Abstract
Background
Retinoic acid (RA) is a powerful differentiation agent. Barrett's oesophagus occurs when duodeno‐gastro‐oesophageal reflux causes squamous epithelium (SE) tissue to become columnar epithelium tissue by an unknown mechanism. The bile acid lithocholic acid (LCA) competes for the retinoid X receptor retinoid binding site. Hence, RA pathways may be implicated in Barrett's oesophagus.
Methods
RA activity in tissues and cell lines treated with all‐trans retinoic acid (ATRA) with or without LCA was assessed using a reporter. Expression of p21 was determined by real‐time PCR in Barrett's oesophagus cell lines with or without LCA. SE and Barrett's oesophagus biopsy specimens were exposed to 100 μM of ATRA or 20 mM of a RA inhibitor, citral, in organ culture for >72 h. Characteristics of treated specimens, compared with untreated controls, were analysed by immunohistochemical analysis (cytokeratins (CKs), vimentin) and RT‐PCR (CKs). Confocal microscopy assessed temporal changes in co‐localisation of CK8/18 and vimentin. Cell proliferation was assessed by bromo‐deoxyuridine incorporation and immunohistochemical analysis for Ki67 and p21.
Results
RA biosynthesis was increased in Barrett's oesophagus compared with SE (p<0.001). LCA and ATRA caused a synergistic increase in RA signalling as shown by increased p21 (p<0.01). Morphological and molecular analysis of SE exposed to ATRA showed columnar differentiation independent of proliferation. Metaplasia could be induced from the stromal compartment alone and vimentin expression co‐localised with CK8/18 at 24 h, which separated into CK8/18‐positive glands and vimentin‐positive stroma by 48 h. Citral‐treated Barrett's oesophagus led to phenotypic and immunohistochemical characteristics of SE, which was independent of proliferation.
Conclusion
RA activity is increased in Barrett's oesophagus and is induced by LCA. Under conditions of altered RA activity and an intact stroma, the oesophageal phenotype can be altered independent of proliferation.
Barrett's oesophagus is a disease that is predominant in Western countries that occurs as a consequence of chronic and severe exposure to duodeno‐gastro‐oesophageal refluxate and, per year, 0.5–1% patients with Barrett's oesophagus will develop adenocarcinoma.1,2 Oesophageal adenocarcinoma has increased by eightfold over the past three decades.3 Histopathologically, Barrett's oesophagus is characterised by a change from the usual stratified, squamous epithelium (SE) to a columnar‐lined epithelium. The process of metaplastic change is rarely observed in vivo and there are no reliable animal models. Therefore, natural history studies to understand the molecular and cellular processes underlying the development of Barrett's oesophagus have not been possible.
The mechanism by which an epithelial cell commits and proceeds to its full differentiation status is not fully understood. In understanding Barrett's oesophagus, it may be relevant to know that during embryogenesis, the oesophagus undergoes a columnar to squamous cell transition at 18 weeks' gestation.4 Hence, it is possible that chronic duodeno‐gastro‐oesophageal reflux in adulthood induces a change in the concentration of a morphogen, or other extracellular factor, which leads to abnormal reactivation of the genes responsible for the physiological transition between squamous and columnar cell types.5
On this basis, retinoic acid (RA) is a candidate molecule for the induction of metaplasia as it is a powerful inducer of differentiation during embryogenesis. Retinoids mediate their biological functions by binding to the nuclear receptors known as retinoic acid receptors and retinoid X receptors, and therefore activating a number of cell‐signalling pathways that are involved in determining the fate of embryonic cells.6,7 Interestingly, the bile acid lithocholic acid (LCA), which is a component of refluxate, has recently been demonstrated to compete efficiently with 9‐cis‐RA for the retinoid X receptor binding site.8
There are many RA target genes, including the vertebrate Cdx genes (Cdx1,2) which encode homeodomain transcription factors and have been implicated in the development of the posterior part of the embryo.9 Cdx factors convey the activity of signalling molecules to Hox genes, which have also been found to contain functional retinoic acid response elements (RAREs).10 As a result, retinoids can mediate the anteroposterior patterning of the gut in embryogenesis.11 The Cdx genes may act as a “master‐switch” gene for the cell differentiation status in adulthood, as shown by the development of intestinal metaplasia of the gastric mucosa in transgenic mice expressing Cdx2 driven by a H+/K+‐ATPase promoter.12 In the oesophagus, Cdx2 expression is observed in the areas of specialised intestinal metaplasia.12,13,14
There is historical evidence to suggest that RA may induce profound alterations in oesophageal epithelial cell differentiation status. In the 1960s, Adeylotte15 demonstrated that oesophageal explants from 13‐day chick embryos grown in the presence of excess all‐trans retinoic acid (ATRA) developed an excess of glands within the lamina propria, and that the epithelium acquired a pseudostratified structure with a greater number of columnar ciliated cells. Around the same time, Lasnitzki16 demonstrated similar effects of ATRA on embryonic rat oesophagus. Now it is known that adult tissues have greater plasticity than was recognised previously,17 and these historical studies were performed without the benefit of modern methods to perform molecular characterisation.
These observations provide a rationale for investigating the possible role of RA in the development of altered differentiation status in the oesophagus. Such studies may be relevant for our understanding of the pathogenesis of Barrett's oesophagus. For these experiments, we used human biopsy specimens in an organ culture system as it has been recognised for some time that the stromal compartment is important for the differentiation to occur.18,19 For example, to achieve asymmetrical stem‐cell divisions of the oesophageal epithelium, it is necessary to reconstitute the oesophageal squamous keratinocytes on the denuded connective tissue that is derived from the oesophagus.20 Furthermore, it is not possible to generate oesophageal or intestinal epithelia in a fully differentiated structural pattern in monolayer culture and conversion of embryonic stem cells to columnar epithelium or squamous epithelium has been shown to depend on the components of the extracellular matrix.21 Organ culture provides a model system in which the oesophageal epithelium with an intact stromal compartment can be manipulated to study the metaplastic process22,23.
The specific aims of this study were to examine the RA levels in normal squamous oesophagus compared with those in Barrett's oesophagus, to determine whether LCA could induce RA biosynthesis and, finally, to determine whether exogenous application of RA or an RA inhibitor could induce any alterations in oesophageal cell differentiation status.
Methods
Patients and tissues
Patients were recruited prospectively from Addenbrooke's Hospital, Cambridge, UK, with approval by the local research ethics committee. Patients included those with Barrett's oesophagus undergoing surveillance and those with an endoscopically normal SE, undergoing evaluation for dyspeptic symptoms. All samples of SE were taken from at least 2 cm above the squamo‐columnar junction (either the normal z‐line or the neo‐squamo‐columnar junction in patients with Barrett's oesophagus). Wherever possible, control experiments (Barrett's oesophagus cultured with or without ATRA or citral) were carried out using biopsy specimens from the same patient as a within‐patient control. Otherwise, all the samples represent independent experimental data from separate patients.
RA biosynthesis
Biopsy specimens (24 SE and 11 Barrett's oesophagus) were maintained in an organ culture system using serum‐free medium containing 100 nM retinol for 24 h (Sigma, St Louis, Missouri, USA). RA levels in the medium were determined using NIH 3T3 cells transfected with 1.2 μg of pRARE‐TA‐SEAP (BD Bioscience, Oxford, UK), which contains two copies of the RARE upstream of a secreted alkaline phosphatase reporter gene and 0.2 μg of pIres‐puro (Clontech, Palo Alto, California, USA). Using 1 µg/ml of puromycin (Sigma‐Aldrich, Dorset, UK), cells stably expressing the reporter were selected, and after 24 h, the phosphatase activity was quantified using a Great EscAPe SEAP detection kit (BD Biocsience, Palo Alto, California, USA). Relative RA content in the medium (bioactivity) was calculated by comparing with a standard curve (serial RA concentrations 10−6–10−12 M) and adjusted for protein content according to the bicinchoninic acid protein assay using a commercially available kit (Pierce, Rockford, Illinois, USA).
Real‐time‐PCR for p21WAF1 mRNA
Two immortalised dysplastic, well‐characterised Barrett's cell lines24 (CP‐C and CP‐D, gift from P Rabinovich, University of Washington, Washington, USA) were cultured in supplemented MDCB media and treated with RA (10−7 M) and LCA (15 μM) or a combination of both. As serum contains RA, cells were cultured for 1 h in low‐serum (0.5%) medium (to adapt the cells to serum‐free conditions), followed by 3 h culture in serum‐free medium. Total RNA was then extracted by using TriZol reagent (Invitrogen, Paisley, UK). Each RNA sample was prepared in triplicate. Real‐time (RT)‐PCR was performed in a Rotor Gene 3000 (Corbett Research, Mortlake, Sydney, New South Wales, Australia) using SYBR Green JumpStart Taq Readymix (Sigma‐Aldrich, St Louis, Missouri, USA). PCR consisted of 40 cycles of 94°C denaturation (10 s), 57°C annealing (20 s) and extension (20 s; primer sequences are given in table 1). Cycle threshold (Ct) was determined for each sample, and the average Ct of triplicate samples was calculated. The expression of p21 relative to glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was determined.
Table 1 Primer sequences.
| Forward | Reverse | |
|---|---|---|
| p21WAF1 | 5′‐CTGTCACTGTCTTGTACCCT‐3′ | 5′‐GGTAGAAATCTGTCATGCTGG‐3′ |
| CK7 | 5′‐TGAATGATGAGATCAACTTCCTCAG‐3′ | 5′‐CAGGCGGAGATCTGGGACTGC‐3′ |
| CK13 | 5′‐AATCCTGCTCTCACCATAGAGAG‐3′ | 5′‐GGTTCTGCATGGTGATCTTC‐3′ |
| CK8/18 | 5′‐GCTCTGTCCAGGCGCCCAGCTACGG‐3′ | 5′‐CAGGCGGTCGTTCAGGCTTTGCATG‐3′ |
| GAPDH | 5′‐GGTTCTGCATGGTGATCTTC‐3′ | 5′‐GGGGTCATTGATGGCAACA‐3′ |
GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
Organ culture and RA differentiation experiments
SE samples were randomly assigned to the treatment group for culture with ATRA (n = 40, of which 5 SE samples were taken from patients with Barrett's oesophagus, mean (SD) age 62.9 (11.75) years; M:F is 1.2:1) or to the control group for culture without RA (n = 9 controls, of which 3 SE samples were from patients with Barrett's oesophagus, mean (SD) age 67.01 (8.29) years; M:F is 1.2:1). Barrett's oesophagus samples were also taken from the same patients with Barrett's oesophagus detailed above and randomly assigned for culture with the RA antagonist citral (n = 10) or to the control group without citral (n = 6). Organ culture was performed using Medium 199 containing 2 mM glutamine (Sigma‐Aldrich, Dorset, UK) at a liquid–oxygen (95%) interface, and viability was checked using a lactate dehydrogenase assay (Sigma‐Aldrich, Dorset, UK) as described previously.22 Media were supplemented with 100 μM ATRA (Sigma‐Aldrich, Dorset, UK) or 20 mM citral (Sigma, Seelze, Germany) for a maximum of 72 h. A supra‐physiological concentration was required for the RA or the citral to penetrate the biopsy specimen. For the bromo‐deoxyuridine (BrdU) incorporation experiments, 30 μM was added to the media for the experiment duration. To separate the epithelial and stromal compartments, the biopsy tissue was incubated in balanced salt solution with 2 U/ml dispase (Gibco, Paisley, UK) for an hour, and then washed vigorously to remove the epithelium.
Histochemical stains and immunohistochemical analysis
Tissues were fixed in 10% formalin and processed for paraffin‐wax embedding. Sections of 5 μM thickness were stained with H&E. An antigen‐retrieval procedure was performed by pressure cooking the specimen in 0.01 M tris‐sodium citrate buffer, pH 6.0, before incubation with the following primary monoclonal antibodies raised against human Minichromosome maintenance protein 2 (gift from Steve Dilworth and Ron Laskey): CK13 (1:200), CK8/18 (1:40), CK7 (1:50), CK14 (1:40), p21WAF1 (1:50; Novocastra Laboratories, Newcastle, UK) and Ki‐67 (1:100; DakoCytomation, Ely, UK). Anti‐BrdU (1:40; AbCam, Cambridge, UK). The avidin–biotin and peroxidase method and a haematoxylin counterstain were used. Negative controls were performed for each slide by omission of the primary antibody. Staining for p21WAF1 was quantified by counting positive cells and negative cells in three high‐power fields (×400) per biopsy.
Immunofluorescence
Sections were incubated with polyclonal anti‐vimentin (1:40) (Santa Cruz biotechnology, Santa Cruz, California, USA) and monoclonal anti‐CK8/18 (1:40; Novocastra, Newcastle Upon Tyne, UK), simultaneously. The secondary antibodies used were FITC‐conjugated anti‐mouse antibody (Vector Labs, Burlingame, California, USA) and Texas red‐conjugated anti‐rabbit antibody (Amersham, Little Chalfont, UK). The DNA staining was performed using TO‐PRO‐3 (Molecular Probes, Eugene, Oregon, USA). The sections were analysed on a Zeiss Axioplan 2 confocal microscope (Carl Zeiss Ltd, Welwyn Garden City, UK).
Real‐time PCR
A cryosection was performed from the RA‐cultured biopsy specimens before RNA extraction to confirm that glandular differentiation had occurred. Total RNA was isolated using Trizol reagent. In all, 2 μg of RNA was reverse transcribed and 2 μl of cDNA was amplified. Amplification conditions consisted of 30 cycles of 94°C for 30 s, 30 cycles of 55°C for 30 s and 30 cycles of 72°C for 45 s for CK7, CK 13 and CK8/18 (table 1). The amplification of GAPDH was 94°C for 45 s, 60°C for 45 s and 72°C for 1 min. PCR products were analysed on 1.5% agarose gels, stained with ethidium bromide and quantified by densitometry.
Statistics
The significance of RA biosynthesis in SE compared with Barrett's oesophagus samples was determined using the Mann–Whitney's U test. LCA synergism with RA was determined by one‐way analysis of variance using the Kruskal–Wallis test. A p value <0.05 was required for significance.
Results
Retinoid bioactivity and effect of LCA
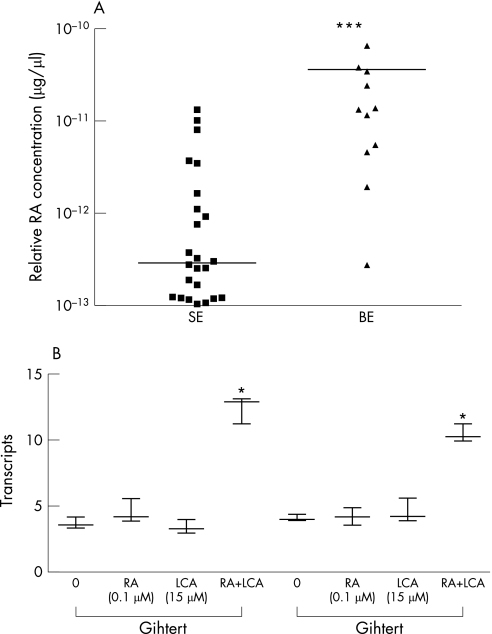
Using RARE reporter cells, we demonstrated that retinoid bioactivity of Barrett's oesophagus was increased by 30‐fold compared with SE samples (p<0.001, fig 1A). The effect of treating two immortalised Barrett's cell lines with physiological concentrations of either LCA or ATRA alone or in combination was assessed by examining the expression of the ATRA response gene p21, which is expressed at an abundance similar to GAPDH (mean delta Ct value of 10 and 15, respectively). For this experiment, we used the concentration of ATRA and LCA that resulted in a significant increase in RA signalling in a preliminary experiment (10−7 M RA and 15 μM LCA, p<0.001, data not shown). ATRA and LCA in combination resulted in a threefold increase in p21 mRNA expression compared with addition of either LCA or ATRA alone (p<0.001; fig 1B). Hence, these data suggest that LCA can augment the effect of local RA to stimulate RA signalling. In subsequent experiments, we added ATRA directly to biopsy specimens ex vivo to determine the effect on the tissue phenotype.
Figure 1 (A) Retinoic acid (RA) bioactivity in patient tissues of normal oesophagus with squamous epithelium (SE) and Barrett's metaplasia (Barrett's oesophagus (BE)) in the presence of the substrate retinol (10−6 M). Bioactivity in the medium was measured using a luciferase reporter assay. The scatter plot demonstrates the mean value for each patient and the overall mean. RA bioactivity is highest in Barrett's tissues (***, p<0.001). (B) Immortalised Barrett's cell lines (CP‐C and CP‐D) were treated with 10−7 M RA or 15 µM lithocholic acid (LCA) or a combination of both for 1 h and SYBR green‐based real‐time PCR was performed for the RA downstream gene, p21WAF1. Increased p21 expression was observed in both cell lines treated by exogenous RA and LCA, which was synergistic (*p<0.001 in comparison to either LCA or all‐trans retinoic acid alone).
RA induces glandular oesophageal mucosa ex vivo
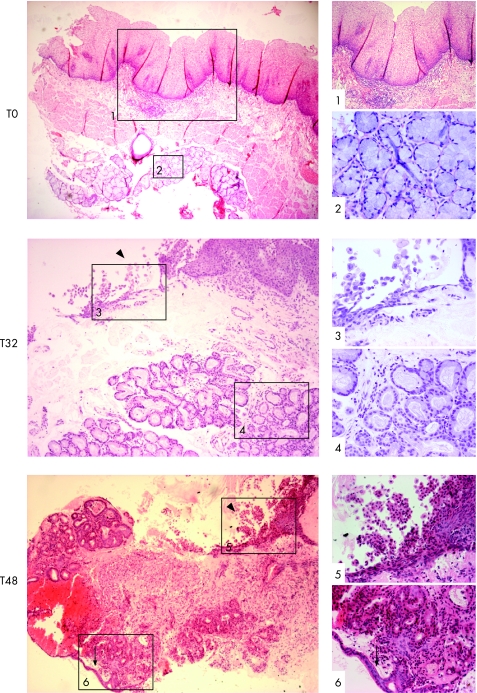
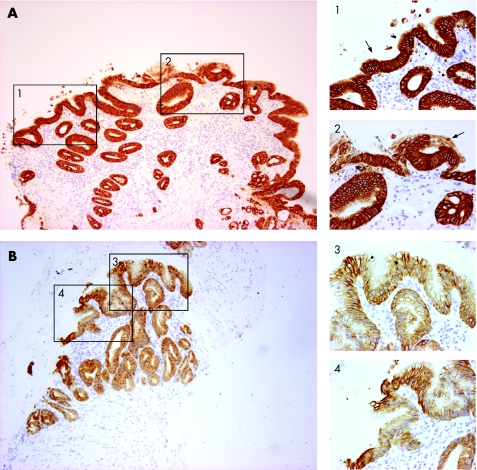
Following the culture of SE samples with exogenous ATRA over a time course, the morphological characteristics were first examined using standard H&E staining. Only well‐orientated sections that included the biopsy specimen's surface were regarded as informative to prevent persistent submucosal glandular structures being erroneously attributed to RA‐induced changes. A representative endoscopic section is shown prior to culture (fig 2, T0). Depending on the thickness of the endoscopic biopsy specimen, submucosal glands may also be observed (fig 2, T0). Following 32 h of culture in the presence of RA, the squamous mucosa is seen sloughing off from the surface (fig 2, T32, arrowhead box 3). The submucosal glands are of varying degrees of differentiation with some enlargement of nuclei (fig 2, T32, box 4). By 48 h, the remnants of the squamous mucosa are again seen sloughed from the surface and there is more extensive remodelling. Glands are seen fusing with the biopsy specimen's surface that, in places, has a columnar‐lined appearance with one or two layers of epithelial cells (fig 2, T48, arrow box 6). In 8 of 40 (20%) treated squamous tissues, there were glandular characteristics, which extended to the surface (fig 3). After ATRA treatment, the epithelial surface and deep glands were strongly positive for cytokeratin (CK)8/18 (fig 4A), which is usually expressed in native columnar epithelium (fig 4B). In addition, we observed that, in several places, glandular structures appeared to have fused with and opened onto the epithelial surface (fig 4A, arrows).
Figure 2 Squamous oesophageal endoscopic biopsy specimens were cultured with 100 μM all‐trans retinoic acid ATRA ex vivo over a 48 h time period (T). These images are representative sections, always orientated in relation to the biopsy surface (top) and stained with H&E. The images in the left‐hand side panel are at ×40 (T0, T48) and ×100 (T32) magnification and the images on the right corresponding to the numbered boxes are at ×100 (1), ×200 (5, 6) and ×400 (2–4) magnification. (T0, prior to culture): native squamous oesophageal mucosa with stratified squamous epithelium, lamina propria containing connective tissue or stroma (box 1), the muscularis mucosa and the submucosal glands (box 2) prior to culture. T32, (32 h of culture): the squamous mucosa is sloughing off from the surface (box 3, arrowhead) and the submucosal glands are of varying degrees of differentiation with some enlargement and rounding‐up of nuclei (box 4). T48, (48 h of culture): by 32 and 48 h of culture, the squamous mucosa has mainly sloughed off (arrowhead), leaving the basal layer (box 5) and glands seen cupping and fusing with the surface, which, in places, has a columnar‐lined appearance with a single layer of epithelial cells (arrow, box 6).
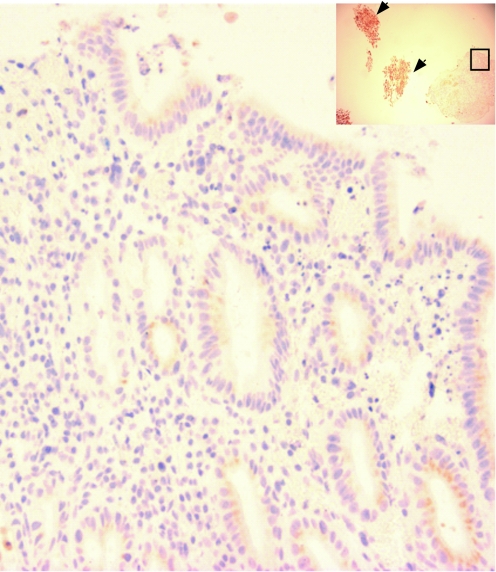
Figure 3 A further example of the effect of 48 h culture of a squamous oesophageal biopsy specimen in 100 μM of all‐trans retinoic acid. The high‐power view (×400) shows a columnar‐lined surface and the low‐power inset (×100) shows that the squamous oesophagus (highlighted using cytokeratin (CK)13 staining, arrowheads) has sloughed off and is separate from the main biopsy. The part of the low‐power image also shown at high power is denoted by the black box.
Figure 4 (A) Following 48 h culture with 100 μM of all‐trans retinoic acid (ATRA), immunohistochemical analysis has been performed using immunoperoxidase‐labelled cytokeratin (CK)8/18 with a haematoxylin counterstain. The epithelial surface and deep glands are strongly CK8/18 positive. At several places, the glands appear to have opened up and fused with the surface (arrows in 1, 2). (B) A CK8/18‐stained section through native Barrett's oesophagus is shown for comparison. The intensity of CK8/18 staining was always much stronger following ATRA culture. For A and B, the areas denoted by the black boxes on the low‐power view (×100 magnification) are also shown at ×400 magnification (boxes 1–4) to highlight the appearances at the surface.
These morphological changes seemed to occur after sloughing of initial surface SE or tissue, resulting in the exposure of submucosal glands to the luminal surface (fig 5A,B). Interestingly, in 2 of 8 of the ATRA‐treated specimens, the columnar epithelium presented as a “multilayered epithelium” in which polarised columnar cells were present in the uppermost layers with multilayered squamoid cells beneath. The oesophageal submucosal gland ducts could be seen nearby or even in continuation with the induced surface epithelium (fig 5C, asterisk).
Figure 5 A further example of the effect of 48 h culture of a squamous oesophageal biopsy specimen in 100 μM of all‐trans retinoic acid (ATRA) to show areas of multilayered epithelium. (A) Sloughing of squamous tissue (arrow) is seen with induced columnar epithelium on the surface (arrowhead). (B) Multilayered epithelium (arrowhead) is seen with attenuation and continuation to a bilayered, polarised columnar epithelium (arrow). (C) Another example of multilayered epithelium after culture in ATRA consists of a columnar surface with basal squamous cells beneath (arrowhead). A submucosal gland duct is marked by the asterisk (A–C ×400).
When the treated biopsy specimens that demonstrated surface columnar differentiation were compared with biopsy specimens showing only primitive gland‐like structures beneath the surface, multivariate analysis did not show any difference in the age, sex or endoscopic diagnoses of the patients (data not shown). In contrast with the changes demonstrated in all samples cultured with RA, none of the control tissues cultured without RA had any evidence of altered differentiation at the biopsy surface (data not shown).
Stromal origin of RA‐induced changes
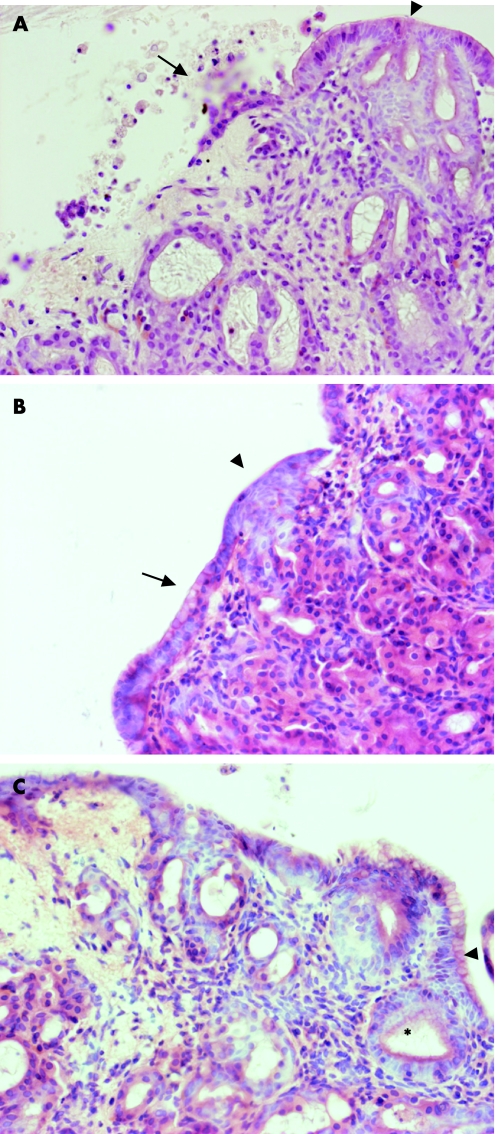
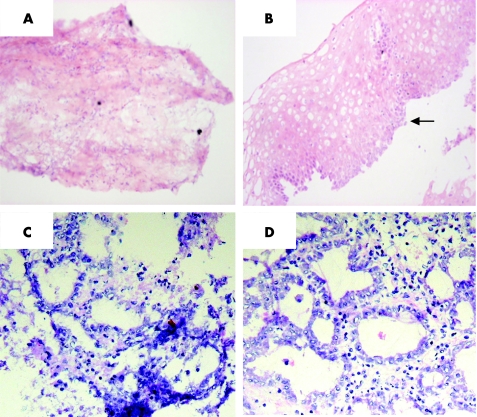
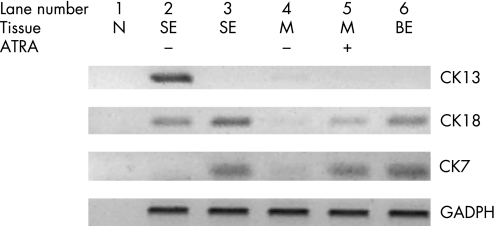
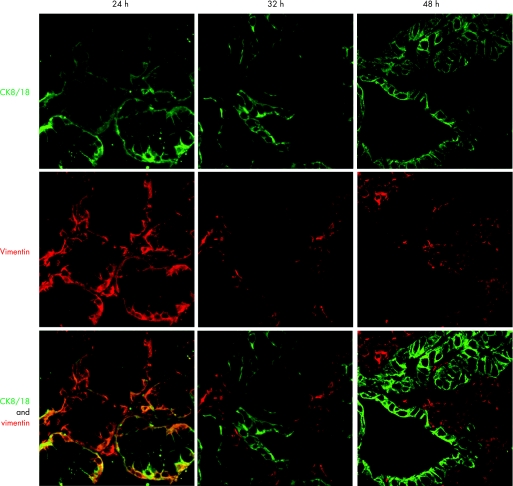
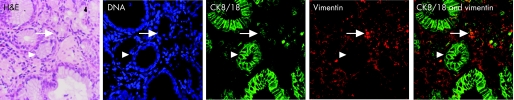
To determine whether the glandular mucosa originated from either the epithelial or the stromal compartments, the endoscopic SE biopsy specimen was separated into epithelium and stroma before culture (fig 6). RT‐PCR was used to determine both the efficacy of the epithelial stripping and the effect of the ATRA culture (fig 7). Hence, as expected, the full‐thickness squamous biopsy (lane 2, labelled SE) expressed CK13 and some CK18, which comes from the basal compartment, with no expression of CK7. After culture of the full‐thickness biopsy specimen with RA, there was an increase in CK18 and CK7, with loss of CK13 expression (lane 3, SE+). This is the expected CK profile of Barrett's epithelium (lane 6, labelled Barrett's oesophagus in fig 7). In contrast, after culture in dispase, the expression of CKs was virtually lost, in keeping with successful removal of the epithelial portion of the biopsy specimen (fig 7, lane 4, labelled M−). Culture of the stroma alone with ATRA still resulted in expression of the CK profile, which is consistent with the development of a columnar‐lined epithelium (fig 7, lane 5, labelled M+). Although it is possible that the RA‐induced increase in CK7 and CK18 synthesis occurred from residual gland ducts or other epithelium still present in the biopsy specimens after the dispase treatment, CK18 was not detectable by RT‐PCR in these samples even after further rounds of amplification (data not shown). In addition, there were no remnant epithelial cells observed in the control dispase‐treated biopsy specimens cultured without ATRA (fig 6A,B). Overall, these data suggest that the ATRA‐induced changes are of stromal origin. The dual‐labelling immunofluorescence shows that, at the early time point (24 h), there is co‐localisation of vimentin and CK8/18, which then separates out at 32 and 48 h into CK8/18‐positive glands and vimentin‐positive stroma (fig 8). Although not conclusive, this would be in keeping with mesenchymal–epithelial transition. Furthermore, these data suggest that the changes observed with ATRA are dynamic over time and are unlikely to result from erroneous sampling of glandular mucosa. In addition, native Barrett's oesophageal biopsy specimens have immature deep glands with weak expression of CK8/18, which colocalises with speckled vimentin expression (fig 9, arrows). These appearances resemble the RA‐induced changes. In contrast, mature glands stained with CK8/18 alone with no expression of vimentin (fig 9, arrowheads).
Figure 6 Dispase‐treated biopsy specimens. The top panel shows the (A) mesenchymal compartment and (B) epithelial compartment from the same biopsy specimen after dispase treatment before culture with all‐trans retinoic acid (ATRA). The black arrow indicates basal squamous cells, suggesting that the totality of the epithelium was separated from the lamina propria. The bottom panels are representative pictures of the mesenchyme after a (C) 32 h and (D) 48 h treatment with ATRA, after which glandular differentiation has occurred.
Figure 7 The effect of all‐trans retinoic acid (ATRA) on the stromal portion of the biopsy specimen (M+) compared with the full thickness squamous epithelial (SE) biopsy specimen was examined by performing real‐time PCR for epithelial markers. ATRA induced the glandular epithelial markers cytokeratin (CK)8/18 and CK7 with reduction of the squamous epithelial marker CK13 in a full thickness SE biopsy specimen. The stroma alone (M+) cultured in the absence of retinoic acid (RA) did not express any of the epithelial cell markers CK13, CK 8/18 or CK7, but after RA treatment (SE+), CK 8/18 and CK7 were again induced. N was a negative control using water in the RT mix, Barrett's oesophagus (BE) was a full‐thickness biopsy specimen from native BE, and GAPDH (glyceraldehyde 3‐phosphate dehydrogenase) confirmed equivalent cDNA loading.
Figure 8 Immunohistochemical staining was performed in biopsy specimens cultured with 100 μM retinoic acid (RA) over 24, 32 and 48 h. Cytokeratin (CK)8/18 (green) and vimentin (red) are viewed using the confocal microscope. Coexpression is seen by dual labelling at 24 h, which separates into glands (CK8/18 positive) and surrounding stroma again at 32 h.
Figure 9 Native Barrett's oesophagus (BE) biopsy specimens have immature deep glands, which resemble the retinoic‐acid‐induced changes. Biopsy specimens have been stained with H&E, and viewed with the light microscope or with TO‐PRO‐3 DNA stain (blue), cytokeratin (CK)8/18 (green), vimentin (red) and dual labelling. The mature glands (arrowheads) in this representative biopsy specimen have nuclei, which are configured in a clear glandular arrangement and stain with CK8/18 with no expression of vimentin. In contrast, there are more immature areas (arrows) in which the nuclei are more loosely palisaded with weak expression of CK8/18 and speckled vimentin in the same areas.
The process is proliferation independent
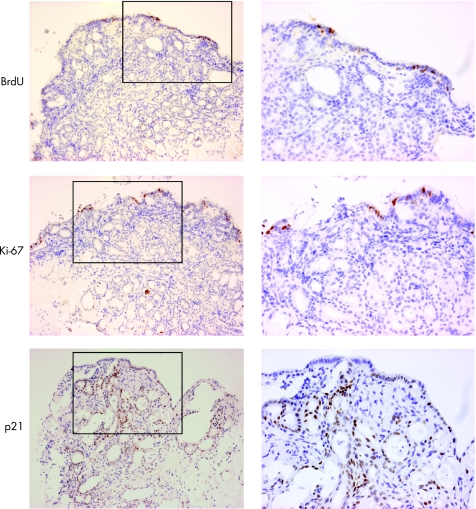
The rapid time course of this phenotypic change suggests a process of transdifferentiation rather than proliferation of a pre‐existing glandular compartment. This was confirmed by the scarcity of BrdU incorporation and of Ki‐67 staining in tissues, which were successfully treated with ATRA (fig 10). In addition, overexpression of p21 (29.3±6.0 positive immunostained cells in ATRA‐treated SE compared with 16.3±7.6 in control tissues not treated with ATRA) was observed in these same tissues, suggesting that active RA signalling resulted in cell‐cycle arrest (fig 9).
Figure 10 Culture with bromo‐deoxyuridine (BrdU) and immunohistochemical analysis with Ki67 and p21 to determine whether the observed phenotypic changes were dependent on proliferation. The low‐power images that include the biopsy surface are shown on the left (×100) and the high‐power images (×200) corresponding to the areas denoted by black boxes are shown on the right. The amount of BrdU incorporation is sparse after culture with all‐trans retinoic acid. The only proliferating cells are seen on the surface. This was confirmed by immunohistochemical analysis of Ki67. There was significant p21 expression, which is in keeping with significant downstream retinoic acid signalling and cell‐cycle arrest.
Reversal of columnar phenotype by RA antagonist
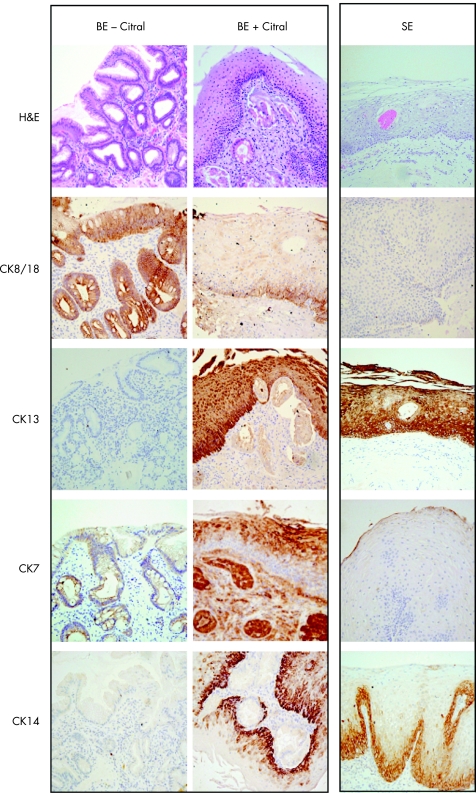
In light of these findings, to determine whether inhibition of RA activity could reverse the columnar phenotype, Barrett's oesophagus biopsy specimens were cultured with the RA inhibitor citral. After 48 h, 5 of 10 (50%) Barrett's explants had evidence of a squamous phenotype (fig 11). The villiform surface could be observed to be sloughed off, with remnant glandular structures underlying the squamous mucosa. The immunohistochemical characteristics resembled native squamous oesophagus with the expression of CK13 and CK14, but not CK8/18. Interestingly, CK7, which is usually expressed in the glandular mucosa, was selectively expressed in the superficial layers of the neo‐squamous epithelium. Ki67 labelling suggested that these changes were occurring independent of proliferation (data not shown).
Figure 11 Barrett's oesophageal epithelium (BE) was cultured ex vivo for 48 h in with or without citral. Squamous epithelium (SE) is uncultured normal for comparison. Routine H&E staining and immunohistochemical analysis using immunoperoxidase‐labelled cytokeratins (CK)8/18, CK13, CK7 and CK14 has been performed. The citral‐treated BE expresses CK13 similar to native SE. There is no expression of the glandular marker CK8/18, but there is some CK14 expressed in the superficial squamous layers. CK7 expression is altered after citral culture of BE. (All ×100 magnification).
Discussion
This is the first report of the induction and reversal of a glandular phenotype from adult, human mucosa ex vivo. Previously, continuous acid exposure ex vivo was shown to increase the degree of differentiation of native Barrett's epithelium.22 The data presented here suggest that ATRA induces the columnar phenotype de novo via a proliferation‐independent process, which may arise within the stromal components of the tissue either from submucosal glands or by mesenchymal–epithelial transition. The findings presented here have important implications for our understanding of metaplasia in general and of the pathogenesis of Barrett's oesophagus.
The organ culture system permits culture of the epithelium with intact stroma. As expected, the short time‐period permitted by the organ culture system meant that there was no evidence of goblet cell or other specialised cell lineages in the ATRA‐treated tissues. The limitations of the culture system meant that it was not possible to view sequential changes in the same biopsy specimen or to confirm the histopathological diagnosis at the beginning. However, we have made every attempt to rule out the possibility of sampling bias. First, the appearance of these ATRA‐treated biopsy specimens is unlikely to have been accounted for by erroneous sampling of the gastric mucosa as specialised gastric cell types were not present and sloughed squamous mucosa expressing CK13 was observed in the same low‐power field (fig 3). Similarly, the changes are unlikely to have been due to erroneous sampling of Barrett's epithelium as the effect of exogenous ATRA on the epithelial phenotype was primarily observed in patients who did not have a diagnosis of Barrett's oesophagus (7 of 8 patients). Furthermore, specialised intestinal metaplasia was not observed and all the patients undergoing surveillance had this histopathological subtype. This would also argue against inadvertent sampling of ultrashort Barrett's oesophagus (ie, non‐endoscopically visible Barrett's with intestinal metaplasia on biopsy).25 If the changes observed were due to sampling error, then one would expect a similar proportion of biopsy samples cultured without RA to have a columnar phenotype and this was not observed in any of the control specimens. Last, the abnormal expression of CK7 in the citral‐treated Barrett's biopsy specimen would not have been expected from inadvertent sampling of normal SE.
It is intriguing that only a proportion of the explants showed a phenotypic alteration, which extended to the surface after culture with either RA or citral. It is possible that the changes were dependent on the presence of submucosal gland ducts or the extent of stromal tissue obtained in the pinch biopsy. It is also possible that there were intrinsic genetically determined differences between patients, which determine metabolic responsiveness to RA.
With regards to the cell of origin of the ATRA‐induced columnar mucosa, all of the squamous biopsy specimens were taken at least 2 cm away from the gastro‐oesophageal junction and this makes it unlikely that the glandular differentiation has originated from pluripotential stem cells at the gastro‐oesophageal junction (transitional zone). It would also seem unlikely that there has been transdifferentiation from the squamous epithelial layer along an abnormal cell lineage pathway as culture of the mesenchymal compartment alone is sufficient for glandular differentiation to occur. Furthermore, the squamous layer is visibly shed from the surface (figs 2, 3) and CK13 is not expressed in the glandular phenotype (fig 7). The question therefore arises as to which cells within the stroma give rise to the new columnar epithelium. It is possible that, as suggested previously, the glandular tissue may arise from the stem cells located in the region of oesophageal glands and associated ducts.26 Support for this theory has come from observational studies of neo‐columnar‐lined epithelium generated in animal models of Barrett's oesophagus27 and it would also be in keeping with recent genetic analysis of neo‐squamous epithelium.28 In addition, neo‐squamous epithelium has been observed extending from the submucosal gland duct after photodynamic therapy. There were clear examples in which submucosal glands were present in the RA‐treated biopsy specimens (figs 2, 5). In addition, multilayered epithelia were noted in two of our specimens, which attenuated to form a single or bilayered columnar epithelia (fig 5C). These appearances are often seen in conjunction with submucosal gland ducts and it is possible that the mucosal gland duct epithelium could contain progenitor cells that can give rise to the metaplastic epithelium. If this is the case, then this process must have occurred by migration or differentiation rather than proliferation, because, a maximum of two cycles of cell division would be possible during the incubation period and, in contrast, the data suggests that there is cell‐cycle arrest (fig 10). The proliferation‐independent effect of RA that we observed follows a similar time course to the rapid RA‐induced differentiation observed in the epidermis of Xenopus, which was also seen in the absence of cell division.29 These data are also in keeping with the observation that goblet‐cell lineages can develop in postmitotic cells within 8 h of induction of Notch signalling.30
In contrast with the possibility that the columnar epithelium is derived from submucosal glands, it is also possible that the morphological changes induced by RA, occurred via a mesenchymal–epithelial transition. The results of the dual labelling studies suggest a transition from mesenchyme to epithelium at 48 h of culture (fig 8). Mesenchymal –epithelial transition is a well‐described mechanism for RA‐induced glandular differentiation and is well described within normal embryonic development.7,15,31,32 Previous work on the cell‐signalling pathways involved in RA‐induced columnar differentiation suggest that the first step in the mesenchymal–epithelial transition involves cell migration, followed by differentiation and cell‐cycle arrest; a process called condensation.
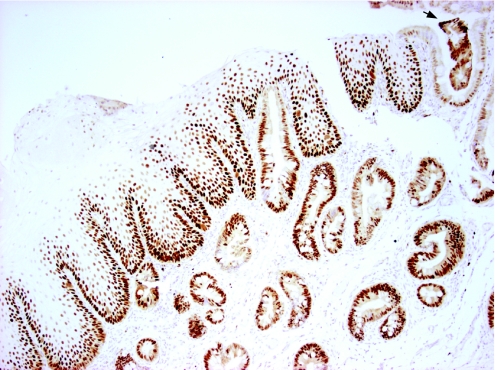
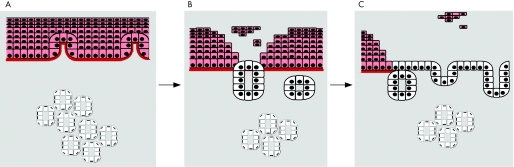
The appearance of glands apparently fusing with the squamous epithelial surface (fig 4) is also seen in native Barrett's oesophagus glandular tissues (fig 12). These observations suggest a hypothetical model for the development of Barrett's metaplasia from the stromal compartment in which an environmental stimulus leads to part of the SE being sloughed. Glands then migrate and fuse with the breached surface. The glands may arise from migration of submucosal glands or from new gland formation secondary to mesenchymal–epithelial transition. As these glands unfold, a new epithelial surface would be formed (fig 13). Alternatively, glands (either from submucosal gland ducts or mesenchymal epithelial transition) that migrate to the basal compartment of the SE may displace squamous stem cells. Therefore, the differentiated squamous cell compartment may become depleted, thus exposing the glands at the luminal surface (fig 13).
Figure 12 Native Barrett's glandular tissues stained with the proliferation protein Minichromosome maintenance protein 2 and counterstained with haematoxylin, demonstrating oesophageal glands fusing with the squamous epithelial surface. A segment of columnar epithelium on the far right contains glandular debris, which could result from the fusion of glands with the new epithelial surface (arrow) (×200).
Figure 13 A hypothetical model for the development of Barrett's metaplasia from the stromal compartment based on the observed effects of retinoic acid (RA) is described here. (A) Intact squamous epithelium (SE) is damaged gastro‐oesophageal reflux disease or affected by an environmental signal (eg, RA), leading to migration of glands or sloughing of SE. (B) The glands were exposed and then fused with breached surface, and as these glands unfold (C), a new epithelial surface is formed.
Collectively, this evidence suggests that the stromal compartment might provide a cell source for the columnar cells induced by RA treatment. We hypothesise that this same process of cell remodelling may be relevant for the development of native Barrett's metaplasia in vivo.
With regards to whether RA is relevant in vivo, we have shown for the first time that LCA, a component of gastro‐oesophageal refluxate, can stimulate RA signalling. LCA is a carcinogenic compound formed via bacterial 7‐dehydroxylation of the primary bile acid, chenodeoxycholic acid,33 which has been implicated in the pathogenesis of Barrett's oesophagus.34 This would provide a potential in vivo mechanism through which RA could be implicated in the induction of Barrett's metaplasia. It is also possible that exogenous RA may be activating the same final common pathway responsible for the induction of Barrett's metaplasia in vivo, which is normally independent of RA. For example, the Cdx2 intestinal homeobox gene, which may be induced by RA, has also been shown to be activated by chronic acid exposure in a long‐term culture of mouse oesophageal keratinocytes9,35 and ectopic Cdx2 expression has been shown to induce intestinal metaplasia in vivo.36 In view of the reversal of columnar differentiation observed with citral, the elucidation of the key pathways involved in the lineage‐specific differentiation pathways will be important for the development of new therapeutic strategies in Barrett's oesophagus and associated carcinogenesis.
Acknowledgements
We are grateful to the staff and patients who participated in endoscopy who made this study possible. Dr Chih‐Long Chang is funded by a scholarship from Mackay Memorial Hospital, Taiwan. This work is supported by the Medical Research Council.
Abbreviations
ATRA - all‐trans retinoic acid
BrdU - bromo‐deoxyuridine
CK - cytokeratin
Ct - cycle threshold
GAPDH - glyceraldehyde‐3‐phosphate dehydrogenase
LCA - lithocholic acid
RA - retinoic acid
RARE - retinoic acid response elements
RT‐PCR - real‐time PCR
SE - squamous epithelium
Footnotes
Competing interests: None.
References
- 1.Murray L, Watson P, Johnston B.et al Risk of adenocarcinoma in Barrett's oesophagus: population based study. BMJ 2003327534–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen N, Ransohoff D F. Gastroesophageal reflux, Barrett's esophagus, and esophageal cancer: scientific review. JAMA 20022871972–1981. [DOI] [PubMed] [Google Scholar]
- 3.Brown L M, Devesa S S. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 200211235–256. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery R K, Mulberg A E, Grand R J. Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology 199916702–731. [DOI] [PubMed] [Google Scholar]
- 5.Tosh D, Slack J M. How cells change their phenotype. Nat Rev Mol Cell Biol 20023187–194. [DOI] [PubMed] [Google Scholar]
- 6.Gronemeyer H, Miturski R. Molecular mechanisms of retinoid action. Cell Mol Biol Lett 200163–52. [PubMed] [Google Scholar]
- 7.Hay E D, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis 199526678–690. [DOI] [PubMed] [Google Scholar]
- 8.Radominska‐Pandya A, Chen G. Photoaffinity labeling of human retinoid X receptor beta (RXRbeta) with 9‐cis‐retinoic acid: identification of phytanic acid, docosahexaenoic acid, and lithocholic acid as ligands for RXRbeta. Biochemistry 2002414883–4890. [DOI] [PubMed] [Google Scholar]
- 9.Houle M, Prinos P, Iulianella A.et al Retinoic acid regulation of Cdx1: an indirect mechanism for retinoids and vertebral specification. Mol Cell Biol 2000206579–6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simeone A, Acampora D, Nigro V.et al Differential regulation by retinoic acid of the homeobox genes of the four HOX loci in human embryonal carcinoma cells. Mech Dev 199133215–227. [DOI] [PubMed] [Google Scholar]
- 11.Huang D, Chen S W, Langston A W.et al A conserved retinoic acid responsive element in the murine Hoxb‐1 gene is required for expression in the developing gut. Development 19981253235–3246. [DOI] [PubMed] [Google Scholar]
- 12.Eda A, Osawa H, Satoh K.et al Aberrant expression of CDX2 in Barrett's epithelium and inflammatory esophageal mucosa. J Gastroenterol 20033814–22. [DOI] [PubMed] [Google Scholar]
- 13.Lord R V, Bradender J, Wickramasinghe K.et al Increased CDX2 and decreased PITX1 homeobox gene expression in Barrett's esophagus and Barrett's‐associated adenocarcinoma. Surgery 2005138924–931. [DOI] [PubMed] [Google Scholar]
- 14.Phillips R W, Frierson H F, Jr, Moskaluk C A. Cdx2 as a marker of epithelial intestinal differentiation in the esophagus. Am J Surg Pathol 2003271442–1447. [DOI] [PubMed] [Google Scholar]
- 15.Adeylotte M B. The effects of vitamin A and citral on epithelial differentiation in vitro. J Embryol Exp Morphol 196311621–635. [PubMed] [Google Scholar]
- 16.Lasnitzki I. The effect of excess vitamin A on the embryonic rat oesophagus in culture. J Exp Med 19631181–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quesenberry P J, Colvin G A, Abedi M.et al Stem cell biology and the plasticity polemic. Exp Hematol 200533389–394. [DOI] [PubMed] [Google Scholar]
- 18.Potter J D. Morphostats: a missing concept in cancer biology. Cancer Epidemiol Biomarkers Prev 200110161–170. [PubMed] [Google Scholar]
- 19.McLoughlin C B. The importance of mesenchymal factors in the differentiation of chick epidermis. J Embryol Exp Morphol 19619370–384. [Google Scholar]
- 20.Seery J P. Stem cells of the oesophageal epithelium. J Cell Sci 20021151783–1789. [DOI] [PubMed] [Google Scholar]
- 21.Takito J, Al‐Awqati Q. Conversion of ES cells to columnar epithelia by hensin and to squamous epithelia by laminin. J Cell Biol 20041661093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald R C, Omary M B, Triadafilopoulos G. Dynamic effects of acid on Barrett's esophagus. An ex vivo proliferation and differentiation model. J Clin Invest 1996982120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krugluger W, Rohrbacher W, Laciak K.et al Reorganization of hair follicles in human skin organ culture induced by cultured human follicle‐derived cells. Exp Dermatol 200514580–585. [DOI] [PubMed] [Google Scholar]
- 24.Palanca‐Wessels M C, Klingelhutz A, Reid B J.et al Extended lifespan of Barrett's esophagus epithelium transduced with the human telomerase catalytic subunit: a useful in vitro model. Carcinogenesis 2003241183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spechler S J, Zeroogian J M, Antonioli D A.et al Prevalence of metaplasia at the gastro‐esophageal junction. Lancet 19943441533–1536. [DOI] [PubMed] [Google Scholar]
- 26.Jankowski J A, Harrison R F, Perry I.et al Barrett's metaplasia. Lancet 20003562079–2085. [DOI] [PubMed] [Google Scholar]
- 27.Gillen P, Keeling P, Byrne P J.et al Implication of duodenogastric reflux in the pathogenesis of Barrett's oesophagus. Br J Surg 198875540–543. [DOI] [PubMed] [Google Scholar]
- 28.Paulson T G, Xu L, Sanchez C.et al Neosquamous epithelium does not typically arise from Barrett's epithelium. Clin Cancer Res 2006121701–1706. [DOI] [PubMed] [Google Scholar]
- 29.Reeves O R, Laskey R A. In vitro differentiation of a homogeneous cell population—the epidermis of Xenopus laevis. J Embryol Exp Morph 19753475–92. [PubMed] [Google Scholar]
- 30.Zecchini V, Domaschenz R, Winton D.et al Notch signaling regulates the differentiation of post‐mitotic intestinal epithelial cells. Genes Dev 2005191686–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birchmeier W, Birchmeier C. Mesenchymal–epithelial transitions. Bioessays 199416305–307. [DOI] [PubMed] [Google Scholar]
- 32.Plateroti M, Freund J N, Leberquier C.et al Mesenchyme‐mediated effects of retinoic acid during rat intestinal development. J Cell Sci 19971101227–1238. [DOI] [PubMed] [Google Scholar]
- 33.Debruyne P R, Bruyneel E A, Karaguni I M.et al Bile acids stimulate invasion and haptotaxis in human colorectal cancer cells through activation of multiple oncogenic signaling pathways. Oncogene 2002216740–6750. [DOI] [PubMed] [Google Scholar]
- 34.Nehra D, Howell P, Williams C P.et al Toxic bile acids in gastro‐oesophageal reflux disease: influence of gastric acidity. Gut 199944598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchetti M, Caliot E, Pringault E. Chronic acid exposure leads to activation of the cdx2 intestinal homeobox gene in a long‐term culture of mouse esophageal keratinocytes. J Cell Sci 20031161429–1436. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto H, Bai Y Q, Yuasa Y. Homeodomain protein CDX2 regulates goblet‐specific MUC2 gene expression. Biochem Biophys Res Commun 2003300813–818. [DOI] [PubMed] [Google Scholar]