Growing evidence suggests that the immune system plays a critical role in all the stages of atherogenesis, from lesion formation to plaque rupture.1 The inflammatory component of atherothrombosis is a topic of intensive research, since it is still unclear whether inflammatory markers measurable in peripheral blood can be useful tools for risk assessment in the general population; it is also unknown whether therapeutic strategies leading to a decrease of these inflammatory markers can modify cardiovascular risk.w1
INFLAMMATORY MECHANISMS IN ATHEROSCLEROSIS
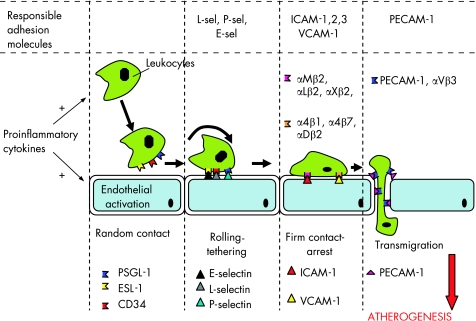
Early in the atherogenesis process, resident or circulating leucocytes bind to the site of a developing lesion in response to oxidised low density lipoprotein cholesterol (LDL‐C), injury, or infection. Proinflammatory cytokines play a central regulatory role in this early stage of atherogenesis, since they induce the migration of these inflammatory cells to the subendothelial space, both by acting directly on these leucocytes and by upregulating the expression of several adhesion molecules (such as vascular cells adhesion molecules, intercellular adhesion molecules and selectins) which participate in leucocyte adhesion, rolling and subendothelial migration (fig 1).w2
Figure 1 Cytokines and adhesion molecules in the early stages of atherogenesis. Circulating leucocytes are activated by proinflammatory cytokines, and they adhere to vascular endothelium. Adhesion molecules, whose expression is also regulated by proinflammatory cytokines, play a key role in the subendothelial migration of leucocytes. ICAM‐1, intercellular adhesion molecule‐1; PECAM‐1, platelet endothelial cell adhesion molecule; Sel, selectin; VCAM‐1, vascular cell adhesion molecule‐1.
As these monocytes accumulate in the subendothelial space, they continue to ingest chemically modified lipids and lipoproteins, they become macrophages and finally develop into foam cells, and initiate fatty streaks. At the same time, other inflammatory cells, such as activated T cells and mast cells, also attach themselves to the endothelium, contributing to the formation of the atheromatous lesion, which consists of a lipid pool covered by a fibrous cap.w3 During the whole process, smooth muscle cells (SMCs) secrete chemoattracting factors that recruit additional monocytes.w4 Local stimulation of SMCs in the artery wall can amplify the inflammatory response and promote a local procoagulant effect.w3 The activation of all these immune cells and SMCs leads to the release of additional mediators, including cytokines, chemokines and growth factors, molecules which lead to further immune activation and trigger further steps in atherogenesis.w5
Interleukin‐6 (IL‐6), interleukin 1b (IL‐1b) and tumour necrosis factor α (TNFα) are the principal pro‐atherogenic cytokines,1 which are also produced in tissues other than the vascular wall and immune system, such as adipose tissue, myocardium, intestine, etc.1 They upregulate the expression of adhesion molecules on vascular endothelium,w2 depress nitric oxide synthesisw6 and promote the subendothelial migration of leucocytes. Further to their local regulatory role at a vascular level, these cytokines induce the liver‐derived synthesis of acute phase proteins, such as fibrinogen, plasminogen, C‐reactive protein (CRP) and serum amyloid α (SAA), which amplify inflammatory and pro‐coagulant responses.w7 w8
Although the role of inflammation is critical in all the stages of atherogenesis, from plaque formation to plaque rupture and the development of acute coronary syndromes (ACS), it is unclear whether measurement of circulating levels of inflammatory molecules can be useful in risk assessment or even in the design of therapeutic approaches against the development and/or progression of coronary atherosclerosis. In this review we examine the clinical importance of the most widely known inflammatory markers, in the assessment of cardiovascular risk.
PROINFLAMMATORY CYTOKINES AS PREDICTORS FOR CARDIOVASCULAR DISEASE
Initial evidence suggested that IL‐6 may be a better predictor of coronary artery disease than CRP,w9 w10 while its effect on the risk for stroke was particularly strong (relative risk (RR) 3.7, 95% confidence interval (CI) 1.67 to 8.21). However, it seems that IL‐6 is also elevated in the presence of subclinical atherosclerosis, and it is hard to distinguish between patients with advanced and subclinical atherosclerosis.w11 The association between subclinical disease and IL‐6 is consistent with data from the Health ABC Study, which showed that both IL‐6 and TNFα are higher in older adults with subclinical cardiovascular disease.w12
Furthermore, in a study by Ridker et alw13 it was found that apparently healthy subjects with IL‐6 at the highest quartile had relative risk for future myocardial infarction, in a 6 year follow up period, 2.3 times higher than those in the lowest quartile (95% CI 1.3 to 4.3), with an increase of risk by 38% for each quartile increase in IL‐6.
Although TNFα is directly implicated in atherogenesis, it has not been frequently measured in epidemiologic studies. In the Health ABC Study, it was moderately correlated with IL‐6 and weakly correlated with CRP,w12 while a stronger relationship between TNFα and coronary heart disease (CHD) than with either IL‐6 or CRP has been reported.w9 Additionally, in a nested case–control study, Ridker et alw14 reported a multivariable‐adjusted relative risk of recurrent coronary events of 2.5 (95% CI 1.3 to 5.1) among men whose TNFα levels exceeded the 95th centile, as compared with men with lower levels. However, TNFα has a limited half‐life and is difficult to measure in large‐scale epidemiologic studies,w15 and this is the main reason for the observed lack of data from prospective studies about the predictive role of TNFα in clinical practice.
Alternatively, the soluble forms of TNF receptors 1 (sTNF‐R1) and 2 (sTNF‐R2) levels can be measured; they may be of greater significance than direct measurement of TNFα, and they can be measured with greater sensitivity and reliability.w15 The soluble TNF receptors may attenuate the bioactivity of TNFα but may also serve as slow‐release reservoirs and promote inflammation in the absence of free TNF ligand.w16 Only a few studies have examined the relationship between levels of sTNF‐R1/sTNFR2 and the risk of CHD.2 w9 w17 However, recent evidence suggested that measurement of these receptors is a rather weak approach to predicting cardiovascular risk compared to classic inflammatory markers, such as CRP or IL‐6.w18
Another cytokine with a potential interest for future clinical research, but with limited clinical data currently available, is IL‐10. IL‐10 is an anti‐inflammatory cytokine that inhibits the production of a variety of inflammatory cytokines such as IL‐2, TNFα and IFNγ, and it is strongly associated with better prognosis in patients with ACS.w10 w20 However, IL‐10 has not been evaluated in any epidemiologic study, and its clinical use is still obscure.
ACUTE PHASE PROTEINS
C‐reactive protein (CRP)
CRP is a widely known acute phase protein, produced by the liver in response to proinflammatory cytokines, and especially IL‐6, TNFα and IL‐1b.w8 Although it is a non‐specific inflammatory marker, it appears to be a stronger predictor of cardiovascular risk compared to most of the other known circulating inflammatory molecules.w21
At a clinical level, measurement of CRP in healthy individuals predicts the future development of coronary artery disease (tables 1 and 2). However, in the Thrombogenic Risk Factor (THROMBO) study, CRP was not a predictor of risk after adjustment for important predictors of prognosis, such as left ventricular ejection fraction and the presence of pulmonary congestion.w22 The potential association between CRP and cardiovascular prognosis was first illustrated in patients presenting with ACS.w23 The Thrombolysis In Myocardial Infarction (TIMI) investigators have since shown that the increased risk associated with high CRP values may be evident as early as 14 days after presentation with an ACS.w24 The CAPTUREw25 trial investigators found that, although only troponin T was predictive in the initial 72 h period, both CRP and troponin T were independent predictors of risk at 6 months, while the FRISCw26 investigators reported that the risk associated with elevated CRP values at the time of index event continues to increase for several years. In each of the above studies, the predictive value of CRP was independent of, and additive to, troponin levels. Most importantly, CRP has been found to have prognostic value among patients without evidence of myocyte necrosis; specifically, even among patients with negative troponin T, an elevated CRP is predictive of future adverse events.w24–26
Table 1 Inflammatory markers in coronary atherosclerosis: available meta‐analyses of long‐term prospective studies in healthy individuals.
| Marker | Meta‐analysis available | Cases | Relative risk (95% CI) |
|---|---|---|---|
| Cytokines/receptors | |||
| IL‐6 | None available | – | – |
| TNFα | None available | – | – |
| TNFα R1 | None available | – | – |
| TNFα R2 | None available | – | – |
| Acute phase proteins | |||
| Fibrinogen | Danesh et al4 | 4018 | 1.8 (1.6 to 2.0)* |
| FSC5 | 7213 | 1.8 (1.6 to 2.0)* | |
| Danesh et al11 | 154211 | 2.4 (2.2 to 2.6)† | |
| CRP | Danesh et al6 | 7068 | 1.49 (1.37 to 1.62)* |
| SAA | Danesh et al12 | 1057 | 1.6 (1.1 to 2.2)* |
| Adhesion molecules | |||
| sVCAM‐1 | Malik et al7 | 1307 | 1.02 (0.81 to 1.29)* |
| sICAM‐1 | Malik et al7 | 1192 | 1.21 (0.95 to 1.55)* |
| sE‐selectin | Malik et al7 | 832 | 1.16 (0.87 to 1.55)* |
| sP‐selectin | Malik et al7 | 627 | 1.18 (0.63 to 2.21)* |
CI, confidence interval; CRP, C‐reactive protein; IL‐6, interleukin‐6; SAA, serum amyloid α; sICAM‐1, soluble intercellular adhesion molecule‐1; sVCAM‐1, soluble vascular cells adhesion molecule‐1; TNFα, tumour necrosis factor α.
*Odds ratio of top third vs bottom third of marker.
†Hazard ratio per 1g/litre increase in usual fibrinogen value.
Table 2 C‐reactive protein (CRP) and cardiovascular risk in healthy individuals.
| Study | End point | Relative risk (95% CI) |
|---|---|---|
| Tracy et al13 | Myocardial infarction | 2.67 (1.04 to 6.81) |
| Ridker et al14 | Myocardial infarction | 2.9 (1.8 to 4.6) |
| Ridker et al14 | Stroke | 1.9 (1.1 to 3.3) |
| Ridker et al15 | Peripheral vascular disease | 2.2 (1.1 to 4.8) |
| Ridker et al16 | Any vascular event | 4.8 (2.3 to 10.1) |
| Ridker et al16 | Myocardial infarction or stroke | 7.3 (2.7 to 19.9 |
| Roivainen et al17 | Coronary heart disease | 3.56 (1.93 to 6.57) |
| Ridker et al2 | Cardiovascular events | 4.4 (2.2 to 8.9) |
| Rost et al18 | Stroke | 1.9 (1.1 to 3.3) |
| Lowe et al19 | Ischaemic heart disease | 2.73 (1.6 to 4.7) |
| Ridker et al20 | Cardiovascular disease | 3.6 (2.5 to 5.2) |
| Danesh et al6 | Coronary heart disease | 2.13 (1.38 to 3.28) |
| Boekholdt et al21 | Coronary artery disease | 2.49 (2.02 to 3.08) |
| Fatal | 2.92 (1.83 to 4.67) | |
| Non‐fatal | 1.25 (0.93 to 1.66) | |
| Ridker et al22 | Coronary artery disease | 2.98 (1.90 to 4.67) |
Measuring and evaluating CRP
On the basis of all these data, the US Centers for Disease Control and Prevention and the American Heart Association issued guidelines in 2003 for the use of high sensitivity CRP (hsCRP) in clinical practice (table 3).3 Subjects are defined as low risk if CRP is <1.0 mg/litre, average risk if CRP is 1.0–3.0 mg/litre, and high risk if CRP is >3.0 mg/litre; however, high CRP values may be associated with an inflammatory disease, and this possibility should be taken into account when evaluating CRP values.
Table 3 Statement from the US Centers for Disease Control and Prevention/American Heart Association (CDC/AHA) on the measurement of C‐reactive protein, 20033.
| 1. hsCRP assay is the assay of choice and should be performed in metabolically stable persons without obvious inflammatory or infectious diseases |
| 2. The results should be expressed in mg/litre and two assays, averaged, fasting or non‐fasting, 2 weeks apart (in this way, they represent the inflammatory status better) |
| 3. The adult population should be stratified in three tertiles, at different cardiovascular risk |
| 4. Subjects at the highest tertile have about a twofold increased risk of future cardiovascular disease compared with those in the lower tertile |
| 5. Subjects with moderate risk (10–20% risk of CHD over 10 years) may benefit from measurement of CRP in addition to traditional cardiovascular risk factors |
CHD, coronary heart disease; hsCRP: high sensitivity C‐reactive protein.
CRP is easily and inexpensively measured, and standardised high sensitivity assays are commercially available.w27 w28 These assays provide similar results in fresh or frozen plasma, while the predictive value of hsCRP measurement may be further improved by repeating several serial measurements.w29
Fibrinogen
Further to its role as an acute phase protein, fibrinogen plays a pivotal role in coagulation mechanisms, as the substrate for thrombin, while there is evidence that it may be involved in multiple mechanisms at all stages of the atherothrombotic process.w30
At a clinical level, the first meta‐analysis4 examining the role of plasma fibrinogen as a risk factor for CHD in 4018 subjects, showed a risk of 1.8 for the top third (>350 mg/dl) versus the bottom third (<250 mg/dl), a finding confirmed in another meta‐analysis in 7213 cases with CHD (table 1).5
Although the direct involvement of acute phase proteins in the progression of coronary atherosclerosis needs to be documented, it is likely that acute phase proteins may be markers reflecting the levels of proinflammatory cytokines, in a more reliable way than the direct measurement of these cytokines in plasma, since they are less variable throughout the day.
Serum amyloid α (SAA)
SAA is an amphipathic, α‐helical apolipoprotein that is transported in the circulation primarily in association with high density lipoprotein (HDL),w31 and like CRP and fibrinogen, is an acute phase protein. SAA can induce the expression of proteinases thought to degrade extracellular matrix,w32 which might be important during tissue injury. In several observational and prospective studies, the risk of cardiovascular disease associated with SAA changed in parallel with that seen with CRP,2w33 although the absolute level of risk was generally smaller. Moreover, elevated plasma SAA is observed in the presence of risk factors such as obesity,w34 insulin resistance,w35 the metabolic syndrome,w36 and diabetes.w35 A recent meta‐analysis6 suggests that subjects with SAA at higher tertile have a significant increase in cardiovascular risk up to 1.49 (95% CI 1.37 to1.62), although its use in clinical practice is still controversial. Although additional studies are needed, these observations raise the possibility that SAA may serve as a marker for an increased risk of cardiovascular disease in humans.
SOLUBLE FORMS OF ADHESION MOLECULES
Cellular adhesion molecules are molecules expressed on the surface of cells that mediate the adhesion of the cell to other cells or to the extracellular matrix.w2 A number of adhesion molecules expressed mainly on endothelial cells, leucocytes and platelets are actively involved in atherogenesis (table 4). The expression of these molecules is upregulated on the surface of these cells under the stimulatory effect of proinflammatory cytokines,w2 and they play a key role in atherogenesis (fig 1).
Table 4 Adhesion molecules in atherosclerosisw2.
| Adhesion molecule | Ligands | Role | Comments | Clinical predictive value of soluble form |
|---|---|---|---|---|
| Selectins | ||||
| P‐selectin | CD24, PSGL‐1, Lewis X | Rolling and tethering | Present on endothelial cells and platelets. ELISA kits for soluble forms commercially available | Weak predictor in healthy individuals, high risk patients and patients with CAD, moderate predictor for patients with acute coronary events |
| E‐selectin | L‐set, PSGL‐1, ESL‐1, Lewis X | Rolling and tethering | Present in endothelial cells. ELISA kits for soluble form commercially available | Weak predictor in healthy individuals, high‐risk subjects, and patients with CAD or ACS |
| L‐selectin | CD34, PSGL‐1, Lewis X, GlyCAM | Rolling and tethering | Present in leucocytes. ELISA kit for soluble form commercially available | – |
| Immunoglobulin‐like molecules | ||||
| ICAM‐1 | αMβ2, αLβ2, αXβ2 | Present in endothelial cells and leucocytes. ELISA kits for soluble‐form commercially available | Moderate predictor for healthy individuals, high‐risk subjects and CAD patients. Weak predictive value for ACS | |
| Firm adhesion | ||||
| ICAM‐2 | αMβ2, αLβ2 | Firm adhesion | Present in endothelial cells, leucocytes and platelets. ELISA kits for soluble form commercially available | – |
| ICAM‐3 | αLβ2, αDβ2, DC‐SIGN | Firm adhesion | Present in endothelial cells and leucocytes. ELISA kits for soluble form commercially available | – |
| VCAM‐1 | α4β1, α4β7, αDβ2 | Firm adhesion | Present in endothelial cells. ELISA kits for soluble form commercially available | No predictive value for healthy individuals. Strong predictor for high risk subjects, CAD patients and patients with ACS |
| PECAM‐1 | PECAM1, αVβ3 | Leucocyte extravasation | Maintains endothelial integrity. Present in endothelial cells, leucocytes and platelets. ELISA kits for soluble forms commercially available | – |
ACS, acute coronary syndromes; CAD, coronary artery disease; ELISA, enzyme‐linked immunosorbent assay; ICAM, intercellular adhesion molecule; PECAM, platelet endothelial cell adhesion molecule; VCAM, vascular cells adhesion molecule.
Adhesion molecules and risk prediction in coronary atherosclerosis
Since the expression of both acute phase proteins and adhesion molecules is largely regulated by proinflammatory cytokines, it is expected that the soluble forms of adhesion molecules (such as sICAM‐l) correlate with acute phase reactants like CRP, and they may provide similar predictive information to CRP in settings of primary prevention.w37 In large prospective studies, sICAM‐l, but not sVCAM‐1, has been consistently related to incident coronary artery disease (CAD), at least in healthy populations (table 1).w37–40 Similarly, data from the Women's Health Study indicated an association between sICAM‐1 values and a combined cardiovascular end point,2 although after controlling for classical risk factors and other inflammatory biomarkers such as CRP, IL‐6, and SAA, this association lost significance. Therefore, sICAM‐l appears to be a general marker of pro‐inflammatory status, and it is expressed in healthy individuals under stable clinical conditions.
Several studies have assessed the prognostic role of soluble adhesion molecules in a setting of secondary prevention. In the AtheroGene study, a prospective cohort of patients with established CAD, sVCAM‐1 was identified as a strong independent predictor of future fatal cardiovascular events, independently of most potential confounders, especially other inflammatory markers including hsCRP.w41 In the Bezafibrate Infarction Prevention (BIP) study based on patients with CAD, baseline sICAM‐1 concentration was an independent predictor for future coronary eventsw42 and stroke.w43 Similarly, in CAD patients and high‐risk subjects (for example, diabetic patients), sVCAM‐1 appears to be a strong predictor of cardiovascular mortality.w44
Only few data are available about the relationship between selectin levels and extent of atherosclerosis or their use for cardiovascular risk assessment. The first evidence, summarised in a recent meta‐analysis,7 suggests that soluble selectin values may be predictors of cardiovascular disease in healthy populations; however, these data are based on small numbers of patients, and further studies are needed to establish their role in risk assessment in the general population.
OTHER NEW INFLAMMATORY MARKERS
sCD40L
CD40 ligand is a transmembrane protein related to TNFα.w45 A variety of cells involved in atherosclerosis, such as endothelial cells, smooth muscle cells, macrophages, T lymphocytes and platelets, express CD40L.w46 Evidence suggests that sCD40L is a strong predictor for clinical outcome in patients with ACS,w20 w47 w48 while high sCD40L was correlated with late restenosis after coronary angioplasty. w49 Although the first results from risk assessment studies were promising, there is still lack of sufficient data about the clinical importance of sCD40L as a predictive marker for coronary atherosclerosis, and further studies are needed to establish its role in the clinical setting.
Lipoprotein‐associated phospholipase A2 (Lp‐PLA2)
Lp‐PLA2 is an enzyme distinct from secretory PLA2 and is transported primarily in LDL.w50 Lp‐PLA2 is secreted by cells of the monocyte–macrophage series, T lymphocytes and mast cells,w51 and hydrolyses oxidised phospholipids, generating lysophosphatidylcholine which upregulates adhesion molecule expression.w51 Recent evidencew52 suggests that Lp‐PLA2 is associated with coronary endothelial dysfunction, implying that it could be used as a predictor for cardiovascular risk.
Clinical evidence suggests that elevated Lp‐PLA2 values are associated with increased risk of CHD and stroke, independently of lipid values (table 5).8,9w50 This association was shown in men enrolled in the West of Scotland Coronary Prevention Study (WOSCOPS), among whom Lp‐PLA2 values in the highest quintile were independently associated with a twofold increase in risk compared with those in the lowest quintile.8 In the Women's Health Study, the baseline Lp‐PLA2 value was significantly higher in women who had a cardiovascular event during a mean 3 year follow up (1.20 mg/litre vs 1.05 mg/litre in controls); however, after adjustment for other cardiovascular risk factors, the relative risk in the highest versus the lowest quartile of Lp‐PLA2 was not statistically significant.9 Recent evidence also suggests that Lp‐PLA2 and CRP have additive effects to increase risk for cardiovascular events. In the ARIC studyw53 which included 12 819 men and women free of CHD at baseline, both Lp‐PLA2 and CRP were associated with increased risk for CHD over a 6 year follow up period. In the presence of low LDL‐C, CHD risk was three times greater for individuals with both Lp‐PLA2 and hsCRP in the highest versus the lowest category.10w28
Table 5 Lp‐PLA2 and cardiovascular risk.
| Study | Measure | Relative risk (95% CI) |
|---|---|---|
| Kardys et al23 | Measures of extracoronary atherosclerosis | 1.86 (1.01 to 3.43) in men |
| 1.60 (1.08 to 2.37) in women | ||
| Ballantyne et al24 | Ischaemic stroke | 1.91 (1.15 to 3.18)† |
| Khuseyinova et al25 | Coronary artery disease | 2.04 (1.19 to 3.48)* |
| Oei et al26 | CHD | 1.97 (1.28 to 3.02)* |
| Stroke | 1.97 (1.03 to 3.79)* | |
| Packard et al8 | CHD, death, myocardial infarction, revascularisation | 1.18 (1.05 to 1.33)‡ |
| Blake et al9 | CHD, death, myocardial infarction, stroke | 1.17 (0.45 to 3.05)* |
| Koening et al27 | CHD, death, myocardial infarction | 1.21 (1.01 to 1.45)‡ |
| Ballantyne et al10 | CHD, death, myocardial infarction, revascularisation | 1.15 (0.81 to 1.63)† |
CHD, coronary heart disease.
*Lowest quartile as reference; †lowest tertile as reference; ‡increase by 1SD of Lp‐PLA2 activity.
Inflammatory markers in atherosclerosis: key points
Inflammation is a key element of atherogenesis
Proinflammatory cytokines, adhesion molecules and acute phase proteins are the main inflammatory markers measured in humans
C‐reactive protein is the strongest inflammatory predictor for atherosclerosis
Fibrinogen and serum amyloid α have been associated with atherosclerosis, but their predictive role is still controversial
Among adhesion molecules, the soluble form of sICAM‐1 is a predictor of cardiovascular risk in healthy individuals, and sVCAM‐1 in high‐risk patients
Lp‐PLA2 and sCD40L are new promising inflammatory markers with predictive value in cardiovascular disease, but their clinical usefulness is still under investigation
Myeloperoxidase (MPO)
MPO is an abundant enzyme stored in the primary granules of neutrophils and monocytes. MPO has been demonstrated in human atherosclerotic lesions and has been implicated as a catalyst for LDL oxidation, leading to increased uptake and foam cell formation,w21 and may also contribute to endothelial dysfunction. MPO values may be elevated among individuals with CAD.w53 Increasing concentrations of leucocyte‐MPO and blood‐MPO were significant predictors of the risk for CAD.11 After adjustment for white blood cell count and Framingham risk score, individuals in the highest quartile of blood‐MPO had a 20‐fold higher risk of CAD than individuals in the lowest quartile.w53 However, further to these initial reports, there is a lack of prospective studies examining its long‐term predictive value in cardiovascular risk.
CONCLUSIONS
The role of inflammation in atherogenesis is now well established. Although a large variety of inflammatory markers participating at several stages of atherogenesis can be measured in plasma or serum, only some of them seem to be clinically important (table 6). The acute phase proteins such as CRP and fibrinogen as well as SAA are the most well studied inflammatory markers, which can be used for cardiovascular risk assessment in the general population. Among the soluble forms of adhesion molecules, sICAM‐1 is a promising inflammatory marker which can predict cardiovascular risk in healthy individuals, while measurement of sVCAM‐1 is more useful for risk assessment in high‐risk subjects and in patients with advanced atherosclerosis. Newer inflammatory markers such as sCD40L, Lp‐PLA2, or MPO also seem promising for the prediction of cardiovascular risk, but further studies are needed to establish their role in cardiovascular disease.
Table 6 Comparison of common inflammatory markers in cardiovascular risk assessment.
| Marker | Advantage | Disadvantage |
|---|---|---|
| Cytokines | ||
| IL‐6 | Direct implication in atherogenesis. It presents rapid variations during acute coronary events | Large daily/seasonal variability. Measurement relatively expensive |
| Hard to distinguish between patients with advanced and subclinical atherosclerosis | ||
| TNFα | Direct involvement in atherogenesis. Probably stronger association with coronary artery disease than IL‐6 | Limited half‐life, difficult to measure in large‐scale epidemiologic studies. Not routinely measured in prospective studies. Lack of sufficient epidemiologic data about its role. Expensive to measure |
| sTNF‐R1 and sTNF‐R2 | Alternative measures to TNFα. Measured with greater sensitivity and reliability than TNFα | Very few data about their predictive role. The first reports show that they are weaker predictors for cardiovascular disease than IL‐6 or CRP. Expensive to measure |
| Acute phase proteins | ||
| CRP | Easy and cheap to measure. Probably the most well established inflammatory marker in cardiovascular risk assessment. Long half‐life, therefore less circadian variability than IL‐6 and less seasonal variability that fibrinogen | Its direct involvement in atherogenesis is not clear. It reflects cytokine expression only in the presence of sufficient liver biosynthetic activity. It is a non‐specific marker of inflammation |
| Fibrinogen | Direct involvement in atherothrombosis. Predictive value comparable to that of CRP. Relatively cheap to measure on a routine basis | Relatively high seasonal variability. Affected by liver biosynthetic ability. It is a non‐specific marker of inflammation |
| SAA | Its variation is in parallel with that of CRP. Elevated in the presence of risk factors such as obesity, insulin resistance, metabolic syndrome and diabetes | No specific advantage over CRP or fibrinogen. The absolute level of risk for subjects with high SAA is generally smaller that that of CRP |
| Adhesion molecules | ||
| sICAM‐1 | Good predictor for healthy individuals, high‐risk subjects and CAD patients. Value comparable to that of CRP. Direct involvement in atherogenesis | Weak predictive value for ACS. Expensive to measure. Not enough data from large population‐based studies |
| sVCAM‐1 | Strong predictor for high risk subjects, CAD patients and patients with ACS. Direct involvement in atherogenesis | No predictive value for healthy individuals. Expensive to measure. Not enough data from large population‐based studies |
| sP‐selectin | Direct involvement in atherothrombosis. Marker of platelet activation. Rapidly increased in ACS | Not enough data for its role as cardiovascular risk factor. Expensive to measure |
| sE‐selectin | Direct involvement in atherogenesis. Marker of endothelial function | Not enough data for its role as cardiovascular risk factor. Expensive to measure |
| Others | ||
| sCD40L | Important pathophysiologic role. Strong predictor for clinical outcome in patients with ACS. High sCD40L is correlated with late restenosis after PTCA. Marker of platelet activation | Relatively new marker. Not enough data for its clinical significance |
| Lp‐PLA2 | Direct involvement in atherogenesis. Useful to identify patients at increased risk for stroke or CHD, including those who have “normal” LDL‐C (<130 mg/dl, <3.4 mmol/l) and are not targeted for drug treatment by the current guidelines. In healthy subjects with moderately raised total cholesterol, Lp‐PLA2 and CRP seem to have additive role in their ability to predict risk of CHD. Very promising marker, when combined with measurement of CRP | Further studies are needed to establish its role, especially in combination with CRP |
| MPO | Role in LDL oxidation. Increased in CAD | No evidence for its role in cardiovascular risk assessment |
CAD, coronary artery disease; CRP, C‐reactive protein; IL‐6, interleukin‐6; LDL, low density lipoprotein; Lp‐PLA2, lipoprotein‐associated phospholipase A2; MPO, myeloperoxidase; SAA, serum amyloid α; sCD40L, soluble CD40‐ligand; sICAM, soluble intercellular adhesion molecule; sVCAM, soluble vascular cell adhesion molecule; TNFα, tumour necrosis factor α.
Additional references appear on the Heart website— http://heart.bmj.com/supplemental
INTERACTIVE MULTIPLE CHOICE QUESTIONS
This Education in Heart article has an accompanying series of six EBAC accredited multiple choice questions (MCQs).
To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group.
If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one‐time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
Supplementary Material
Footnotes
In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article
Additional references appear on the Heart website—http://heart.bmj.com/supplemental
References
- 1.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999340115–126.This is a key review article, providing the most important steps of atherogenesis and focusing on the impact of inflammation in the whole process. [DOI] [PubMed] [Google Scholar]
- 2.Ridker P M, Hennekens C H, Buring J E.et al C‐Reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000342836–843.This is one of the first studies providing strong data about the role of CRP in the prediction of cardiovascular risk. [DOI] [PubMed] [Google Scholar]
- 3.Pearson T A, Mensah G A, Alexander R W.et al Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003107499–511.This article provides a good summary of the inflammatory markers implicated in atherogenesis, explaining their predictive value in cardiovascular disease, in the form of a statement from the American Heart Association. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J, Collins R, Appleby P.et al Association of fibrinogen, C‐reactive protein, albumin, or leukocyte count with coronary heart disease: meta‐analyses of prospective studies. JAMA 19982791477–1482. [DOI] [PubMed] [Google Scholar]
- 5.Anon Collaborative meta‐analysis of prospective studies of plasma fibrinogen and cardiovascular disease. Eur J Cardiovasc Prev Rehabil 2004119–17.This is a key meta‐analysis examining the predictive role of fibrinogen, as a risk factor for cardiovascular disease. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Wheeler J G, Hirschfield G M.et al C‐reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 20043501387–1397. [DOI] [PubMed] [Google Scholar]
- 7.Malik I, Danesh J, Whincup P.et al Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta‐analysis. Lancet 2001358971–976. [DOI] [PubMed] [Google Scholar]
- 8.Packard C J, O'Reilly D S J, Caslake M J.et al Lipoprotein‐associated phospholipase A2 as an independent predictor of coronary heart disease. N Engl J Med 20003431148–1155. [DOI] [PubMed] [Google Scholar]
- 9.Blake G J, Dada N, Fox J C.et al A prospective evaluation of lipoprotein‐associated phospholipase A2 levels and the risk of future cardiovascular events in women. J Am Coll Cardiol 2001381302. [DOI] [PubMed] [Google Scholar]
- 10.Ballantyne C M, Hoogeveen R C, Bang H.et al Lipoprotein‐associated phospholipase A2, high‐sensitivity C‐reactive protein, and risk for incident coronary heart disease in middle‐aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2004109837–842. [DOI] [PubMed] [Google Scholar]
- 11.Danesh J, Lewington S, Thompson S G.et al Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta‐analysis. JAMA 20052941799–1809. [DOI] [PubMed] [Google Scholar]
- 12.Danesh J, Whincup P, Walker M.et al Low grade inflammation and coronary heart disease: prospective study and updated meta‐analyses. BMJ 2000321199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tracy R P, Lemaitre R N, Psaty B M.et al Relationship of C‐reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol 1997171121–1127. [DOI] [PubMed] [Google Scholar]
- 14.Ridker P M, Cushman M, Stampfer M J.et al Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997336973–979. [DOI] [PubMed] [Google Scholar]
- 15.Ridker P M, Cushman M, Stampfer M J.et al Plasma concentration of C‐reactive protein and risk of developing peripheral vascular disease. Circulation 199897425–428. [DOI] [PubMed] [Google Scholar]
- 16.Ridker P M, Buring J E, Shih J.et al Prospective study of C‐reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 199898731–733. [DOI] [PubMed] [Google Scholar]
- 17.Roivainen M, Viik‐Kajander M, Palosuo T.et al Infections, inflammation, and the risk of coronary heart disease. Circulation 2000101252–257. [DOI] [PubMed] [Google Scholar]
- 18.Rost N S, Wolf P A, Kase C S.et al Plasma concentration of C‐reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke 2001322575–2579. [DOI] [PubMed] [Google Scholar]
- 19.Lowe G D, Yarnell J W, Rumley A.et al C‐reactive protein, fibrin D‐dimer, and incident ischemic heart disease in the Speedwell study: are inflammation and fibrin turnover linked in pathogenesis? Arterioscler Thromb Vasc Biol 200121603–610. [DOI] [PubMed] [Google Scholar]
- 20.Ridker P M, Rifai N, Rose L.et al Comparison of C‐reactive protein and low‐density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 20023471557–1565. [DOI] [PubMed] [Google Scholar]
- 21.Boekholdt S M, Hack C E, Sandhu M S.et al C‐reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC‐Norfolk prospective population study 1993–2003. Atherosclerosis 2006187415–422. [DOI] [PubMed] [Google Scholar]
- 22.Ridker P M, Rifai N, Cook N R.et al Non‐HDL cholesterol, apolipoproteins A‐I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA 2005294326–333. [DOI] [PubMed] [Google Scholar]
- 23.Kardys I, Oei H ‐ H S, van der Meer I M.et al Lipoprotein‐associated phospholipase A2 and measures of extracoronary atherosclerosis. The Rotterdam Study. Arterioscler Thromb Vasc Biol 200526631–636. [DOI] [PubMed] [Google Scholar]
- 24.Ballantyne C M, Hoogeveen R C, Bang H.et al Lipoprotein‐associated phospholipase A2, high‐sensitivity C‐reactive protein, and risk for incident ischemic stroke in middle‐aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med 20051652479–2484. [DOI] [PubMed] [Google Scholar]
- 25.Khuseyinova N, Imhof A, Rothenbacher D.et al Association between Lp‐PLA2 and coronary artery disease: focus on its relationship with lipoproteins and markers of inflammation and hemostasis. Atherosclerosis 2005182181–188. [DOI] [PubMed] [Google Scholar]
- 26.Oei H H, van der Meer I M, Hofman A.et al Lipoprotein‐associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation 2005111570–575. [DOI] [PubMed] [Google Scholar]
- 27.Koenig W, Khuseyinova N, Lowel H.et al Lipoprotein‐associated phospholipase A2 adds to risk prediction of incident coronary events by C‐reactive protein in apparently healthy middle‐aged men from the general population: results from the 14‐year follow‐up of a large cohort from southern Germany. Circulation 20041101903–1908.This is one of the most important studies examining the additive role of Lp‐PLA2 and CRP in the prediction of the risk for coronary artery events in the general population. This is one of the prospective studies with the longer follow‐up (14 years in total). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.