Abstract
Background
Protease‐activated receptor‐2 (PAR‐2) is present in the pancreas, where it has been shown to play a protective role during pancreatitis. However, the mechanism by which it protects against pancreatitis still remains to be elucidated. Acute pancreatitis is associated with premature zymogen activation and a blockage in digestive enzyme secretion.
Aim
To examine the effects of PAR‐2 activation on the severity of pancreatitis, and to determine whether its protective effects are mediated by affecting either premature activation or secretory blockage, or both.
Results
The results confirmed that PAR‐2 −/− mice have more severe pancreatitis than wild‐type mice. Interestingly, intrapancreatic trypsin levels in the PAR‐2 knockouts remained high after 6 h of pancreatitis, whereas they reverted to normal in the wild types. During pancreatitis, PAR‐2 mRNA levels were upregulated in wild‐type mice in response to supramaximal caerulein administration. Further, after a single injection of supramaximal caerulein, PAR‐2 mRNA levels were also elevated, reaching a peak at 3 h. Stimulating PAR‐2 with trypsin or the PAR‐2‐activating peptide, serine‐leucine‐isoleucine‐glycine‐arginine‐leucine (SLIGRL), induced significantly more secretion from the acini of these caerulein‐sensitised mice compared with the controls. PAR‐2 activation also reversed the inhibition of secretion observed in both the caerulein and arginine models.
Conclusions
Trypsin released during the early stages of pancreatitis activates PAR‐2 receptors on the acinar cells and stimulates secretion from these cells. Thus, PAR‐2 activation may decrease pancreatic injury and limit the severity of pancreatitis by allowing extracellular trypsin to act as a secretagogue.
Acute pancreatitis varies in severity and may be associated with considerable systemic complications and mortality. Although its pathogenesis is not understood completely, the initiating events are believed to be a blockage in secretion of the digestive enzyme from pancreatic acini and premature intra‐acinar cell zymogen (trypsinogen) activation in both clinical and experimental systems.1,2 Several studies designed to inhibit either of these two early phenomena have been shown to result in amelioration of the disease.3,4,5,6,7 However, during the late phase, a plethora of factors and signalling cascades may extend the disease systemically to multiple organs, thereby making it difficult to design pharmacological approaches to reduce its severity and multiple organ dysfunction.
The critical roles of several factors in the initiation and extension of the disease have been elucidated.8,9,10,11,12,13 One such factor focused on in recent studies14,15 is the protease‐activated receptor 2 (PAR‐2). PAR‐2 is a tethered ligand G‐protein‐coupled receptor with seven transmembrane domains that is activated proteolytically by trypsin and mast cell tryptase, and non‐proteolytically by its peptide ligand serine‐leucine‐isoleucine‐glycine‐arginine‐leucine (SLIGRL). It is widely expressed on many cell types in different organs in both humans and animals, including the acinar16 and ductal17 cells of the exocrine pancreas, where it modulates digestive enzyme secretion and electrolyte (chloride/bicarbonate) transport, respectively.
Although PAR‐2 plays a proinflammatory role in certain disease systems,18,19 it also reduces bronchoconstriction and plays a protective role in the bowel.20,21 Although it is predominantly protective in the pancreas during acute pancreatitis,14,15,22 it exacerbates the associated systemic complications.15 Though a number of mechanisms have been proposed to explain these contrasting effects during acute pancreatitis, several questions still remain unanswered.
The present studies were undertaken to explore the link between PAR‐2‐induced digestive enzyme secretion and the secretagogue (caerulein)‐induced premature intra‐acinar cell activation of trypsinogen. We hypothesised that trypsin released from injured acinar cells during acute pancreatitis activates PAR‐2 in the neighbouring cells. Once activated, PAR‐2 stimulates enzyme secretion out of these cells, including potentially harmful activated enzymes or their precursors, thereby reducing further damage. Our results confirm this hypothesis and provide insights into designing therapeutic approaches for modulating the severity of acute pancreatitis through the activation of PAR‐2.
Materials and methods
Animals
All experiments were carried out according to the protocols approved by the Institutional Animal Care and Use Committees of the University of Massachusetts Medical School, Worcester, Massachusetts, USA, and Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA. Male C57Bl/6 mice (18–20 g) were obtained from Taconic Laboratories (Cambridge, Massachusetts, USA) and PAR‐2 knockouts were the kind gift of Dr Patricia Andrade Gordon (Johnson and Johnson Pharmaceutical Research and Development, Spring House, Pennsylvania, USA). They were housed with a 12 h light/dark cycle, at 23 (2)°C, fed standard laboratory chow, and allowed water ad libitum. Caerulein was from Research Plus (Bayonne, New Jersey, USA) and the trypsin substrate Boc‐Gln‐Ala‐Arg‐MCA from Peptides International (Louisville, Kentucky, USA). SLIGRL and leucine‐arginine‐glycine‐isoleucine‐leucine‐serine (LRGILS) were synthesised at the peptide synthesis facility, University of Massachusetts Medical School. Collagenase (type IV) was from Worthington Biochemicals (Freehold, New Jersey, USA), while RNA Later, Totally RNA kit and Universal 18S Internal Standards (yielding a 315 bp band) were obtained from Ambion (Austin, Texas, USA). Powerscript for reverse transcription was from Clontech (Mountain View, California, USA). Platinum PCR Supermix was purchased from Life Technologies (Gaithersberg, Maryland, USA), while Cyber Gold was obtained from Bio Whittaker (Walkersville, Maryland, USA). Other chemicals and reagents, which were of the highest purity commercially available, were purchased from Sigma‐Aldrich (St Louis, Missouri, USA).
In vivo models of pancreatitis
A total of 20 wild types and 20 knockouts were divided into groups of 4 controls and 8 animals each, for 6 h and 12 h pancreatitis, respectively. Secretagogue‐induced pancreatitis was elicited by hourly (×6 or ×12) intraperitoneal injections of caerulein (50 μg/kg) to mice, whereas control animals received an equal amount of saline. Unless stated otherwise, animals were killed by CO2 asphyxiation 1 h after the final caerulein injection and tissue samples were appropriately collected for study. For arginine‐induced pancreatitis, mice were administered or 8 g/kg arginine dissolved in saline intraperitoneally and killed 24 h later.
Evaluation of trypsin activity
Pancreas specimens were homogenised in 3‐(N‐morpholino)‐propane sulphonic acid buffer (4°C), pH 7.0 (250 mM sucrose, 5 mM MOPS, 1 mM MgSO4) using a motorised glass‐Teflon homogeniser and centrifuged (50 g, 5 min). Trypsin activity in the supernatant was measured fluorometrically using Boc‐Glu‐Ala‐Arg‐MCA as described by Kawabata et al23 and expressed as activity per μg DNA.6
Evaluation of the severity of pancreatitis
Neutrophil sequestration within the pancreas was evaluated by quantitating tissue myeloperoxidase activity using a modification of the bromide‐dependent chemiluminescence technique described by Haqqani et al.24 Pancreatic oedema was quantitated by measuring tissue water content and expressing it as a percentage of tissue wet weight. Serum amylase activity was quantitated as described previously.6 The extent of pancreatic acinar cell necrosis was quantitated morphometrically by an observer who was not aware of the sample identity. For these studies, 5 μm paraffin‐wax‐embedded samples were sectioned and stained with H&E. A total of 10 randomly chosen microscopic fields (×125) were examined for each tissue sample and the extent of acinar cell injury/necrosis was expressed as a percentage of total acinar tissue.
Semiquantitative RT‐PCR for PAR‐2
RNA was extracted using the Totally RNA kit as per the manufacturer's protocol. In all, 5 μg of non‐degraded RNA was used for reverse transcription using random primers. For PCR, gene‐specific intron‐spanning primers were added to the samples, initially denatured at 94°C for 4 min, followed by cyclic denaturation at 94°C for 45 s, annealing at 60°C for 45 s and extension at 72°C for 60 s (28 cycles), and a final extension at 72°C for 10 min, giving a single band of 471 bp. The sequences of the PAR‐2 primers were as follows: sense—5′‐CAT GTT CAA TTA CTT CCT CTC ACT, antisense—5′‐GGT TTT AAC ACT GGT TGA GCT TGA. As per the manufacturer's instructions, the 18S primer/competimers ratio was kept at 1:7, and this was added to each PCR tube along with 1 μl of cDNA from the reverse transcription reaction. The ratio of the PAR‐2:18S band was measured in each case and was plotted as fold increase of basal.
Evaluation of nuclear factor kappa B, AP‐1 activation by electrophoretic mobility‐shift assay
Nuclear and cytoplasmic protein extracts were prepared as described by Dyer and Herzog.25 Protein concentrations were determined by the method of Bradford.26 Electrophoretic mobility‐shift assay (EMSA): 7.5–10.0 μg of nuclear protein in 5 mM Tris, 100 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, 4% (v/v) glycerol and 0.08 mg/ml salmon sperm DNA were incubated with the nuclear factor kappa B (NFκB) oligonucleotide probe (5′‐AGT TGA GGG GAC TTT CCC AGG C‐3′), or the activator protein‐1 (AP‐1) oligonucleotide probe (5′‐CGC TTG ATG AGT CAG CCG GAA‐3′; Promega, Madison, Wiscosin, USA) previously end‐labelled with [γ‐32P]ATP using T4 polynucleotide kinase and purified over two successive 1 ml G‐50 columns (Amersham Pharmacia Biotech) for 20 min at room temperature. DNA–protein complexes were resolved in 6% non‐denaturing polyacrylamide gels in Tris‐borate buffer (TBE) at 140 V for 2–3 h. Gels were dried and exposed to Kodak Bio Max MR films at –70°C.
Ex‐vivo preparation
Pancreatic acini were prepared from C57Bl/6 mice (18–20 g) by collagenase digestion as described previously.6 These were taken from mice that were either normal untreated controls or those that had received a single sensitising injection of 50 μg/kg of caerulein 6 h earlier. Freshly prepared acini were suspended in oxygen‐saturated HEPES (4‐(2‐hydroxyethyl)‐1‐piperazine ethane sulphonic acid)‐Ringer buffer (pH 7.4). The viability of acini, evaluated by trypan blue exclusion, was >95% at the start of each experiment. Acini were stimulated with the indicated concentrations of caerulein, trypsin, SLIGRL or LRGILS. Amylase secretion was measured at 37°C as indicated in the legends. For culture studies, acini harvested under sterile conditions were suspended in RPMI 1640 supplemented with HEPES, 10% fetal calf serum and 100 IU/ml penicillin–streptomycin mix. Acini were cultured overnight and viability was checked the next morning by trypan blue exclusion (normally >98%) before stimulation. Acini were stimulated with trypsin (3 μM) for 4 h, washed and used immediately or stored in RNA later for semiquantitative RT‐PCR analysis of PAR‐2 RNA as described above. The concentration of trypsin chosen was based on the net amount of trypsin activity measured in the medium at the end of 4 h. This was in the range normally observed in pancreatic homogenates during pancreatitis.
Analysis of data
The results reported in this communication represent means (SE) of the values obtained from three or more separate experiments. In all figures, vertical bars denote SE values. Statistical evaluation of data was accomplished by analysis of variance, and p values <0.05 were considered significant.
Results
Effect of genetic deletion of PAR‐2 on pancreatic injury during caerulein‐induced pancreatitis
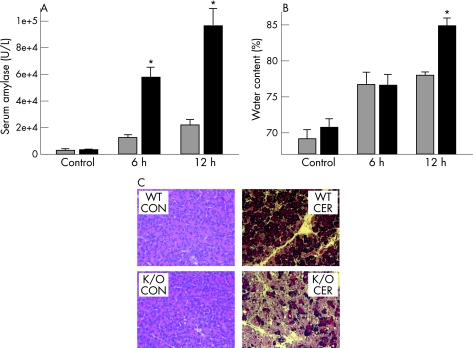
Secretagogue‐induced pancreatitis in mice is associated with a rise in serum amylase levels, pancreatic oedema, acinar cell necrosis and inflammatory cell infiltration. As shown in fig 1, the extent of pancreatic injury in PAR‐2 knockout mice was more severe than that in the wild‐type controls. Serum amylase levels were similar in both PAR‐2 −/− and PAR‐2 +/+ control mice (fig 1A). However, serum amylase levels at both 6 and 12 h of pancreatitis were significantly higher in the PAR‐2 knockout mice (12 590 (1177) U/L and 21 470 (2267) U/L in wild types vs 58 777 (7318) U/L and 98 135 (9573) U/L in knockouts at 6 and 12 h, respectively). Pancreatic oedema increased after 6 h of pancreatitis, but the increase was similar in both PAR‐2 wild‐type (76.7 (1.8)%) and knockout (76.7 (1.6)%) mice. However, pancreatic water content was significantly higher in the knockouts at 12 h (78.2 (0.3)% vs 84.9 (1.2)%, p<0.01). Similarly, pancreatic necrosis was significantly higher in the PAR‐2 knockouts at 12 h (29.7 (2.8)% in knockouts vs 18.3 (1.9)% in wild types, p<0.05) and, as seen in the representative photomicrographs in fig 1C, was associated with increased acinar cell vacuolisation and oedema.
Figure 1 Protease‐activated receptor‐2 knockouts have more severe pancreatic injury: wild types (WT; grey bars) or PAR‐2 knockouts (black bars) were given 6‐ or 12‐ hourly intraperitoneal injections of 50 μg/kg caerulein (CER) or saline (CON) and killed 1 h after the last injection. Serum amylase (A), or pancreatic water content (B) were measured as described in the text. Values are means (SE). *p value <0.05 for PAR‐2 knockouts (K/O) given caerulein versus PAR‐2 wild types given caerulein. (C) Representative H&E‐stained pancreas sections from control mice receiving saline (CON) or those that had received 12‐hourly injections of 50 μg/kg caerulein (CER).
Effect of PAR‐2 activation on intrapancreatic trypsin levels after 30 min and 6 h of caerulein‐induced pancreatitis
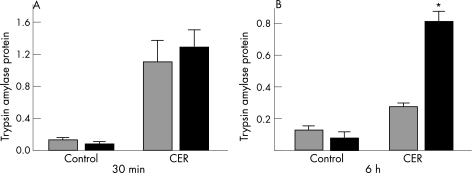
Intrapancreatic trypsinogen activation is an early event during secretagogue‐induced pancreatitis. We were interested in determining whether it was affected by the genetic deletion of PAR‐2. Figure 2A shows that intrapancreatic trypsin levels were increased to a similar extent in both wild‐type and PAR‐2 knockout mice after 30 min of supramaximal caerulein administration. Interestingly, trypsin activity was significantly higher in PAR‐2 knockouts compared with wild type counterparts at 6 h (0.81 (0.06) vs 0.28 (0.02), p<0.05; fig 2B).
Figure 2 Trypsin activity in protease‐activated receptor‐2 wild types and knockout mice after 30 min (A) and 6 h (B) of saline (CON) or supramaximal caerulein (CER) administration. Trypsin activity in the pancreatic homogenates was determined spectrofluorometrically as described in the Materials and methods section, and expressed as trypsin activity/μg DNA. Although the extent of trypsinogen activation was similar in both PAR‐2 wild‐type (grey bars) and knockout (black bars) mice after 30 min of supramaximal caerulein administration, PAR‐2 knockout mice had significantly more trypsin activity after 6‐hourly injections of supramaximal caerulein. *p value <0.05 for PAR‐2 knockouts given caerulein versus PAR‐2 wild types given caerulein.
Effect of PAR‐2 activation on transcription factor activation during caerulein‐induced pancreatitis
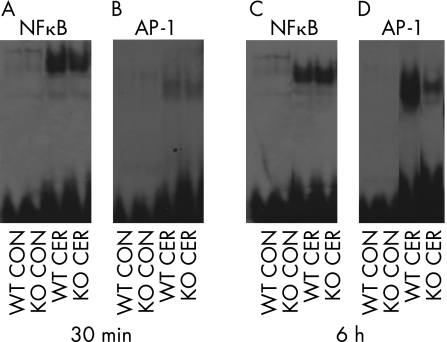
During secretagogue‐induced pancreatitis, several transcription factors including NFκB and AP‐1 are activated in the pancreas within 30 min of supramaximal caerulein administration. We monitored the extent of NFκB and AP‐1 activation and nuclear translocation by EMSA at 30 min (fig 3A,B) and 6 h (fig 3C,D). As shown in fig 3A,B, both transcription factors were activated in the pancreas to a similar extent at 30 min in both PAR‐2 wild‐type and knockout mice. At 6 h, whereas the extent of NFκB activation and translocation in the pancreas was similar in both the wild types and PAR‐2 knockouts (fig 3C), AP‐1 activation was reduced in PAR‐2 knockouts compared with the wild types (fig 3D).
Figure 3 Transcription factor activation in protease‐activated receptor‐2 wild types (WT) and knockouts (KO) after 30 min (A,B) and 6 h (C,D) of saline (CON) or supramaximal caerulein (CER) administration. Nuclear translocation of nuclear factor kappa B (NFκB; A,C) and activator‐protein 1 (AP‐1; B,D) was measured in the nuclear extracts by EMSA as described in the text. Note that while the extent of NFκB activation was similar in PAR‐2 knockouts and wild types at both the time points, AP‐1 translocation was reduced in the PAR‐2 knockouts at 6 h.
Effect of supramaximal caerulein administration on pancreatic PAR‐2 mRNA expression
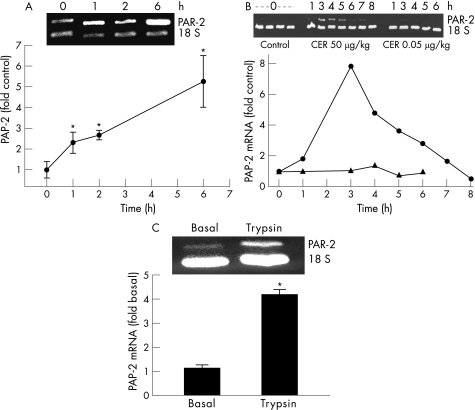
PAR‐2 mRNA levels in the pancreas were measured by semiquantitative PCR, and the results are expressed as fold increase in PAR‐2 mRNA over controls. As shown in fig 4A, PAR‐2 mRNA levels increased in the pancreas during the course of pancreatitis induced by hourly injections of caerulein. To determine whether the increase in PAR‐2 mRNA was due to its upregulation in pancreatic cells or was caused by the infiltration of inflammatory cells that also express PAR‐2, we measured the levels of PAR‐2 mRNA at varying times after a single injection of caerulein (50 μg/kg). As shown in fig 4B, following a single injection of caerulein, PAR‐2 mRNA gradually increased, peaked at 3 h and then decreased. In contrast, a physiological dose of caerulein (0.05 μg/kg) did not cause any increase in the levels of PAR‐2 mRNA. It has previously been reported that a single intraperitoneal injection of supramaximal caerulein in mice neither causes pancreatitis nor is associated with the infiltration of inflammatory cells into the pancreas.27 Thus, the observed increase in PAR‐2 mRNA seen in fig 4B is probably due to its upregulation in pancreatic acinar cells. To determine whether the acinar cells were the source of this observed increase, we cultured acini, stimulated them with trypsin and measured PAR‐2 mRNA as described in the Materials and methods section. Figure 4C shows that incubation of acini with trypsin causes an increase in the levels of PAR‐2 mRNA.
Figure 4 Protease‐activated receptor‐2 (PAR‐2) mRNA expression levels in the pancreas in vivo and in vitro in response to caerulein and trypsin. (A). Pancreatic PAR‐2 mRNA in wild‐type mice given 1‐, 2‐, or 6‐hourly intraperitoneal injections of 50 μg/kg of caerulein. The graph shows the fold increase over control at each time point. A representative gel with PAR‐2 and 18S bands is also shown. (B). PAR‐2 mRNA in the pancreas of wild‐type mice killed at different times after administration of a single injection of 50 μg/kg caerulein (circles) or 0.05 μg/kg caerulein (triangles). The levels are plotted as fold control, and the graph and gel image from a representative experiment are shown. (C). PAR‐2 mRNA in cultured pancreatic acini after 4 h of incubation with trypsin (3 μM). *p value <0.05 versus control.
Effect of PAR‐2 activation on amylase secretion from caerulein‐sensitised acinar cells
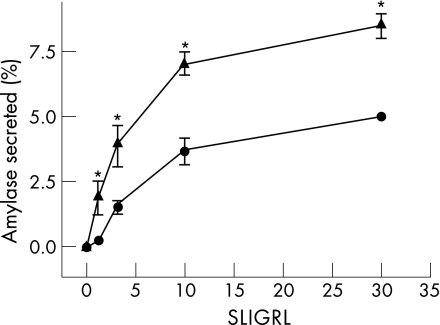
To determine whether the decrease in intrapancreatic trypsin along with the protection noted in caerulein‐induced pancreatitis in wild‐type mice could be mediated by an increase in secretion from acinar cells in response to PAR‐2 stimulation, we prepared pancreatic acini from untreated wild‐type controls and wild‐type mice that had received a single dose of caerulein (50 μg/kg) 6 h earlier (caerulein‐sensitised mice), and measured amylase secretion from these in response to different concentrations of the PAR‐2 ligand SLIGRL. Figure 5 shows a representative experiment. As shown in fig 5, acini prepared from caerulein‐sensitised mice secrete significantly more amylase compared with unsensitised acini. The response was specific, as incubation of acini with the inactive peptide LRGILS did not result in enhanced secretion (data not shown). Notably, SLIGRL induced higher secretion from the caerulein‐sensitised mouse acini at all tested concentrations (p<0.02).
Figure 5 Pancreatic acini prepared from caerulein‐sensitised mice secrete more amylase in response to Ser‐Leu‐Ile‐Gly‐Arg‐Leu‐amide. Acini from control mice (circles) or sensitised mice—that is, those that had received a single intraperitoneal injection of caerulein (50 μg/kg) 6 h earlier (triangles)—were prepared and stimulated with varying concentrations of SLIGRL over 60 min, and the percentage of total amylase released into the medium was determined as described in the text. *p value <0.05 versus control.
Effect of PAR‐2 activation on supramaximal caerulein‐mediated inhibition of secretion in sensitised acini
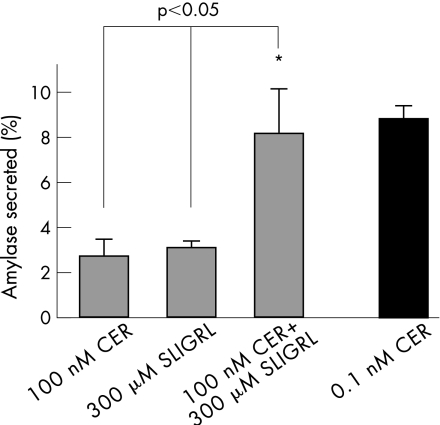
Since PAR‐2 knockouts had higher levels of intrapancreatic trypsin at 6 h and acini from sensitised wild‐type mice (which had upregulated PAR‐2 mRNA) secreted more amylase after PAR‐2 stimulation compared with unsensitised mice (fig 5), we tested whether the inhibition of secretion caused by supramaximal caerulein could be reversed by stimulation of PAR‐2 by its ligand SLIGRL. For this, acini from sensitised mice were prepared as described above and were incubated with supramaximal (100 nM) caerulein, SLIGRL (300 μM) or a combination of the two, and the secretion of amylase into the medium over 30 min was compared with that observed with maximal caerulein (0.1 nM; 8.8 (0.6)%). As shown in fig 6, acini incubated with the combination secreted significantly more amylase (8.2 (2.0)%) than the supramaximal (100 nM) caerulein (2.7 (0.7)%, p<0.05), which inhibits secretion, and the effect of the combination was at least additive of the two agents alone. SLIGRL alone caused 3.1 (0.3)% secretion.
Figure 6 Effect of serine‐leucine‐isoleucine‐glycine‐arginine‐leucine (SLIGRL) on supramaximal caerulein (CER)‐mediated inhibition of secretion. Acini prepared from sensitised wild‐type mice that had received a single dose (50 μg/kg) of caerulein intraperitoneally 6 h earlier were incubated with SLIGRL (300 μM), caerulein (100 nM), a combination of the two (all grey bars) or maximal caerulein (0.1 nM; black bar) for 30 min, and the amount of amylase released into the medium was measured. Net secretion over unstimulated acini was measured and expressed as a percentage of total amylase content. *p value <0.05 versus secretion from acini stimulated with 100 nM caerulein or SLIGRL.
Effect of trypsin on amylase secretion from acini prepared from control and caerulein‐sensitised animals
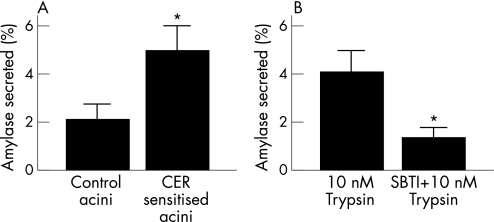
To confirm that the increase in amylase secretion noted in response to PAR‐2 stimulation by SLIGRL would translate to increased secretion in response to trypsin during pancreatitis, we prepared acini from control and caerulein‐sensitised mice and stimulated them with 10 nM trypsin over 30 min as described previously.28 As shown in fig 7A, trypsin caused 2.1 (0.6)% secretion from acini prepared from unsensitised wild‐type mice (control acini). This was significantly increased (p<0.05) to 5.0 (1.0)% in acini from caerulein‐sensitised mice (CER‐sensitised mice). The secretion in response to trypsin was significantly reduced (p<0.05) with soybean trypsin inhibitor (SBTI) in the incubation medium (4.1 (0.9)% vs 1.2 (0.4)%), as shown in fig 7B. The data shown in fig 7B are pooled from six independent experiments performed with acini from both control and caerulein‐sensitised wild‐type mice.
Figure 7 Protease‐activated receptor‐2‐mediated secretion by trypsin is enhanced in acini from caerulein‐sensitised mice. (A) Acini from control wild‐type mice (control acini) or acini prepared from wild‐type mice which had received a single dose (50 μg/kg) of caerulein intraperitoneally 6 h earlier (CER‐sensitised acini) were incubated with 10 nM trypsin for 30 min and secretion into the medium was measured and expressed as a percentage of total amylase content. (B) Amylase secretion in response to 10 nM trypsin in medium with or without soybean trypsin inhibitor (SBTI) (0.1 mg/ml). *p value <0.05 between the two groups.
Effect of PAR‐2 stimulation on secretion during arginine pancreatitis
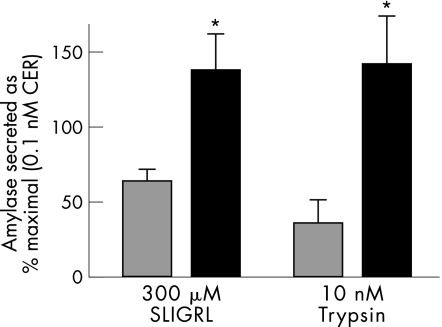
To confirm whether the increased secretion in response to PAR‐2 stimulation during caerulein pancreatitis is true in other models too, we prepared acini from mice administered 8 g/kg of l‐arginine 1 day before, and compared the secretion from these acini with that from control acini. Although the control acini showed robust amylase secretion in response to maximal (0.1 nM) caerulein (14.2 (2.0)%), this was significantly reduced in acini prepared from mice with arginine pancreatitis (4.5 (0.1)%). However, as seen in fig 8, amylase secretion in response to PAR‐2 stimulation by both 300 μM SLIGRL and 10 nM trypsin was increased significantly in acini prepared from mice with arginine pancreatitis (black bars) compared with control acini (grey bars).
Figure 8 Enhanced secretion in response to protease‐activated receptor‐2 stimulation during arginine pancreatitis. Acini from control wild‐type mice (grey bars) or acini prepared from wild‐type mice which had received a single dose (8 g/kg) of arginine intraperitoneally 24 h earlier (black bars) were incubated with 300 μM serine‐leucine‐isoleucine‐glycine‐arginine‐leucine (SLIGRL) (left) or 10 nM trypsin (right) for 30 min, and secretion into the medium was measured and expressed as a percentage of the response to maximal caerulein (0.1 nM). *p value <0.05 between the two groups of each set.
Discussion
PAR‐2 has been shown to occur on pancreatic acinar and ductal cells, and plays an important role in regulating digestive enzyme secretion and ion channel function. Recently, using both genetic and pharmacological approaches,14,15,22 it has been shown to protect against acute pancreatitis. In this study, we have extended those observations and explored the mechanism(s) by which PAR‐2 exerts a protective effect and limits the severity of secretagogue‐induced pancreatitis in mice. We first employed the secretagogue‐induced model of pancreatitis in PAR‐2 wild‐type and knockout mice and monitored various early and late parameters of pancreatitis. Our observations confirmed the findings reported by Sharma et al14 that genetic deletion of PAR‐2 worsens the severity of caerulein‐induced pancreatitis (fig 1). In addition, we observed that intrapancreatic trypsin activity at 6 h was higher in PAR‐2 knockouts compared with wild types (fig 2B). This was an intriguing finding, as we have previously shown that intrapancreatic trypsin activity peaks at 30 min after the induction of caerulein pancreatitis in wild‐type mice and is followed by a rapid decline to near basal values by 6 h.29
We hypothesised that the ability of PAR‐2 to stimulate digestive enzyme secretion from acinar cells could be linked to its ability to reduce accumulation of trypsin activity, thereby limiting pancreatic injury. Our hypothesis is substantiated by earlier studies from Gorelick's group,30 who showed that both premature activation and retention of activated enzymes within the acinar cell are a prerequisite for pancreatic injury and ensuing pancreatitis. Those authors showed that, although acinar cell stimulation with either supramaximal caerulein or bombesin causes premature zymogen activation, stimulation with the latter did not result in pancreatic injury and pancreatitis because the active enzymes were secreted out of and not retained within the acinar cell.
Furthermore, although pancreatitis is thought to be associated with a decrease in secretion from the pancreas (in volume, protein or enzyme content),27,31 we believe that in the PAR‐2‐deficient mice, this secretion is probably further reduced, resulting in the accumulation of intracellular trypsin, worse injury and more severe pancreatitis. These observations are also supported by the fact that there is increased secretion in response to PAR‐2 stimulation by trypsin or SLIGRL from acini prepared from either caerulein‐ or arginine‐sensitised animals. Complementarily, the duodenal output of trypsin from the pancreas has been shown to be increased in patients with acute pancreatitis,32,33,34 and attempts to decrease this with glucagon were associated with a complication rate higher than that in controls .34 Conversely, patients with severe pancreatitis have reduced duodenal output of trypsin compared with patients with mild/moderate pancreatitis.35 Similar causes may explain the ineffectiveness of somatostatin treatment for pancreatitis.
PAR‐2 stimulation with SLIGRL has been shown to cause secretion of pancreatic enzymes into the duodenum16 in control mice. Here, we show that in wild‐type mice PAR‐2‐mediated secretion is not blocked by supramaximal caerulein (fig 6). In fact, PAR‐2 stimulation may reverse the inhibition of secretion caused by supramaximal concentrations of caerulein. This is consistent with our finding that intrapancreatic trypsin levels were high at 6 h in the PAR‐2 knockouts, which cannot secrete zymogens (or activated trypsin) in response to PAR‐2 stimulation, and have more severe pancreatic injury after supramaximal caerulein administration (fig 1). The fact that there is increased secretion in response to PAR‐2 activation in arginine pancreatitis also points to the generality of the phenomenon. Human pancreatitis has been shown to be associated with secretion of trypsin into the duodenum;32,33,34 the secretion is reduced in severe pancreatitis,35 and interference with this secretion,34 possibly causing activated zymogen retention within the pancreas, worsens its severity.
Because PAR‐2 is activated by trypsin and has been shown to be expressed on both pancreatic acinar and ductal cells,15,16,17 we further designed experiments to find out whether PAR‐2 expression was altered during caerulein‐induced pancreatitis and whether it could result in altered digestive enzyme secretion. We observed that PAR‐2 mRNA was upregulated in the pancreas in response to hourly injections of supramaximal caerulein (fig 4A). As multiple cells (immune cells, acinar, ductal and islet cells) may be responsible for PAR‐2 mRNA increase in pancreatitis, it was important to measure PAR‐2 levels after a single injection of supramaximal caerulein (which does not cause inflammatory cell infiltration)27,36 to exclude the effect of inflammatory cells. Indeed, PAR‐2 mRNA increased within 1 h of a single injection of 50 μg/kg caerulein and peaked at 3 h (fig 4B). This enhanced expression in turn caused increased secretion from the “caerulein‐sensitised” acini in response to SLIGRL 6–8 h after the single injection (fig 5).
The increase in PAR‐2 mRNA was also observed in cultured acini in response to trypsin (fig 4C). These findings clearly indicate that (a) PAR‐2 mRNA expression is increased on acinar cells under both in vivo and in vitro conditions and that (b) trypsin acts as a secretagogue and also upregulates the level of its own receptor. The mechanism by which trypsin causes this increase in PAR‐2 mRNA remains to be elucidated.
Prevention or inhibition of trypsinogen activation has been associated with a reduction in the severity of pancreatitis.3,5,6,7,37 The site of trypsin‐induced damage is controversial, and different authors have proposed that it is intracellular37,38 or, opposingly, extracellular.39 We believe that both the sites of trypsin generation6,7 and trypsin‐mediated damage37 are intracellular. We hypothesise that injured or dying cells release trypsin, which activates PAR‐2 on the neighbouring cells and stimulates secretion that empties them of intracellular, activated and potentially deleterious trypsin, thus protecting them against damage. In the absence of PAR‐2, this does not occur, resulting in accumulation of intracellular trypsin and worse injury such as that seen in PAR‐2 knockouts. This also explains why attempts at reducing the severity of pancreatitis with serine protease inhibitors such as gabexate mesilate that would inhibit extracellular trypsin (and in turn inhibit the protective effect of the PAR‐2) before gaining intracellular access (thereby inhibiting intracellular trypsin) have had limited success.40,41 This hypothesis is further supported by the findings that administration of PAR‐2 activation peptide SLIGRL protects against experimental pancreatitis.14 Our hypothesis is in accord with that of Namkung et al,15 who suggest that activation of PAR‐2 in the pancreas serves as an acute local defence mechanism until the cells get rid of the imposing stress in the form of active trypsin, and that once the PAR‐2 system is overwhelmed pancreatitis ensues.
Our present studies allude to one potential pathway by which PAR‐2 exerts its protective effect during pancreatitis. As postulated by Namkung et al15 and Sharma et al,14 PAR‐2 could be protective in pancreatitis through other mechanisms. These include, but are not limited to, the activation of signalling pathways that may be short‐lived (such as Ca2+ signalling) or long‐lasting (extracellular signal‐related kinase) that protects against both caerulein‐induced and bile salt‐induced cell damage. Regardless of the postulated mechanisms, the net effect is pancreatic protection in dissimilar models of pancreatitis.
In conclusion, we have shown that PAR‐2, whose expression increases during secretagogue‐induced pancreatitis and in response to trypsin in vitro, enhances secretion from sensitised acinar cells, and this may represent a mechanism by which the pancreas rids itself of potentially damaging, prematurely activated zymogens such as trypsin. Thus, in this scenario, trypsin plays contrasting roles by initiating pancreatitis intracellularly and by limiting its severity through the extracellular activation of PAR‐2.
Acknowledgements
We thank Dr Michael Steer for his helpful suggestions during the initial stages of the study which were carried out at Beth Israel Deaconess Medical Center, Boston, Massachusetts, USA. This study is supported in part by NIH grant # DK58649.
Abbreviations
AP‐1 - activator‐protein 1
CER - caerulein
EMSA - electrophoretic mobility‐shift assay
LRGILS - leucine‐arginine‐glycine‐isoleucine‐leucine‐serine
MOPS - 3‐(N‐morpholino)‐propane sulphonic acid
NFκB - nuclear factor kappa B
PAR‐2 - protease‐activated receptor 2
SLIGRL - serine‐leucine‐isoleucine‐glycine‐arginine‐leucine
TBE - tris‐borate buffer
Footnotes
Competing interests: None declared.
References
- 1.Renner I G, Rinderknecht H, Douglas A P. Profiles of pure pancreatic secretions in patients with acute pancreatitis: the possible role of proteolytic enzymes in pathogenesis. Gastroenterology 1978751090–1098. [PubMed] [Google Scholar]
- 2.Steer M L, Saluja A K. Experimental acute pancreatitis: studies of the early events that lead to cell injury. Pancreas Biol, Pathobiol Disease 1993489–500.
- 3.Bhagat L, Singh V P, Song A M.et al Thermal stress‐induced hsp70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology 2002122156–165. [DOI] [PubMed] [Google Scholar]
- 4.Halangk W, Lerch M M, Brandt‐Nedelev B.et al Role of cathepsin b in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest 2000106773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H S, Bhagat L, Frossard J L.et al Water immersion stress induces heat shock protein 60 expression and protects against pancreatitis in rats. Gastroenterology 2000119220–229. [DOI] [PubMed] [Google Scholar]
- 6.Singh V P, Saluja A K, Bhagat L.et al Phosphatidylinositol 3‐kinase‐dependent activation of trypsinogen modulates the severity of acute pancreatitis. J Clin Invest 20011081387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Acker G J, Saluja A K, Bhagat L.et al Cathepsin b inhibition prevents trypsinogen activation and reduces pancreatitis severity. Am J Physiol Gastrointest Liver Physiol 2002283G794–G800. [DOI] [PubMed] [Google Scholar]
- 8.Grady T, Liang P, Ernst S A.et al Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology 19971131966–1975. [DOI] [PubMed] [Google Scholar]
- 9.Gukovsky I, Gukovskaya A S, Blinman T A.et al Early nf‐kappab activation is associated with hormone‐induced pancreatitis. Am J Physiol 1998275G1402–G1414. [DOI] [PubMed] [Google Scholar]
- 10.Halangk W, Lerch M M. Early events in acute pancreatitis. Gastroenterol Clin North Am 200433717–731. [DOI] [PubMed] [Google Scholar]
- 11.Pandol S J. Acute pancreatitis. Curr Opin Gastroenterol 200521538–543. [DOI] [PubMed] [Google Scholar]
- 12.Puneet P, Moochhala S, Bhatia M. Chemokines in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 2005288L3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waterford S D, Kolodecik T R, Thrower E C.et al Vacuolar ATPase regulates zymogen activation in pancreatic acini. J Biol Chem 20052805430–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma A, Tao X, Gopal A.et al Protection against acute pancreatitis by activation of protease‐activated receptor‐2. Am J Physiol Gastrointest Liver Physiol 2005288G388–G395. [DOI] [PubMed] [Google Scholar]
- 15.Namkung W, Han W, Luo X.et al Protease‐activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology 20041261844–1859. [DOI] [PubMed] [Google Scholar]
- 16.Kawabata A, Kuroda R, Nishida M.et al Protease‐activated receptor‐2 (par‐2) in the pancreas and parotid gland: immunolocalization and involvement of nitric oxide in the evoked amylase secretion. Life Sci 2002712435–2446. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen T D, Moody M W, Steinhoff M.et al Trypsin activates pancreatic duct epithelial cell ion channels through proteinase‐activated receptor‐2. J Clin Invest 1999103261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vergnolle N, Hollenberg M D, Sharkey K A.et al Characterization of the inflammatory response to proteinase‐activated receptor‐2 (par2)‐activating peptides in the rat paw. Br J Pharmacol 19991271083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanke T, Takizawa T, Kabeya M.et al Physiology and pathophysiology of proteinase‐activated receptors (pars): par‐2 as a potential therapeutic target. J Pharmacol Sci 20059738–42. [DOI] [PubMed] [Google Scholar]
- 20.Kong W, McConalogue K, Khitin L M.et al Luminal trypsin may regulate enterocytes through proteinase‐activated receptor 2. Proc Natl Acad Sci USA 1997948884–8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorucci S, Mencarelli A, Palazzetti B.et al Proteinase‐activated receptor 2 is an anti‐inflammatory signal for colonic lamina propria lymphocytes in a mouse model of colitis. Proc Natl Acad Sci USA 20019813936–13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh V, Saluja A, Navina S.et al Par‐2 plays a protective role in pancreatitis. Gastroenterology 2003124
- 23.Kawabata S, Miura T, Morita T.et al Highly sensitive peptide‐4‐methylcoumaryl‐7‐amide substrates for blood‐clotting proteases and trypsin. Eur J Biochem 198817217–25. [DOI] [PubMed] [Google Scholar]
- 24.Haqqani A S, Sandhu J K, Birnboim H C. A myeloperoxidase‐specific assay based upon bromide‐dependent chemiluminescence of luminol. Anal Biochem 1999273126–132. [DOI] [PubMed] [Google Scholar]
- 25.Dyer R B, Herzog N K. Isolation of intact nuclei for nuclear extract preparation from a fragile b‐lymphocyte cell line. Biotechniques 199519192–195. [PubMed] [Google Scholar]
- 26.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem 197672248–254. [DOI] [PubMed] [Google Scholar]
- 27.Niederau C, Ferrell L D, Grendell J H. Caerulein‐induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology 1985881192–1204. [DOI] [PubMed] [Google Scholar]
- 28.Bohm S K, Kong W, Bromme D.et al Molecular cloning, expression and potential functions of the human proteinase‐activated receptor‐2. Biochem J 19963141009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Acker G, Saluja A, Singh V.et al Intrapancreatic activation of trypsinogen precedes and is required for neutrophil sequestration in the pancreas during secretagogue‐induced pancreatitis in mice. Gastroenterology 20011202742 [Google Scholar]
- 30.Grady T, Mah'Moud M, Otani T.et al Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol 1998275G1010–G1017. [DOI] [PubMed] [Google Scholar]
- 31.Saluja A K, Saluja M, Printz H.et al Experimental pancreatitis is mediated by low‐affinity cholecystokinin receptors that inhibit digestive enzyme secretion. Proc Natl Acad Sci USA 1989868968–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyr K, Aenishanslin H W, Kayasseh L.et al Raised trypsin concentration in duodenal secretion after a test meal (lundh test) (author's transl). Dtsch Med Wochenschr 19751001419–1422. [DOI] [PubMed] [Google Scholar]
- 33.Brugge W R, Burke C A, Brand D L.et al Increased interdigestive pancreatic trypsin secretion in alcoholic pancreatic disease. Dig Dis Sci 198530431–439. [DOI] [PubMed] [Google Scholar]
- 34.Regan P T, Malagelada J R, Go V L.et al A prospective study of the antisecretory and therapeutic effects of cimetidine and glucagon in human acute pancreatitis. Mayo Clin Proc 198156499–503. [PubMed] [Google Scholar]
- 35.O'Keefe S J, Lee R B, Li J.et al Trypsin secretion and turnover in patients with acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2005289G181–G187. [DOI] [PubMed] [Google Scholar]
- 36.van Acker G, Saluja A, Bhagat L.et al Relationship between intrapancreatic activation of trypsinogen and intrapancreatic sequestration of neutrophils following supramaximal stimulation with caerulein in mice. Pancreas 200021486 [Google Scholar]
- 37.Saluja A K, Bhagat L, Lee H S.et al Secretagogue‐induced digestive enzyme activation and cell injury in rat pancreatic acini. Am J Physiol 1999276G835–G842. [DOI] [PubMed] [Google Scholar]
- 38.Hofbauer B, Saluja A K, Lerch M M.et al Intra‐acinar cell activation of trypsinogen during caerulein‐induced pancreatitis in rats. Am J Physiol 1998275G352–G362. [DOI] [PubMed] [Google Scholar]
- 39.Hartwig W, Jimenez R E, Werner J.et al Interstitial trypsinogen release and its relevance to the transformation of mild into necrotizing pancreatitis in rats. Gastroenterology 1999117717–725. [DOI] [PubMed] [Google Scholar]
- 40.Singh V P, Chari S T. Protease inhibitors in acute pancreatitis: lessons from the bench and failed clinical trials. Gastroenterology 20051282172–2174. [DOI] [PubMed] [Google Scholar]
- 41.Buchler M, Malfertheiner P, Uhl W.et al Gabexate mesilate in human acute pancreatitis. German pancreatitis study group. Gastroenterology 19931041165–1170. [DOI] [PubMed] [Google Scholar]