Abstract
Objective
To compare the effectiveness of statins of different treatment intensity used to treat elderly patients with acute coronary syndrome (ACS) in typical care settings.
Design
Retrospective cohort study using linked hospital and pharmacy claims data.
Setting
Statewide pharmacy benefits programmes in Pennsylvania and New Jersey.
Participants
18 311 Medicare patients discharged alive after ACS who received a prescription for a statin within 90 days of hospital discharge.
Main outcome measures
Using multivariable and propensity‐matched Cox proportional hazards regression models, patients who were prescribed high‐intensity and moderate‐intensity statins were compared based on the drug–dose combination that they initially received. Individual drug–dose combinations were also compared. Our primary outcome was the composite of all‐cause death or recurrent ACS.
Results
Patients who received moderate‐intensity statins were as likely to experience a primary outcome as patients treated with high‐intensity statins (adjusted HR 1.02, 95% CI 0.96 to 1.08). Propensity matching did not change the results. Individually, all moderate‐intensity statins were as effective as high‐intensity atorvastatin with the exception of lovastatin (adjusted HR 1.22, 95% CI 1.09 to 1.36). Similarly, all high‐intensity statins seem as effective as high‐intensity atorvastatin but the CIs surrounding these estimates were wide.
Conclusions
This analysis of elderly patients with ACS treated in typical care settings does not demonstrate the superiority of high‐intensity over moderate‐intensity statin treatment or significant differences among individual statins.
The use of statins for the prevention of ischaemic events in patients with coronary artery disease has been extensively evaluated. For patients with stable coronary artery disease, placebo‐controlled trials have demonstrated the benefit of moderate‐intensity statins (ie, statins at doses that would be expected to lower low‐density lipoprotein (LDL)‐cholesterol (LDL‐C) levels by 30–40%)1,2 and comparative trials have shown that high‐intensity statins (ie, statins at doses that would be expected to lower LDL‐C levels by >40%) provide even more benefit.3,4 In patients with acute coronary syndromes (ACSs), early treatment with moderate‐intensity statins offers no short‐term benefit compared with placebo,5,6 but high‐intensity atorvastatin is superior to moderate‐intensity pravastatin7 and placebo.8 Another trial comparing early intensive simvastatin (40 mg daily for 1 month followed by 80 mg daily) with delayed conservative simvastatin (placebo for 4 months followed by 20 mg daily) found less clear but generally supportive results.9
A “lower is better” cholesterol‐lowering strategy has been widely advocated and incorporated into treatment guidelines for patients with ACS from the National Cholesterol Education Program10 and other professional organisations.11 Although these guidelines recommend target LDL‐C levels rather than specific drugs or doses, others suggest that the use of high‐intensity statins, regardless of cholesterol level, would be a more evidence‐based approach.12 In keeping with this, atorvastatin has become the dominant statin used to treat patients with ACS in actual practice.13
We sought to address two unresolved issues regarding the appropriate use of statins. First, frail elderly patients with ACS, who face the largest burden of cardiovascular disease, are typically under‐represented in clinical trials. Thus, although older patients clearly benefit from achieving optimal LDL‐C levels,14 it is unclear whether they derive the same benefit from high‐intensity (vs moderate‐intensity) statin treatment as their younger counterparts. In fact, high‐intensity atorvastatin was not superior to moderate‐intensity pravastatin among patients aged ⩾65 years enrolled in the Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis in Myocardial Infarction 22 (PROVE IT‐TIMI 22) Trial.7
Second, although statins are considered to be members of one therapeutic class and largely interchangeable,15 they do differ with respect to metabolism, excretion, half‐life and cholesterol‐lowering effects.16 Therefore, statins of equivalent cholesterol‐lowering intensity may have different clinical effects. This may be particularly relevant for high‐intensity statins, given the differences in cost.
Methods
Setting and design
We assembled a retrospective cohort of Medicare patients who were prescribed statins after being discharged from hospital after an ACS between 1 January 1997 and 30 September 2004 by linking Medicare files to data from the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE)17 and the New Jersey Pharmaceutical Assistance to the Aged and Disabled (PAAD)18 programmes. Both PACE and PAAD provide prescription drug benefits to lower middle‐income individuals aged ⩾65 years, whose yearly earnings are above the threshold to qualify them for Medicaid. Participants pay co‐payments between US$5 (£2.53, €3.74) and US$10 (£5.06, €7.483) per prescription without any deductibles. The programmes cover all medications that require a prescription and do not restrict which medications can be prescribed (ie, the programmes do not use formularies, preferred drug lists or prior authorisation).
We incorporated data from PACE and PAAD into a relational database consisting of data for all filled prescriptions, procedures, physician encounters, hospitalisations, long‐term care admissions and deaths for the patients in our cohort. These data sources have been used extensively to study population‐based health outcomes.19,20,21 All traceable person‐specific identifying factors were transformed into anonymous, coded study numbers to protect subjects' privacy.
Cohort
We included all patients discharged alive from hospital after an ACS (International classification of diseases—ninth revision 410.x or 411.x) during the study period, who filled a prescription for a statin within 90 days of hospital discharge. This time window was chosen to permit sufficient time for patients to fill prescribed prescriptions after hospital discharge and, therefore, to minimise statin misclassification. We excluded patients who were not active users of Medicare and either drug benefit programme during the 1‐year period before their ACS hospitalisation. In specific, patients were excluded if they did not fill ⩾1 prescription and have ⩾1 healthcare encounter in each of the two 6‐month periods before their ACS hospitalisation. We also excluded patients who received prescriptions for cerevastatin, as this drug was withdrawn from the market. The date of the first statin prescribed after discharge was considered as the index date for our analysis.
Statin classification
Patients were classified into drug and dose categories based on the first statin prescription that they filled after hospital discharge. “High‐intensity” doses were those that would be expected to lower LDL‐C levels by >40% (table 1).10,22 Subsequent to this first prescription, we assessed whether patients changed medication doses, switched statins or discontinued treatment. We classified patients as having discontinued treatment if they did not fill another prescription for the same medication within 150% of the days of medication supplied from their previous prescription. For example, if a patient was prescribed 30 days of medication, they were assumed to have discontinued this drug if they did not re‐fill their prescription within 45 days from the date of their initial prescription. If a patient filled a new prescription before the number of days of medication prescribed from their last prescription had elapsed, then we carried forward the extra days supplied to the patient's next prescription.
Table 1 Drug–dose classification of statins based on daily dose prescribed.
| Statin | High‐intensity daily doses* (mg) | Moderate‐intensity daily doses (mg) |
|---|---|---|
| Atorvastatin | >10 | ⩽10 |
| Fluvastatin | † | Any dose |
| Lovastatin | >40 | ⩽40 |
| Pravastatin | † | Any dose |
| Rosuvastatin | >5 | ⩽5 |
| Simvastatin | >40 | ⩽40 |
*Expected to reduce low‐density lipoprotein‐cholesterol (LDL‐C) level by >40%.
†No doses achieve LDL‐C reductions of >40%.
Covariates
We determined patient comorbidities by searching physician service claims and hospitalisation records for relevant diagnostic codes in the 1 year before the index date. In this manner, we identified the following characteristics: age at index date, gender, prior ACS, hypertension, diabetes, congestive heart failure, cerebrovascular disease, peripheral vascular disease, chronic kidney disease, chronic obstructive pulmonary disease, malignancy, arthritis and dementia. Similarly, we assessed concurrent use of the following medications, including those prescribed after being discharged from the index admission: ACE inhibitors or angiotensin II receptor blockers, β‐blockers, calcium‐channel blockers, diuretics, nitrates, digoxin, warfarin and clopidogrel. We also determined whether patients had been prescribed a statin before the index hospitalisation. Finally, we evaluated whether patients underwent angiography, angioplasty, coronary stent insertion or bypass surgery when they were hospitalised for their index event.
Statistical analysis
We compared the baseline characteristics of patients in each drug–dose category using Student's t tests and χ2 tests. Our primary outcome was the composite of death (as recoded in the patient eligibility files) or hospitalisation for recurrent ACS (determined in the same manner as our index admissions were identified). We examined the univariate relationship between the time to the occurrence of this outcome among high‐intensity and moderate‐intensity statin users, regardless of which specific statin they received, using Kaplan–Meier plots and the log rank test. We then compared the relative risk of death or re‐infarction in high‐intensity and moderate‐intensity statin users using Cox proportional hazards regression. Our estimates were adjusted for differences in patient comorbidity and demographics, and their use of cardiovascular procedures and medications. We performed our analysis on intention‐to‐treat principles, assuming that patients continued to take their index statin throughout follow‐up, and only censored patients at the time of an outcome or administratively on 31 December 2004.
To assess the robustness of our results, we repeated our analyses but additionally censored patients if they switched medications, changed doses or discontinued treatment. We also performed a propensity‐matched analysis to adjust for patients with certain observable characteristics preferentially receiving one treatment or another.23 We first fit a non‐parsimonious multivariable logistic regression model with demographic and clinical predictors to estimate each patient's probability of receiving high‐intensity as opposed to moderate‐intensity treatment. This probability represents their five‐digit “propensity score” (ie, with five decimal points). We matched each patient who was prescribed a high‐intensity statin with a patient who received a moderate‐intensity using a “greedy 5‐to‐1 digit algorithm”. By this method, we first searched for patients who matched on all five digits of their propensity scores. If no such match was found, we moved to those who matched on the first four and fewer digits in an iterative process. This technique minimised the bias introduced by incomplete or inexact matching.24 After matching, we re‐evaluated our Cox proportional hazards models.
We performed two secondary analyses. First, to assess the equivalence of statins with similar potencies, we repeated our Cox models stratifying patients on the basis of the specific drug–dose combination that they were prescribed. High‐intensity atorvastatin was used as the reference statin (referent) for this analysis. Second, in an attempt to replicate the results of the PROVE IT‐TIMI 22 Trial, we directly compared patients who received atorvastatin 80 mg daily with those who received pravastatin 40 mg daily.
All analyses were performed using SAS V.8.2.
Results
Our cohort consisted of 18 311 patients with ACS, of whom 3066 were prescribed a high‐intensity statin and 15 245 were prescribed a moderate‐intensity statin within 90 days of hospital discharge. A total of 8906 patients were rehospitalised for ACS or died during follow‐up. Table 2 shows the median follow‐up times.
Table 2 Baseline characteristics for patients treated with high‐intensity and moderate‐intensity statins.
| Characteristic | Entire cohort | Propensity‐matched cohort | ||
|---|---|---|---|---|
| High‐intensity statin users (n = 3066) | Moderate‐intensity statin users (n = 15245) | High‐intensity statin users (n = 3062) | Moderate‐intensity statin users (n = 3062) | |
| Demographics | ||||
| Median age, years | 77 | 78 | 77 | 77 |
| Female, % | 76.0 | 75.8 | 76.0 | 75.2 |
| Comorbid conditions, % | ||||
| Prior acute coronary syndrome | 4.5 | 6.2 | 4.5 | 4.6 |
| Congestive heart failure | 60.7 | 59.5 | 60.6 | 60.7 |
| Cerebrovascular disease | 41.2 | 40.4 | 41.2 | 41.8 |
| Peripheral vascular disease | 32.5 | 29.4 | 32.4 | 31.8 |
| Hypertension | 85.6 | 83.6 | 85.6 | 85.3 |
| Diabetes | 55.5 | 49.8 | 55.4 | 56.7 |
| Chronic kidney disease | 34.1 | 30.9 | 34.0 | 34.3 |
| Chronic obstructive pulmonary disease | 42.6 | 40.5 | 42.6 | 43.3 |
| Malignancy | 7.1 | 6.3 | 7.1 | 6.7 |
| Dementia | 9.7 | 10.2 | 9.7 | 9.8 |
| Concurrent medication use, % | ||||
| Prior statin | 72.9 | 60.9 | 72.8 | 72.5 |
| ACE inhibitor or angiotensin II receptor blocker | 58.7 | 53.9 | 58.7 | 59.0 |
| Clopidogrel | 30.1 | 23.4 | 30.1 | 30.6 |
| β‐blocker | 67.0 | 61.5 | 67.0 | 67.7 |
| Calcium‐channel blocker | 49.4 | 50.3 | 49.3 | 50.2 |
| Diuretics | 16.8 | 14.1 | 16.8 | 16.2 |
| Nitrates | 58.7 | 59.1 | 58.7 | 58.8 |
| Warfarin | 17.9 | 16.7 | 17.9 | 18.4 |
| Procedures on index hospitalisation | ||||
| Angiography | 47.8 | 45.4 | 47.8 | 47.7 |
| Angioplasty | 2.9 | 4.2 | 2.9 | 3.4 |
| Stent insertion | 18.4 | 14.3 | 18.3 | 17.8 |
| Bypass surgery | 9.7 | 10.5 | 9.7 | 9.2 |
| Median length of hospital stay, days | 5 | 5 | 5 | 5 |
| Median (IQR) length of follow‐up, days | 487 (211–1018) | 633 (245–1276) | 488 (211–1022) | 529 (228–1005) |
| Median (IQR) number of days between discharge and prescription | 16 (3–34) | 15 (2–33) | 16 (3–34) | 16 (3–34) |
IQR, interquartile range (25th–75th centiles).
High‐intensity vs moderate‐intensity statin treatment
At baseline, patients who received high‐intensity statins appeared sicker than moderate‐intensity users (table 2). They were more likely to have peripheral vascular disease, chronic kidney disease, diabetes and hypertension, to have received a statin before their index admission and to be receiving concurrent treatment with an ACE inhibitor/angiotensin II receptor blocker, clopidogrel and a β‐blocker. They were also more likely to have undergone stent insertion during their index hospitalisation. Propensity matching eliminated any meaningful differences.
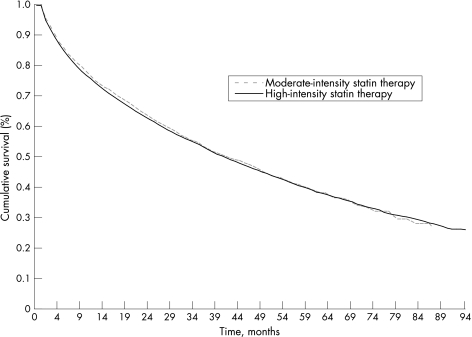
In our unadjusted analysis, moderate‐ and high‐intensity statin users were equally likely to die or be re‐admitted for ACS during follow‐up (log rank, p = 0.66; fig 1). Table 3 shows the results of our multivariable analyses. Patients treated with moderate‐ and high‐intensity statins had equivalent risks of death or readmission for ACS, even after adjusting for other prognostic variables (adjusted hazard ratio (HR) 1.02, 95% CI 0.96 to 1.08, p = 0.62). Similar results were obtained in our sensitivity analyses, including when propensity matching was used.
Figure 1 Kaplan–Meier estimates of time to death or rehospitalisation for acute coronary syndrome for high‐intensity and moderate‐intensity statin users.
Table 3 Adjusted hazard ratios for death or rehospitalisation for acute coronary syndrome.
| Analysis | Adjusted HR (95% CI) of moderate‐intensity compared with high‐intensity statin treatment |
|---|---|
| Intention to treat with censoring at outcome or administratively at the end of follow‐up | 1.02 (0.96 to 1.08) |
| Censoring at medication discontinuation, switching or dose change as well as at outcome or administratively at the end of follow‐up | 1.02 (0.93 to 1.12) |
| Propensity‐matched analysis | 0.98 (0.92 to 1.07) |
Individual drug–dose comparisons
Table 4 shows the baseline characteristics of our cohort stratified by the index drug–dose combinations. Moderate‐intensity simvastatin and atorvastatin were the dominant statins prescribed. Few patients received high‐intensity regimens with medications other than atorvastatin. Patient characteristics differed slightly by statin received. Rosuvastatin users were most likely to have undergone angiography and stent insertion on their index hospitalisation and were also most likely to be concurrent users of clopidogrel and β‐blockers. Lovastatin users were most likely to have received a statin before their index admission. Treatment groups were otherwise comparable.
Table 4 Baseline characteristics by drug–dose category.
| Characteristic | Moderate‐intensity atorvastatin (n = 4136) | Moderate‐intensity fluvastatin (n = 422) | Moderate‐intensity lovastatin (n = 677) | Moderate‐intensity pravastatin (n = 2405) | Moderate‐intensity simvastatin (n = 7605) | High‐intensity atorvastatin (n = 2,776) | High‐intensity lovastatin (n = 37) | High‐intensity rosuvastatin (n = 67) | High‐intensity simvastatin (n = 186) |
|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||
| Median age, years | 78 | 77 | 79 | 78 | 79 | 77 | 79 | 78 | 77 |
| Female, % | 76.4 | 68.5 | 78.9 | 78.3 | 74.8 | 76.3 | 70.3 | 79.1 | 72.0 |
| Comorbid conditions, % | |||||||||
| Prior acute coronary syndrome | 7.2 | 4.5 | 2.8 | 6.5 | 6.1 | 4.5 | 0.0 | 7.5 | 4.3 |
| Congestive heart failure | 59.8 | 54.3 | 56.4 | 56.8 | 60.7 | 60.8 | 56.8 | 52.2 | 61.8 |
| Cerebrovascular disease | 40.4 | 40.0 | 35.9 | 40.0 | 41.0 | 41.1 | 43.2 | 35.8 | 44.6 |
| Peripheral vascular disease | 29.0 | 30.0 | 30.3 | 28.5 | 29.9 | 32.2 | 37.8 | 29.9 | 36.0 |
| Hypertension | 83.8 | 78.7 | 80.2 | 82.9 | 84.3 | 85.6 | 75.7 | 92.5 | 84.4 |
| Diabetes | 51.7 | 47.9 | 44.6 | 49.8 | 49.3 | 55.7 | 46.0 | 56.7 | 53.3 |
| Chronic renal insufficiency | 31.0 | 24.2 | 24.7 | 30.1 | 32.0 | 34.0 | 27.0 | 31.3 | 37.6 |
| Chronic obstructive pulmonary disease | 40.9 | 44.1 | 33.5 | 41.3 | 40.6 | 42.4 | 27.0 | 52.2 | 45.7 |
| Malignancy | 6.1 | 8.5 | 6.7 | 6.2 | 6.3 | 7.2 | 2.7 | 3.0 | 9.1 |
| Dementia | 9.4 | 10.0 | 6.5 | 9.0 | 4.7 | 9.8 | 16.2 | 5.8 | 7.5 |
| Concurrent medication use, % | |||||||||
| Prior statin | 59.4 | 80.8 | 90.8 | 63.0 | 57.2 | 72.2 | 91.9 | 68.7 | 80.1 |
| ACEI or ARB | 22.6 | 53.8 | 52.3 | 52.9 | 53.3 | 58.3 | 67.6 | 56.7 | 63.4 |
| Clopidogrel | 24.9 | 25.4 | 20.7 | 26.4 | 21.9 | 29.9 | 18.9 | 43.3 | 30.7 |
| β‐blocker | 62.9 | 66.6 | 67.0 | 63.1 | 59.5 | 66.6 | 62.2 | 74.6 | 71.5 |
| Calcium‐channel blocker | 49.4 | 47.9 | 57.0 | 52.5 | 49.6 | 49.5 | 54.1 | 50.8 | 45.2 |
| Diuretics | 14.8 | 10.7 | 12.1 | 14.1 | 14.0 | 16.8 | 8.1 | 22.4 | 15.6 |
| Nitrates | 60.6 | 67.1 | 68.1 | 60.3 | 56.6 | 58.7 | 70.3 | 58.2 | 58.1 |
| Warfarin | 16.9 | 14.7 | 19.7 | 18.0 | 16.1 | 17.9 | 21.6 | 17.9 | 17.8 |
| Procedures on index hospitalisation | |||||||||
| Angiography | 44.7 | 42.9 | 39.1 | 46.2 | 46.2 | 48.4 | 32.4 | 49.3 | 41.9 |
| Angioplasty | 4.2 | 3.3 | 6.1 | 4.5 | 4.0 | 2.9 | 5.4 | 1.5 | 3.2 |
| Stent insertion | 14.3 | 10.0 | 10.2 | 15.6 | 14.5 | 18.4 | 8.1 | 31.3 | 15.6 |
| Bypass surgery | 10.6 | 13.3 | 7.7 | 9.3 | 10.9 | 10.0 | 5.4 | 6.0 | 7.0 |
| Median length of hospital stay, days | 5 | 5 | 5 | 5 | 6 | 5 | 5 | 5 | 6 |
| Mean daily dose, mg | 10.0 | 40.7 | 25.9 | 27.6 | 21.1 | 29.5 | 73.6 | 11.0 | 79.2 |
| Median (IQR) length of follow‐up, days | 669 (260–1312) | 715 (288–1437) | 714 (236–1632) | 606 (240–1346) | 616 (237–1198) | 505 (218–1047) | 654 (268–1504) | 178 (113–288) | 466 (228–872) |
| Median (IQR) number of days between discharge and prescription | 18 (3–37) | 24 (10–41) | 19 (8–32) | 17 (2–34) | 13 (2–30) | 16 (3–34) | 24 (11–40) | 11 (3–31) | 16 (3–29) |
ACEI, ACE inhibitor; ARB, angiotensin B receptor blocker; IQR, interquartile range (25th–75th centiles).
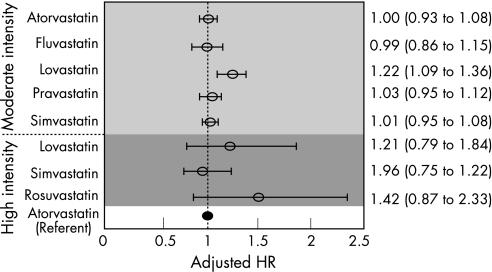
Patients treated with moderate‐intensity atorvastatin, fluvastatin, pravastatin and simvastatin had equivalent rates of death or recurrent ACS as patients treated with high‐intensity atorvastatin; the 95% confidence limits accompanying these estimates were very narrow for most comparisons (fig 2). By contrast, patients treated with moderate‐intensity lovastatin were at a greater risk of death or recurrent ACS than high‐intensity atorvastatin users (adjusted HR 1.22, 95% CI 1.09 to 1.36). Users of all high‐intensity statins had adjusted HRs that did not significantly differ from those of the users of high‐intensity atorvastatin but the CIs surrounding these estimates were relatively wide.
Figure 2 Likelihood of death or rehospitalisation for acute coronary syndrome from different drug–dose combinations compared with high‐intensity atorvastatin (referent). The circles and error bars represent adjusted hazard ratios (HRs) and 95% CIs, respectively.
Comparison of atorvastatin 80 mg daily and pravastatin 40 mg daily
Patients who received pravastatin at a dose of 40 mg had the same rate of death or re‐admission for ACS as patients who received atorvastatin 80 mg daily (adjusted HR 0.84, 95% CI 0.66 to 1.07). We found similar results using propensity‐score methods (HR 0.90, 95% CI 0.66 to 1.23).
Discussion
High‐ and moderate‐intensity statins seem to be equally effective in preventing major cardiovascular events in elderly patients with ACS. Although these results are at odds with the overall results of the PROVE‐IT TIMI 22 Trial,7 our cohort consisted of patients who were an average of 20 years older than the PROVE IT subjects. Epidemiological studies show that the association between cholesterol levels and coronary risk diminishes with age.25,26 In addition, elderly patients have a greater risk of reinfarction and death after an ACS than younger patients with equivalent cholesterol levels and risk factor profiles.14 Accordingly, a pre‐specified subgroup analysis of the 1230 PROVE IT trial participants aged ⩾65 years demonstrated equivalent outcomes in patients randomised to intensive and moderate lipid‐lowering strategies.7 Our findings are in keeping with this.
Our results do not imply a lack of benefit of cholesterol lowering in elderly patients; in PROVE IT, elderly patients with ACS who reached the National Cholesterol Education Program optional LDL‐C goal of <70 mg/dl (1.8 mmol/l) derived as much benefit as younger patients who also achieve this goal.14 Rather, because older patients with ACS tend to have lower cholesterol levels than younger patients,14 it may be that less intensive cholesterol lowering is required to achieve a target LDL‐C level. In the A to Z trial, patients treated with simvastatin 40 mg daily reached an LDL of 68 mg/dl with 1 month of post‐ACS treatment.9 Interestingly, patients in PROVE IT treated with moderate‐intensity lipid lowering only had a 10% LDL reduction. If these patients achieved the 29% LDL reduction seen in other trials using pravastatin 40 mg daily,22 they would have reached an average LDL of 75 mg/dl; this may have had a substantial impact on the trial results. It remains to be demonstrated whether any incremental benefit of intensive statin exists among patients who achieve their LDL goals.
Results from our secondary analyses are also notable. Patients treated with moderate‐intensity atorvastatin, fluvastatin, pravastatin and simvastatin had equivalent outcomes to patients treated with high‐intensity atorvastatin. Similar results were observed in Zhou et al's27 analysis of statins in a cohort of Canadian patients who had had myocardial infarction and in a recent meta‐analysis of randomised trials.28 By contrast, patients treated with moderate‐intensity lovastatin were more likely to die or have a reinfarction than users of high‐intensity atovastatin. A slight trend favouring atorvastatin over lovastatin was observed by Zhou et al,27 but the difference between treatment groups was not statistically significant. Because lovastatin shares many lipid and non‐lipid properties with other statins, particularly with simvastatin,16,29 and would be expected to have similar clinical effects at comparable dose intensities, it is possible that this specific finding is spurious.
Our comparison of high‐intensity statins also suggests a class effect for statins. Unfortunately, because relatively few patients were treated with statins at this dose intensity, we cannot exclude the possibility of small but clinically meaningful differences between these medications.
There are several limitations to our analyses. First, “confounding by indication”, which is a common concern in non‐randomised observations,30,31 may have biased our results. Patients who received high‐intensity statins, especially before the widespread dissemination of recently published trial data, may have been at a higher risk of post‐ACS events than patients who received moderate‐intensity statins. For example, high‐intensity statins may have preferentially been prescribed to patients with higher baseline cholesterol levels. The greater use of other treatments primarily used in patients at a higher risk of ACS (eg, coronary stents and clopidogrel) among the high‐intensity statin users in our study may be in keeping with this concern. As such, standard comparisons of patients who received statins of different intensity would bias the potential protective effect of high‐intensity statins towards the null. To minimise this effect, we performed a propensity‐score analysis that balanced all measured prognostic variables and found results that were practically identical to those of our primary analyses. Nevertheless, because administrative data do not contain detailed clinical information as cholesterol levels or the reasons for doctors' prescription choices, confounding by indication remains a potential concern.
Second, patients in our high‐intensity cohort may not have received sufficiently intensive treatment to enable us to observe the incremental benefit of aggressive statin treatment found in clinical trials. Subjects randomised to intensive lipid‐lowering in PROVE‐IT7 received 80 mg of atorvastatin daily; high‐intensity atorvastatin users in our analysis received a mean daily dose of 29.5 mg. By contrast, the incremental gain in lipid‐lowering effect between 30 and 80 mg daily is relatively modest,32 and older patients achieve higher plasma concentrations of atorvastatin than younger patients.33 Thus, patients treated with high‐intensity treatment in our analysis should still have derived the incremental benefit of aggressive treatment, should such a benefit truly exist. The results of our sensitivity analysis in which we compared patients who specifically received atorvastatin 80 mg daily and pravastatin 40 mg daily also argues against the bias of inadequate treatment intensity.
Third, consistent with other studies,27 we assessed the use of statin based on prescriptions filled within the first 90 days of hospital discharge. By contrast, patients in PROVE IT started receiving treatment within 10 days of ACS. If the incremental benefit of intensive statin treatment begins early in the post‐ACS course34 and if starting high‐intensity treatment later reduces its incremental benefit, then the ascertainment strategy we used would bias our results towards the null. A delay in initiation of high‐intensity treatment may explain why the A to Z trial failed to achieve its pre‐specified end point.9 The median time to statin initiation after hospital discharge was 16 days in our study and should have minimised the effect of this potential bias.
Fourth, we conducted our analysis using data from two state‐wide pharmacy assistance programmes that provide prescription drug benefits to lower middle‐income individuals aged ⩾65 years. As a result, our cohort consisted of predominantly female patients with a high prevalence of comorbid medical conditions, such as congestive heart failure and cerebrovascular disease. Therefore, the results of our analysis may not be generalisable to other populations with different demographic and clinical characteristics.
Finally, we categorised patients into drug‐intensity categories based on the initial statin that they were prescribed and assumed that patients continued this treatment until they had an outcome or were administratively censored. Although this should produce conservative results, our intention‐to‐treat assumption may not have been valid. In actual practice, patients often discontinue treatment or change drugs and doses; such patients probably differ in important ways from patients who continue their initially prescribed treatment. To account for this, we repeated our analysis and censored patients who changed medications, changed doses and discontinued treatment, in addition to those who experienced an outcome, and found identical results to our main analysis.
In summary, elderly patients who had had ACS treated with high‐intensity and moderate‐intensity statins in typical care settings seem to have similar rates of death and recurrent ACS. Our findings are in keeping with subgroup analyses from PROVE IT, and suggest that lower‐intensity treatment may be needed to achieve optimal LDL levels in elderly patients. Our results also demonstrate a class‐effect of moderate‐intensity statins, with the exception of lovastatin. Our analysis of high‐intensity statins is suggestive of a class effect for these drugs as well, but thus far too few patients in routine practice have been treated with these medications to make robust conclusions.
Abbreviations
ACS - acute coronary syndrome
PAAD - Pharmaceutical Assistance to the Aged and Disabled
PACE - Pharmaceutical Assistance Contract for the Elderly
PROVE IT‐TIMI 22 - Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis in Myocardial Infarction 22
Footnotes
Competing interests: We received no financial support for this research. NKC and RL receive salary support from Brigham and Women's Hospital and do not have any financial arrangements with any other organisations or companies. WCW is recipient of a Scientist Development Grant from the American Heart Association and has received additional salary support through research grants from GlaxoSmithKline, Amgen and Astellas.
Contributors: NKC, RL, WCW contributed to the study concept and design, acquisition of data, critical revision of the manuscript for important intellectual content and statistical expertise; NKC and RL analysed and interpreted the data; and NKC drafted the manuscript, provided administrative, technical or material support, and supervised the study.
References
- 1.Sacks F M, Pfeffer M A, Moye L A.et al The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med 19963351001–1009. [DOI] [PubMed] [Google Scholar]
- 2.Anon Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4s). Lancet 19943441383–1389. [PubMed] [Google Scholar]
- 3.Pedersen T R, Faergeman O, Kastelein J J.et al High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 20052942437–2445. [DOI] [PubMed] [Google Scholar]
- 4.LaRosa J C, Grundy S M, Waters D D.et al Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 20053521425–1435. [DOI] [PubMed] [Google Scholar]
- 5.Liem A H, van Boven A J, Veeger N J.et al Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J 2002231931–1937. [DOI] [PubMed] [Google Scholar]
- 6.Thompson P L, Meredith I, Amerena J.et al Effect of pravastatin compared with placebo initiated within 24 hours of onset of acute myocardial infarction or unstable angina: the Pravastatin in Acute Coronary Treatment (PACT) trial. Am Heart J 2004148e2. [DOI] [PubMed] [Google Scholar]
- 7.Cannon C P, Braunwald E, McCabe C H.et al Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 20043501495–1504. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz G G, Olsson A G, Ezekowitz M D.et al Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 20012851711–1718. [DOI] [PubMed] [Google Scholar]
- 9.de Lemos J A, Blazing M A, Wiviott S D.et al Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase X of the A to Z trial. JAMA 20042921307–1316. [DOI] [PubMed] [Google Scholar]
- 10.Grundy S M, Cleeman J I, Merz C N.et al Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004110227–239. [DOI] [PubMed] [Google Scholar]
- 11.Antman E M, Anbe D T, Armstrong P W.et al ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction; a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol 200444E1–211. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz G G, Olsson A G. The case for intensive statin therapy after acute coronary syndromes. Am J Cardiol 20059645F–53F. [DOI] [PubMed] [Google Scholar]
- 13.Austin P C, Mamdani M M. Impact of the pravastatin or atorvastatin evaluation and infection therapy—thrombolysis in myocardial infarction 22/reversal of atherosclerosis with aggressive lipid lowering trials on trends in intensive versus moderate statin therapy in Ontario, Canada. Circulation 20051121296–1300. [DOI] [PubMed] [Google Scholar]
- 14.Ray K K, Bach R, Cannon C P.et al Validating the new NCEP III LDL target of <70 mg/dl among elderly patients: an analysis from PROVE IT‐TIMI 22. J Am Coll Cardiol 200545S412A [Google Scholar]
- 15.Furberg C D, Herrington D M, Psaty B M. Are drugs within a class interchangeable? Lancet 19993541202–1204. [DOI] [PubMed] [Google Scholar]
- 16.Knopp R H. Drug treatment of lipid disorders. N Engl J Med 1999341498–511. [DOI] [PubMed] [Google Scholar]
- 17.Pharmaceutical assistance contract for the elderly act 62 p.S. 2901‐2908. http://www.pacode.com/secure/data/006/006toc.html (accessed 9 Apr 2007)
- 18.Provision of pharmaceutical services under the Pharmaceutical Assistance to the Aged, Disabled program (PAAD) http://www.state.nj.us/health/seniorbenefits/documents/reg_njac_%208‐83c.pdf (accessed 9 Apr 2007)
- 19.Wang P S, Schneeweiss S, Avorn J.et al Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 20053532335–2341. [DOI] [PubMed] [Google Scholar]
- 20.Solomon D H, Schneeweiss S, Glynn R J.et al Relationship between selective cyclooxygenase‐2 inhibitors and acute myocardial infarction in older adults. Circulation 20041092068–2073. [DOI] [PubMed] [Google Scholar]
- 21.Winkelmayer W C, Charytan D M, Levin R.et al Poor short‐term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis 200647301–308. [DOI] [PubMed] [Google Scholar]
- 22.Law M R, Wald N J, Rudnicka A R. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta‐analysis. BMJ 20033261423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin D B. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997127757–763. [DOI] [PubMed] [Google Scholar]
- 24.Parsons L S. Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Twenty‐sixth Annual SAS Users Group International Conference, Long Beach, California 2001214–226.
- 25.Weverling‐Rijnsburger A W, Blauw G J, Lagaay A M.et al Total cholesterol and risk of mortality in the oldest old. Lancet 19973501119–1123. [DOI] [PubMed] [Google Scholar]
- 26.Shipley M J, Pocock S J, Marmot M G. Does plasma cholesterol concentration predict mortality from coronary heart disease in elderly people? 18 year follow up in Whitehall study. BMJ 199130389–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z, Rahme E, Abrahamowicz M.et al Effectiveness of statins for secondary prevention in elderly patients after acute myocardial infarction: an evaluation of class effect. Can Med Assoc J 20051721187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention. Am Heart J 2006151273–281. [DOI] [PubMed] [Google Scholar]
- 29.Bonetti P O, Lerman L O, Napoli C.et al Statin effects beyond lipid lowering—are they clinically relevant? Eur Heart J 200324225–248. [DOI] [PubMed] [Google Scholar]
- 30.Grobbee D E, Hoes A W. Confounding and indication for treatment in evaluation of drug treatment for hypertension. BMJ 19973151151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker A M, Stampfer M J. Observational studies of drug safety. Lancet 1996348489. [DOI] [PubMed] [Google Scholar]
- 32.Jones P, Kafonek S, Laurora I.et al Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). Am J Cardiol 199881582–587. [DOI] [PubMed] [Google Scholar]
- 33.Gibson D M, Bron N J, Richens A.et al Effect of age and gender on pharmacokinetics of atorvastatin in humans. J Clin Pharmacol 199636242–246. [DOI] [PubMed] [Google Scholar]
- 34.Ray K K, Cannon C P. Early time to benefit with intensive statin treatment: could it be the pleiotropic effects? Am J Cardiol 20059654F–60F. [DOI] [PubMed] [Google Scholar]