Supported by the Japanese Ministry of Health, Labour and Welfare, the first Clinical practice guidelines for hepatocellular carcinoma (the JHCC guidelines) were compiled in Japan, using an evidence‐based methodology. Covering six major research fields for hepatocellular carcinoma (HCC), this set of guidelines consists of 58 pairs of research questions and recommendations, and practical algorithms for the surveillance and treatment of HCC (fig 1).1,2 A complete English translation of the main body of the guidelines has recently been uploaded on the website of The Japanese Society of Hepatology (http://www.jsh.or.jp/). The JHCC guidelines are primarily intended to support both physicians and patients in their decision making on the management of HCC.
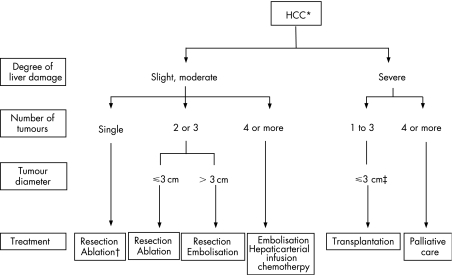
Figure 1 Treatment algorithm for hepatocellular carcinoma (HCC) (cited with permission from the Group formed to establish “Guidelines for evidence‐based clinical practice for the treatment of liver cancer”1). *The presence of vascular invasion or extrahepatic metastasis is indicated separately. †Selected when the severity of liver damage is moderate and the tumour diameter is ⩽2 cm. ‡Tumour diameter ⩽5 cm, when there is only one tumour.
In March 2006, approximately a year after the publication of the JHCC guidelines book, a questionnaire survey was conducted to investigate the level of awareness and influence of the guidelines among 2279 members of the Liver Cancer Study Group of Japan. Of the 843 (37.0%) responders, 467 (55.4%) were hepatologists and 320 (38.0%) were liver surgeons. More than 70% were working at university hospitals or designated teaching hospitals. Over the previous 3 months, 533 (63.2%) and 357 (42.3%) of the responders had treated ⩾10 HCC patients at outpatient and inpatient bases, respectively.
The same questionnaire was sent out to 689 primary care physicians in Osaka and Hyogo prefectures, and 332 (48.2%) responded; 159 (48%) were internists, 146 (44%) were surgeons and 209 (63%) were working at clinics. The majority (86.4%) had treated <10 patients with HCC in the previous 3 months.
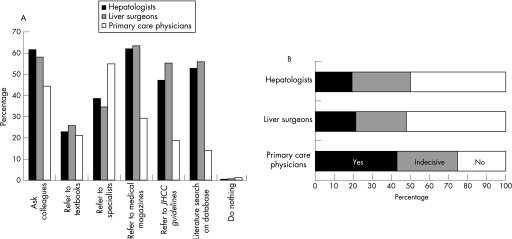
The JHCC guidelines have been acknowledged by 71.9% of hepatologists, 75.6% of liver surgeons and 61.0% of primary care physicians. Answers obtained regarding the possible actions taken for clinical questions on HCC are shown in fig 2A. Medical magazines, literature search, the JHCC guidelines and colleagues' opinions were the main sources of information, both for hepatologists and for liver surgeons, whereas specialists' or colleagues' opinions were the major sources of information for primary care physicians. After the introduction of the JHCC guidelines, only 19–21% of hepatologists or liver surgeons changed their practice pattern (fig 2B); 50–52% did not change their practise pattern, and were convinced that their choice of treatment was similar to the recommendations in the guidelines. In total, 43% of primary care physicians changed their practice pattern as per the recommendations in the guidelines, or by paying more attention to patients' preferences.
Figure 2 (A) What are your possible actions when you have clinical questions or problems in regard to the management of patients with hepatocellular carcinoma (HCC)? (a multiple‐choice question). (B) Have you changed your practice pattern for HCC after reading the JHCC guidelines? (Responders who did not acknowledge the guidelines were excluded.)
In conclusion, 1 year after its publication, the JHCC guidelines have become well disseminated among both specialists and primary care physicians in Japan. As expected, these guidelines have begun to be applied at every level of clinical decision making for HCC.
Acknowledgements
We thank Mr Hiromichi Suzuki and the International Medical Information Center (Tokyo) for their clerical work for the questionnaire survey.
Footnotes
Competing interests: None declared.
References
- 1.Group formed to establish “Guidelines for evidence‐based clinical practice for the treatment of liver cancer” Clinical practice guidelines for hepatocellular carcinoma. Tokyo: Kanehara, 2005 [in Japanese],
- 2.Makuuchi M, Kokudo N. Clinical practice guidelines for hepatocellular carcinoma: the first evidence based guidelines from Japan. World J Gastroenterol 200612828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]