the very least, the presence of EPP should be regarded as a risk factor for liver disease which should be added to other risk factors when deciding if liver biopsy is needed and can be justified.
There should be no argument about the need for liver biopsy in patients with EPP who have abnormal liver function tests or in those with acute liver decompensation. Finally, it is likely that some patients may, after frank discussion of the risk of liver disease and our current inability to predict it with certainty, request liver biopsy in order to attain certainty and perhaps to allay anxiety. This is not unreasonable. Thus, in the absence of a clear consensus on this subject, we propose the following as indications for liver biopsy in patients with EPP:
Patients with null mutations or autosomal recessive disease (for patients in countries where FECH genotyping studies are widely available).
Patients with a family history of EPP‐related liver disease.
The presence of other risk factors for liver disease such as viral hepatitis, haemochromatosis, non‐alcoholic fatty liver disease and alcohol.
Abnormal liver function tests (although sensitivity unknown).
Evidence for liver decompensation: sudden worsening of photosensitivity associated with rising protoporphyrin levels.
Patient anxiety or preference.
A repeat biopsy may be necessary to assess incremental changes in patients shown initially to have early liver disease or following a change in status regarding risk factors.
In patients without an indication for immediate liver biopsy, there is still a need to provide a systematic programme of non‐invasive monitoring of the liver. This might include some or all of the following:
Investigations to exclude other causes of hepatic dysfunction such as hepatitis viral serology and tests for haemochromatosis.
Liver function tests including aminotransferases, alkaline phosphatase and γ‐glutamyl transferase.
Red cell and plasma protoporphyrin levels.
Measurement of hyaluronate, YKL‐40, Fibrotest, Fibroscan and serum aminoterminal propeptide of type III procollagen (PIIINP) as non‐specific serum markers for hepatic fibrosis.
Ultrasound scan for gall stones.
CT and MRI studies of the liver.
However, it should be noted that none of these tests has been adequately assessed prospectively as a tool for identification of liver disease in EPP.
Conclusions
Recent years have seen significant advances in our understanding of EPP, yet fundamental insight into the factors governing clinical expression and progression remains elusive. A review of all reported series suggests that the incidence of significant liver disease in patients with EPP is approximately 3%, although this is not truly a population‐based estimate. Recent work using murine models has provided additional insights which may prove helpful in advancing more effective treatments for EPP. In particular, ex vivo haematopoietic stem cells transduced with retrovirus expressing human FECH resulted in complete and long‐term correction of skin photosensitivity in transplanted mice.111 A bone marrow transplant resulted in cure of symptoms of EPP; interestingly, transplantation was indicated for acute myelogenous leukaemia rather than the coexisting autosomal recessive EPP, which was in fact previously undiagnosed.117 A recent report on 20 patients with EPP who underwent liver transplantation, in most of whom liver disease recurred, led the authors to stress the importance of considering bone marrow transplantation in liver transplant recipients in order to forestall this.84 However, the risks inherent in allogeneic bone marrow transplantation make this an unattractive option for the prevention of EPP‐associated liver disease. It is not unrealistic to anticipate future correction of the protoporphyria phenotype by autologous transplantation of haematopoietic stem cells transfected in vitro with normal FECH DNA.
Until better evidence is available on the optimal method for detecting liver disease in EPP, clinicians caring for patients with EPP should remain vigilant and make an individualised assessment of risk based on the factors listed above, rather than relying arbitrarily on a standard set of tests to determine the need for liver biopsy or for specific therapeutic interventions. The consequences of EPP‐related liver disease are sufficiently serious to justify every effort being made to prevent factors with the potential to exacerbate cholestasis. Thus, alcohol should be avoided altogether, and all patients with EPP should be vaccinated against hepatitis A and B. Where the risk is believed to be high, a liver biopsy is mandatory. Because of the specialised nature of this problem, there are significant advantages to patients with EPP if hepatic monitoring is carried out in collaboration with an experienced hepatologist.
Acknowledgements
The authors thank Dr Mike Badminton, Professor George Elder and Ms Michelle Parker for their helpful comments on the manuscript and acknowledge Mr Stefan Hubscher and Professor Elwyn Elias for the figures of the explanted protoporphyrin‐damaged liver and its histology.
Footnotes
Funding: Dr Alex Anstey was supported by grants from the British Association of Dermatologists and the Welsh Assembly Government.
Competing interests: None.
References
- 1.Elder G H. The cutaneous porphyrias. In: Hawk JLM, ed. Photodermatology. London: Chapman & Hall, 1999;171–97,
- 2.Poh‐Fitzpatrick M B. Porphyrias. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, eds. Cutaneous medicine and surgery: an integrated program in dermatology . Philadelphia: WB Saunders, 19951753–1762.
- 3.Todd D J. Review: erythropoietic protoporphyria. Br J Dermatol 1994131751–766. [DOI] [PubMed] [Google Scholar]
- 4.Anderson K E, Sassa S, Bishop D F.et al X‐linked sideroblastic anaemias and the porphyrias. In: Scriver CR, Beaudet AL, Sly WS, et al eds. The metabolic and molecular basis of inherited disease. Vol II. New York: McGraw‐Hill, 20012991–3062.
- 5.Cox T M. Erythropoietic protoporphyria. J Inherit Metab Dis 199720258–269. [DOI] [PubMed] [Google Scholar]
- 6.Magnus I A, Jarrett A, Prankerd T A J.et al Erythropoietic protoporphyria. A new porphyria syndrome with solar urticaria due to protoporphyrinaemia. Lancet 19612448–451. [DOI] [PubMed] [Google Scholar]
- 7.Bloomer J R, Hill H D, Kools A M.et al Heme synthesis in protoporphyria. Curr Probl Dermatol 199120135–147. [PubMed] [Google Scholar]
- 8.Bonkowsky H L, Bloomer J R, Ebert P S.et al Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest 1975561139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel D, Boboc B, Bernuau J.et al Liver transplantation for protoporphyria. Evidence for the predominant role of the erythropoietic tissue in protoporphyrin overproduction. Gastroenterology 198895816–819. [PubMed] [Google Scholar]
- 10.Ibrahim G W, Watson C J. Enterohepatic circulation and conversion of protoporphyrin to bile pigment in man. Proc Soc Exp Biol Med 1968127890–895. [DOI] [PubMed] [Google Scholar]
- 11.Wells M M, Golitz L E, Bender B J. Erythropoietic protoporphyria with hepatic cirrhosis. Arch Dermatol 1980116429–432. [PubMed] [Google Scholar]
- 12.Lamoril J, Boulechfar S, de Verneuil H.et al Human erythropoietic protoporphyria: two point mutations in the ferrochelatase gene. Biochem Biophys Res Commun 1991181594–599. [DOI] [PubMed] [Google Scholar]
- 13.Sarkany R P E, Alexander G J M A, Cox T M. Recessive inheritance of erythropoietic protoporphyria with liver failure. Lancet 19943431394–1395. [DOI] [PubMed] [Google Scholar]
- 14.Goerz G, Bunselmeyer S, Bolsen K.et al Ferrochelatase activities in patients with erythropoietic protoporphyria and their families. Br J Dermatol 1996134880–885. [PubMed] [Google Scholar]
- 15.Norris P G, Nunn A V, Hawk J L M.et al Genetic heterogeneity in erythropoietic protoporphyria: a study of the enzyme defect in nine affected families. J Invest Dermatol 199195260–263. [DOI] [PubMed] [Google Scholar]
- 16.Went L N, Klasen E C. Genetic aspects of erythropoietic protoporphyria. Ann Hum Genet 198448105–117. [DOI] [PubMed] [Google Scholar]
- 17.Gouya L, Deybach J Ch, Lamoril J.et al Modulation of the phenotype in dominant erythropoietic protoporphyria by a low expression of the normal ferrochelatase allele. Am J Hum Genet 199658292–299. [PMC free article] [PubMed] [Google Scholar]
- 18.Gouya L, Puy H, Lamoril J.et al Inheritance in erythropoietic protoporphyria: a common wild‐type ferrochelatase allelic variant with low expression accounts for clinical manifestation. Blood 1999932105–2110. [PubMed] [Google Scholar]
- 19.Gouya L, Puy H, Robreau A ‐ M.et al The penetrance of dominant erythropoietic protoporphyria is modulated by expression of wildtype FECH. Nat Genet 20023027–28. [DOI] [PubMed] [Google Scholar]
- 20.Wiman Å, Floderus Y, Harper P. Novel mutations and phenotypic effect of the splice site modulator IVs3‐48C in nine Swedish families with erythropoietic protoporphyria. J Hum Genet 20034870–76. [DOI] [PubMed] [Google Scholar]
- 21.Yasui Y, Muranaka S, Tahara T.et al A new ferrochelatase mutation combined with low expression alleles in a Japanese patient with erythropoietic protoporphyria. Clin Sci 2002102501–506. [PubMed] [Google Scholar]
- 22.Schneider‐Yin X, Rufenscht U B, Hergersberg M et a l. Haplotype analysis in determination of the heredity of erythropoietic protoporphyria among Swiss families. J Invest Dermatol 20011171521–1525. [DOI] [PubMed] [Google Scholar]
- 23.Whatley S D, Mason N G, Khan M.et al Autosomal recessive erythropoietic protoporphyria in the United Kingdom: prevalence and relationship to liver disease. J Med Genet 200441105e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aplin C, Whatley S D, Thompson P.et al Late‐onset erythropoietic porphyria caused by a chromosome 18q deletion in erythroid cells. J Invest Dermatol 20011171647–1649. [DOI] [PubMed] [Google Scholar]
- 25.Magness S T, Maeda N, Brenner D A. An exon 10 deletion in the mouse ferrochelatase gene has a dominant‐negative effect and causes mild protoporphyria. Blood 20021001470–1477. [DOI] [PubMed] [Google Scholar]
- 26.Nakahashi Y, Taketani S, Okuda M.et al Molecular cloning and sequence analysis of cDNA encoding human ferrochelatase. Biochem Biophys Res Commun 1990173748–755. [DOI] [PubMed] [Google Scholar]
- 27.Whitcombe D M, Carter N P, Alvertson D G.et al Assignment of the human ferrochelatase gene (FECH) and a locus for protoporphyria to chromosome 18q22. Genomics 1991111152–1154. [DOI] [PubMed] [Google Scholar]
- 28.Taketani S, Inazawa J, Nakahashi Y.et al Structure of the human ferrochelatase gene: exon/intron gene organisation and location of the gene to chromosome 18. Eur J Biochem 1992205217–222. [DOI] [PubMed] [Google Scholar]
- 29.Ohgari Y, Sawamoto M, Yamamoto M.et al Ferrochelatase consisting of wild‐type and mutated subunits from patients with a dominant‐inherited disease, erythropoietic protoporphyria, is an active but unstable dimmer. Hum Mol Genet 200514327–334. [DOI] [PubMed] [Google Scholar]
- 30.Rüfenacht U B, Gouya L, Schneider‐Yin X.et al Systematic analysis of molecular defects in the ferrochelatase gene from patients with erythropoietic protoporphyria. Am J Hum Genet 1998621341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minder E I, Gouya L, Schneider‐Yin X.et al A genotype‐phenotype correlation between null‐allele mutations in the ferrochelatase gene and liver complications in patients with erythropoietic protoporphyria. Cell Mol Biol 20024891–96. [PubMed] [Google Scholar]
- 32.Bloomer J, Bruzzone C, Zhu L.et al Molecular defects in ferrochelatase in patients with protoporphyria requiring liver transplantation. J Clin Invest 1998102107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen F ‐ P, Risheg H, Liu Y.et al Ferrochelatase gene mutations in erythropoietic protoporphyria: focus on liver disease. Cell Mol Biol 20024883–89. [PubMed] [Google Scholar]
- 34.Schoenfeld N, Mamet R, Minder E I.et al A “null allele” mutation is responsible for erythropoietic protoporphyria in an Israeli patient who underwent liver transplantation: relationships among biochemical, clinical, and genetic parameters. Blood Cell Mol Dis 200330298–301. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt H, Snitker G, Thomsen K.et al Erythropoietic protoporphyria. A clinical study based on 29 cases in 14 families. Arch Dermatol 197411058–64. [DOI] [PubMed] [Google Scholar]
- 36.DeLeo V A, Poh‐Fitzpatrick M, Mathews‐Roth M.et al Erythropoietic protoporphyria: 10 years experience. Am J Med 1976608–22. [DOI] [PubMed] [Google Scholar]
- 37.Baart de la Faille H, Bijlmer‐Iest J C, van Hattum J.et al Erythropoietic protoporphyria: clinical aspects with emphasis on the skin. Curr Probl Dermatol 199120123–134. [DOI] [PubMed] [Google Scholar]
- 38.Murphy G M, Hawk J L M, Corbett M F. The UK erythropoietic protoporphyria register: a progress report. Br J Dermatol 1985113(Suppl 29)11 [Google Scholar]
- 39.Frank M, Doss M O. Severe liver disease in prortoporphyria. Curr Probl Dermatol 199120160–167. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann P, Scharffetter K, Kind P.et al Erythropoetische protoporphyrie: synopsis von 20 patienten. Hautarzt 199142570–574. [PubMed] [Google Scholar]
- 41.Gross U, Frank M, Doss M O. Hepatic complications of erythropoietic protoporphyria. Photodermatol Photoimmunol Photomed 19981452–57. [DOI] [PubMed] [Google Scholar]
- 42.Poh‐Fitzpatrick M B. Erythropoietic protoporphyria. Semin Dermatol 1986588–105. [DOI] [PubMed] [Google Scholar]
- 43.Lee R G L, Avner D L, Berenson M M. Structure‐function relationships of protoporphyrin‐induced liver injury. Arch Pathol Lab Med 1984108744–746. [PubMed] [Google Scholar]
- 44.Cox T M, Alexander J M, Sarkany R P E. Protoporphyria. Semin Liver Dis 19981885–93. [DOI] [PubMed] [Google Scholar]
- 45.Bloomer J R. The liver in protoporphyria. Hepatology 19888402–407. [DOI] [PubMed] [Google Scholar]
- 46.Nordmann Y. Erythropoietic protoporphyria and hepatic complications. J Hepatol 1992164–6. [DOI] [PubMed] [Google Scholar]
- 47.Todd D J. Gallstones in children. Am J Dis Child 1991145971–972. [DOI] [PubMed] [Google Scholar]
- 48.Mooyaart B R, de Jong G M T, van der Veen S.et al Hepatic disease in erythropoietic protoporphyria. Dermatologica 1986173120–130. [DOI] [PubMed] [Google Scholar]
- 49.Cripps D J, Scheuer P J. Hepatobilliary changes in erythropoietic protoporphyria. Arch Pathol 196580500–508. [PubMed] [Google Scholar]
- 50.Rademakers L H P M, Cleton M I, Kooijman C.et al Early involvement of hepatic parenchymal cells in erythropoietic protoporphyria? An ultrastructural study of patients with and without overt liver disease and the effect of chenodeoxycholic acid treatment. Hepatology 199011449–457. [DOI] [PubMed] [Google Scholar]
- 51.Macdonald D M, Germain D, Perrot H. The histopathology and ultrastructure of liver disease in erythropoietic protoporphyria. Br J Dermatol 19811047–17. [DOI] [PubMed] [Google Scholar]
- 52.van Hattum J, Baart de la Faille H, Van Den Berg J W O.et al Chenodeoxycholic acid therapy in erythrohepatic protoporphyria. J Hepatol 19863407–412. [DOI] [PubMed] [Google Scholar]
- 53.Gordeuk V R, Brittenham G M, Hawkins C W.et al Iron therapy for hepatic dysfunction in erythropoietic protoporphyria. Ann Intern Med 198610527–31. [DOI] [PubMed] [Google Scholar]
- 54.Mercurio M G, Prince G, Weber F L.et al Terminal hepatic failure in erythropoietic protoporphyria. J Am Acad Dermatol 199329829–833. [DOI] [PubMed] [Google Scholar]
- 55.van Wijk H J, van Hattum J, Baart de la Faille H.et al Blood exchange and transfusion therapy for acute cholestasis in protoporphyria. Dig Dis Sci 1988331621–1625. [DOI] [PubMed] [Google Scholar]
- 56.Bloomer J R, Pierach C A. Effect of hematin administration to patients with protoporphyria and liver disease. Hepatology 19822817–821. [DOI] [PubMed] [Google Scholar]
- 57.Sarkany R P E, Cox T M. Autosomal recessive erythropoietic protoporphyria: a syndrome of photosensitivity and liver failure. Q J Med 199588541–549. [PubMed] [Google Scholar]
- 58.Meerman L, Verwer R, Sloof M J H.et al Perioperative measures during liver transplantation for erythropoietic protoporphyria. Transplantation 199457155–158. [PubMed] [Google Scholar]
- 59.Ishibashi A, Ogata R, Sakisaka S.et al Erythropoietic protoporphyria with fatal liver disease. J Gastroenterol 199934405–409. [DOI] [PubMed] [Google Scholar]
- 60.Sarkany R P E, Norris P G. Hepatic complications of erythropoietic protoporphyria. Br J Dermatol 1994130258–259. [DOI] [PubMed] [Google Scholar]
- 61.Stathers G M. Porphyrin‐binding effect of cholestyramine. Results of in vitro and in vivo studies. Lancet 1966ii780–783. [DOI] [PubMed] [Google Scholar]
- 62.Gorchein A, Foster G R. Liver failure in protoporphyria: long‐term treatment with oral charcoal. Hepatology 199929995–996. [DOI] [PubMed] [Google Scholar]
- 63.Komatsu H, Ishii K, Imamura K.et al A case of erythropoietic protoporphyria with liver cirrhosis suggesting therapeutic value of supplementation with alpha‐tocopherol. Hepatol Res 200018298–309. [DOI] [PubMed] [Google Scholar]
- 64.Mion F B, Faure J ‐ L, Berger F.et al Liver transplantation for erythropoietic protoporphyria. Report of a new case with subsequent medium‐term follow‐up. J Hepatol 199216203–207. [DOI] [PubMed] [Google Scholar]
- 65.Reicheld J H, Katz E, Banner B F.et al The value of intravenous heme‐albumin and plasmapheresis in reducing postoperative complications of orthotic liver transplantation for erythropoietic protoporphyria. Transplantation 199967922–928. [DOI] [PubMed] [Google Scholar]
- 66.Doss M O, Frank M. Hepatobilliary implications and complications in protoporphyria: a 20 year study. Clin Biochem 198922223–229. [DOI] [PubMed] [Google Scholar]
- 67.Milligan A, Graham‐Brown R A C, Sarkanay I.et al Erythropoietic protoporphyria exacerbated by oral iron therapy. Br J Dermatol 198811963–66. [DOI] [PubMed] [Google Scholar]
- 68.McClements B M, Bingham A, Callender M E.et al Erythropoietic protoporphyria and iron therapy. Br J Dermatol 1990122423–424. [DOI] [PubMed] [Google Scholar]
- 69.Anwar N, Mehta S, Bonkovsky H. Erythropoietic protoporphyria in post‐liver transplant setting‐ a case for indefinite hematin infusions. Am J Gastroenterol. 2004; abstract P67.
- 70.Conley C L, Chisholm J J., Jr Recovery from hepatic decompensation in protoporphyria. John Hopkins Med J 1979145237–240. [PubMed] [Google Scholar]
- 71.Bechtel M A, Bertolone S J, Hodge S J. Transfusion therapy in a patient with erythropoietic protoporphyria. Arch Dermatol 198111799–101. [PubMed] [Google Scholar]
- 72.Dobozy A, Csató M, Siklósi C.et al Transfusion therapy for erythropoietic protoporphyria. Br J Dermatol 1983109571–576. [DOI] [PubMed] [Google Scholar]
- 73.Spiva D A, Lewis C E. Erythropoietic protoporphyria. Therapeutic response to combined erythrocyte exchange and plasmapheresis. Photodermatol 19841211–220. [PubMed] [Google Scholar]
- 74.Eichbaum Q G, Dzik W H, Chung R T.et al Red blood cell exchange transfusion in two patients with advanced erythropoietic protoporphyria. Transfusion 200545208–213. [DOI] [PubMed] [Google Scholar]
- 75.Key N S, Rank J M, Freese D.et al Hemolytic anemia in protoporphyria: possible precipitating role of liver failure and photic stress. Am J Hematol 199239202–207. [DOI] [PubMed] [Google Scholar]
- 76.Brun A, Hørding G, Romslo I. Protoporphyrin‐induced photohemolysis: differences related to the subcellular distribution protoporphyrin in erythropoietic protoporphyria and when added to normal cells. Int J Biochem 198113225–228. [DOI] [PubMed] [Google Scholar]
- 77.Todd D J, Callender M E, Mayne E E.et al Erythropoietic protoporphyria, transfusion therapy and liver disease. Br J Dermatol 1992127534–537. [DOI] [PubMed] [Google Scholar]
- 78.Lamon J M, Poh‐Fitzpatrick M B, Lamola A A. Hepatic protoporphyrin production in human protoporphyria: effects of intravenous hematin and analysis of erythrocyte protoporphyrin distribution. Gastroenterology 198079115–125. [PubMed] [Google Scholar]
- 79.Potter C, Tolaymat N, Bobo R.et al Hematin therapy in children with protoporphyric liver disease. J Pediatr Gastroenterol Nutr 199623402–407. [DOI] [PubMed] [Google Scholar]
- 80.Bloomer J R. Pathogenesis and therapy of liver disease in protoporphyria. Yale J Biol Med 19795239–48. [PMC free article] [PubMed] [Google Scholar]
- 81.McCullough A J, Barron D, Mullen K D.et al Fecal protoporphyrin excretion in erythropoietic protoporphyria: effect of cholestyramine and bile acid feeding. Gastroenterology 198894177–181. [DOI] [PubMed] [Google Scholar]
- 82.Herbert A, Corbin D, Williams A.et al Erythropoietic protoporphyria: unusual skin and neurological problems after liver transplantation. Gastroenterology 19911001753–1757. [DOI] [PubMed] [Google Scholar]
- 83.Harper P, Thunell S, Ericzon B G.et al Risk of liver failure in erythropoietic protoporphyria. Be alert for signs of cholestatic development. Larartidningen 1998953051–3056. [PubMed] [Google Scholar]
- 84.McGuire B M, Bonkovsky H L, Carithers R L.et al Liver transplantation for erythropoietic protoporphyria liver disease. Liver Transpl 2005111590–1596. [DOI] [PubMed] [Google Scholar]
- 85.Reisenauer A K, Soon S L, Lee K K.et al Erythropoietic protoporphyria presenting with liver failure in adulthood. Dermatology 20052172–73. [DOI] [PubMed] [Google Scholar]
- 86.Komatsu K, Shimosegawa T, Uchi M.et al Erythropoietic protoporphyria with severe liver dysfunction and acute pancreatitis. J Gastroenterol 200035391–395. [DOI] [PubMed] [Google Scholar]
- 87.Bonkovsky H L, Schned A R. Fatal liver failure in protoporphyria: synergism between ethanol excess and genetic defect. Gastroenterology 198690191–201. [PubMed] [Google Scholar]
- 88.Onaga Y, Ido A, Uto H.et al Hypermethylation of the wild‐type ferrochelatase allele is closely associated with severe liver complication in a family with erythropoietic protoporphyria. Biochem Biophys Res Commun 2004321851–858. [DOI] [PubMed] [Google Scholar]
- 89.de Torres I, Demetris A J, Randhawa P S. Recurrent hepatic allograft injury in erythropoietic protoporphyria. Transplantation 1996611412–1413. [DOI] [PubMed] [Google Scholar]
- 90.Dellon E S, Szczepiorkowski Z M, Dzik W H.et al Treatment of recurrent allograft dysfunction with intravenous hematin after liver transplantation for erythropoietic protoporphyria. Transplantation 200273911–915. [DOI] [PubMed] [Google Scholar]
- 91.Polson R J, Lim C K, Rolles K.et al The effect of liver transplantation in a 13‐year‐old boy with erythropoietic protoporphyria. Transplantation 198846386–389. [DOI] [PubMed] [Google Scholar]
- 92.Bloomer J R, Weimer M K, Bossemaier I C.et al Liver transplantation in a patient with protoporphyria. Gastroenterology 198997188–194. [DOI] [PubMed] [Google Scholar]
- 93.Wagner S, Doss M O, Wittekind C.et al Erythrohepatische protoporphyrie mit rasch progredienter leberzirrhose. Dtsch Med Wschr 19891141837–1841. [DOI] [PubMed] [Google Scholar]
- 94.Shehade S A, Chalmers R G J, Prescott R J. Predictable and unpredictable hazards of erythropoietic protoporphyria. Clin Exp Dermatol 199116185–187. [DOI] [PubMed] [Google Scholar]
- 95.Steinmüller T, Doss M O, Steffen R. Lebertransplantation bei erythrohepatischer protoporphyrie. Dtsch Med Wschr 19921171097–1102. [DOI] [PubMed] [Google Scholar]
- 96.Ozawa K, Uemoto S, Tanaka K.et al An appraisal of pediatric liver transplantation from living relatives. Ann Surg 1992216547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schleiffenbaum B E, Miner E I, Möhr P.et al Cytofluorometry as a diagnosis of protoporphyria. Gastroenterology 19921021044–1048. [DOI] [PubMed] [Google Scholar]
- 98.Rank J M, Carithers R, Bloomer J. Evidence for neurological dysfunction in end‐stage protoporphyric liver disease. Hepatology 1993181404–1409. [PubMed] [Google Scholar]
- 99.Lock G, Holstege A, Mueller A R.et al Liver failure in erythropoietic protoporphyria associated with choledocholithiasis and severe post‐transplantation polyneuropathy. Liver 199616211–217. [DOI] [PubMed] [Google Scholar]
- 100.Thunell S, Harper P, Brun A. Porphyrins, porphyrin metabolism and porphyrias. IV. Pathophysiology of erythropoietic protoporphyria – diagnosis, care and monitoring of the patient. Scand J Clin Lab Invest 200060581–604. [PubMed] [Google Scholar]
- 101.Nguyen L, Blust M, Bailin M.et al Photosensitivity and perioperative polyneuropathy complicating orthotic liver transplantation in a patient with erythropoietic protoporphyria. Anesthesiology 1999911173–1175. [DOI] [PubMed] [Google Scholar]
- 102.Do K D, Banner B F, Katz E.et al Benefits of chronic plasmapheresis and intravenous heme‐albumin in erythropoietic protoporphyria after orthotopic liver transplantation. Transplantation 200273469–472. [DOI] [PubMed] [Google Scholar]
- 103.Jimenez‐Saenz M, Caunedo‐Alvarez A, Rojas M.et al Severe liver failure in erythropoietic protoporphyria. A report of a case treated by liver transplantation. Med Clin (Barc) 1999113176–179. [PubMed] [Google Scholar]
- 104.Meerman L, Haagsma E B, Gouw A S H.et al Long‐term follow‐up after liver transplantation for erythropoietic protoporphyria. Eur J Gastroenterol Hepatol 199911431–438. [DOI] [PubMed] [Google Scholar]
- 105.Leone N, Marzano A, Cerutti E.et al Liver transplantation for erythropoietic protoporphyria: report of a case with medium‐term follow‐up. Dig Liver Dis 200032799–802. [DOI] [PubMed] [Google Scholar]
- 106.Bloomer J R, Rank J M, Payne W D. Follow‐up after liver transplantation for protoporphyric liver disease. Liver Transpl Surg 19962269–275. [DOI] [PubMed] [Google Scholar]
- 107.Avner D L, Berenson M M. Effect of choleretics on canalicular transport of protoporphyrin in the rat liver. Am J Physiol 1982242G347–G353. [DOI] [PubMed] [Google Scholar]
- 108.Berenson M M, Garcia Marin J J, Gunther C. Effect of bile acid hydroxylation on biliary protoporphyrin excretion in rat liver. Am J Physiol 1988255G382–G388. [DOI] [PubMed] [Google Scholar]
- 109.Merenson M M, Gunther C, Samowitz W S.et al Formation of biliary thrombi in protoporphyrin‐induced cholestasis in perfused rat liver. Hepatology 199011757–763. [DOI] [PubMed] [Google Scholar]
- 110.Tutois S, Montagutelli, Da Silva V.et al Erythropoietic protoporphyria in the house mouse. A recessive inherited ferrochelatase deficiency with anaemia, photosensitivity, and liver disease. J Clin Invest 1991881730–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pawliuk R, Bachelot T, Wise R J.et al Long‐term cure of the photosensitivity of murine erythropoietic protoporphyria by selective gene therapy. Nat Med 19995768–773. [DOI] [PubMed] [Google Scholar]
- 112.Richard E, Robert E, Cario‐Andre M.et al Hematopoietic stem cell gene therapy of murine protoporphyria by methylguanine‐DNA‐methyltransferase‐mediated in vivo drug selection. Gene Ther 2004111638–1647. [DOI] [PubMed] [Google Scholar]
- 113.Pawliuk R, Tighe R, Wise R J.et al Prevention of murine erythropoietic protoporphyria‐associated skin photosensitivity and liver disease by dermal and hepatic ferrochelatase. J Invest Dermatol 2005124256–262. [DOI] [PubMed] [Google Scholar]
- 114.Davies R, Schuurman D R, Barker C R.et al Hepatic gene expression in protoporphyric Fech mice is associated with cholestatic injury but not a marked depletion of the heme regulatory pool. Am J Pathol 20051661041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abitbol M, Bernex F, Puy H.et al A mouse model provides evidence that genetic background modulates anaemia and liver injury in erythropoietic protoporphyria. Am J Physiol Gastrointest Liver Physiol 2005288G1208–G1216. [DOI] [PubMed] [Google Scholar]
- 116.Mathews‐Roth M M. The consequences of not diagnosing erythropoietic protoporphyria. Arch Dermatol 1980116407. [DOI] [PubMed] [Google Scholar]
- 117.Poh‐Fitzpatrick M B, Wang X, Andreson K E.et al Erythropoietic protoporphyria: altered phenotype after bone marrow transplantation for myelogenous leukaemia in a patient heteroallelic for ferrochelatase gene mutations. J Am Acad Dermatol 200246861–866. [DOI] [PubMed] [Google Scholar]
- 118.Bloomer J R, Bonkovsky H L. The porphyrias. Dis Mon 1989351–54. [DOI] [PubMed] [Google Scholar]
- 119.Thompson R P H, Molland E A, Nicholson D C.et al ‘Erythropoietic' protoporphyria and cirrhosis in sisters. Gut 197314934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]