Abstract
Background
Conflicting data have been reported about the correlation between plaque composition assessed by virtual histology (VH) and remodelling index (RI).
Aim
To evaluate, in a larger patient population, the relationship between plaque morphology obtained by VH and arterial remodelling.
Methods and results
VH intravascular ultrasound was performed on 95 non‐bifurcation native significant lesions (>75% stenosis) in 85 patients. Positive remodelling (defined as RI ⩾1.05) was present in 28 lesions, whereas intermediate/negative remodelling (RI <1.05) was present in 67 lesions. Compared with intermediate/negative remodelling, positive remodelling was associated with an increased frequency of patients with acute coronary syndrome (n = 13 (52%) vs n = 15 (25%); p = 0.017), and with a greater plaque burden (mean (SD) 78.3 (6.3)% vs 73.2 (6.8)%, p = 0.001). At the minimal lumen site, necrotic core was significantly smaller in lesions with positive remodelling (median (interquartile range) 5.0% (2.2–11.0%)) than in lesions with intermediate/negative remodelling (median (interquartile range) 9.0% (4.0–16.0%); p = 0.048). No differences were observed in the rate of thin‐cap fibroatheroma or in the presence of multiple necrotic core layers, and there were no statistical differences for fibrous, fibro fatty and dense calcium percent plaque area at the minimum lumen diameter (MLD), or for the entire lesion length between both groups.
Conclusions
In vivo VH analysis shows that lesions with positive remodelling have statistically less necrotic core percent area at the MLD site compared with intermediate/negative remodelling lesions.
Changes in size of coronary arteries occur as a response to the growth of atherosclerotic plaques.1 Increase in vessel size due to outward expansion of the vessel wall is called positive remodelling, and a decrease in vessel size due to vessel shrinkage is called negative remodelling.2 Autopsy and intravascular ultrasound (IVUS) studies have shown that the extent of plaque burden and positive remodelling are linked to an increased frequency of vulnerable plaques and acute coronary syndromes (ACSs).3,4,5,6,7
Greyscale IVUS is a useful modality to evaluate the morphology of atherosclerotic plaques and the vessel wall.8,9 However, evaluation of plaque morphology is limited—particularly, the evaluation of the low echogenicity region, which is thought to represent the composition of lipid‐containing and mixed plaque. Virtual histology (VH) IVUS using spectral analysis of the radiofrequency (RF) ultrasound back‐scatter signals allows identification of four different types of atherosclerotic plaques: fibrous, fibro‐fatty, dense calcium and necrotic core.10,11 Ex vivo validation studies, analysing the selected region of interest representing homogeneous plaque components on the histology specimen, have reported high accuracy for the classification of those plaque components.
Recently, two middle‐sized studies using VH reported conflicting data about plaque composition according to the remodelling index (RI).12,13 In this study, we sought to evaluate, in a larger patient population, the relationship between plaque morphology obtained by VH and arterial remodelling.
Methods
Study population
Between October 2004 and July 2005, VH IVUS using the motorised pull‐back system was performed on 95 native de novo target lesions (>75% angiographic stenosis by visual estimation) in 85 patients undergoing clinically indicated percutaneous coronary intervention. Exclusion criteria were ostial and/or bifurcation lesions. Clinical presentation was classified either as stable angina pectoris (SAP) or as ACS. The Canadian Cardiovascular Society classification was used for patients with SAP. ACS includes unstable angina according to the Braunwald classification, or acute myocardial infarction (with or without ST elevation). Secondary causes for angina (Braunwald classification class IA, IIA and IIIA) were excluded. The present study was approved by the hospital ethics committee, and written informed consent was obtained from all patients.
Data acquisition and medication
All patients, apart from patients with ACS, were given aspirin (100 mg/day) and ticlopidine (200 mg/day) for at least 1 week before the procedure. During the procedure, heparin was given as a bolus of 150 U/kg, with additional boluses to 2000 U/h. All baseline angiography and IVUS imaging were performed after administration of 200 μg of nitroglycerin. Angiography was taken so that each lesion was viewed from more than two angles. For the IVUS procedure, a 20 MHz, 3.2 F, phased‐array IVUS catheter (Eagle Eye, Volcano Therapeutics, Rancho Cordova, California, USA) was used. After placing the IVUS catheter at a point distal to the lesion, the catheter was pulled back to the aortic ostium using the motorised pull‐back system at 0.5 cm/s. During pullback, greyscale IVUS was recorded, and raw RF data were captured at the top of the R wave for reconstruction of the colour‐coded map by a VH IVUS data recorder (Volcano Therapeutics). The greyscale IVUS movie and captured RF data were written on CD‐R and DVD‐R, respectively.
Greyscale and VH IVUS analyses
The smallest lumen at the culprit lesion was identified from axial and longitudinal plaque distribution. At the smallest lumen site of the culprit lesion, vessel cross‐sectional area (CSA) and lumen CSA were calculated, and the difference between the two values was defined as plaque and media CSA. Percent plaque and media CSA (plaque burden) was defined as plaque and media CSA divided by vessel CSA. RI was calculated as the external elastic membrane CSA at the minimum lumen diameter (MLD) divided by the proximal reference external elastic membrane CSA. Positive remodelling was defined as an RI ⩾1.05, and intermediate/negative remodelling as an RI <1.05. On grey scale, echo‐lucent plaque was defined visually as a zone of reduced echogenicity occupying approximately greater than or equal to half the plaque area. Atherosclerotic coronary plaques were characterised by classification trees based on mathematical autoregressive spectral analysis of IVUS back‐scattered data (IVUS Lab software, Volcano Therapeutics), as described previously.11 Fibrous areas were marked in green, fibro fatty in yellow, dense calcium in white and necrotic core in red on the reconstructed colour‐coded tissue map. The area and percent area of each plaque component in the tissue map were calculated automatically using IVUS Lab software.
Measurements were made for the region of interest, which was defined as the segment between distal and proximal reference sites that looked most normal within 5 mm proximal and distal to the lesion. The volume of vessel and each plaque component was calculated using Simpson's method and averaged over the length of the lesion to also generate a mean plaque area.
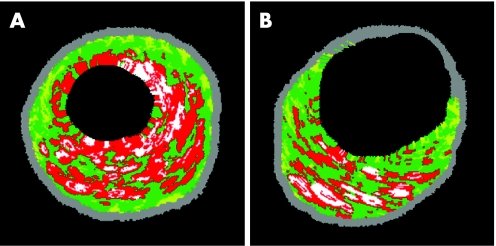
VH IVUS‐derived thin‐cap fibroatheroma (TCFA) were defined, according to previously published data, as lesions fulfilling the following criteria in at least three consecutive cross‐sectional images, by two experienced, independent observers14,15 (fig 1): TCFA: plaque burden >40%, percent necrotic core area >20% without evidence of fibrous cap. Greyscale and VH IVUS analyses were performed by an experienced analyst who was blinded to the quantitative analysis, and baseline clinical and lesion characteristics.
Figure 1 Virtual histology examples for (A) thin‐cap fibroatheroma and (B) multiple necrotic core layers. Red, necrotic core; yellow, fibro fatty; white, dense calcium; green, fibrous.
Statistical analysis
The distribution normality of continuous data was assessed using the Shapiro–Wilk W test. Continuous data were reported as mean (SD), or as median (25th, 75th centile). Categorical data were expressed as number or frequencies of occurrence. Comparison of continuous variables was performed by the two‐sided unpaired Student's t test for normally distributed data, and by the Wilcoxon/Kruskal–Wallis test for non‐normally distributed data, as indicated. The χ2 test, or Fisher's exact test by sparse data, was used for comparing the frequency of occurrence, as indicated. The software JMP V.5.1.2 (SAS Institute) was used for data analysis. A probability value of <0.05 was considered to indicate statistical significance.
Results
Baseline patient and lesion characteristics
Positive remodelling was present in 28 lesions (25 patients), whereas intermediate/negative remodelling was present in 67 lesions (60 patients). The only difference between the two groups in baseline patient and lesion characteristics was the presence of significantly more patients with ACS in the positive remodelling group than in the intermediate/negative remodelling groups (52% vs 25%, p = 0.017; table 1). The mean (SD) diameter of stenosis measured by quantitative coronary angiography was 74.7 (16.1)%.
Table 1 Baseline patient and lesion characteristics.
| Positive remodelling (n = 25; lesions = 28) | Intermediate/negative remodelling (n = 60; lesions = 67) | p Value | |
|---|---|---|---|
| Mean (SD) age (years) | 63.9 (14.7) | 65.1 (11.3) | 0.99* |
| Sex, male | 21 (84) | 50 (83.3) | 1.0† |
| Acute coronary syndrome | 13 (52) | 15 (25) | 0.017 |
| Diabetes mellitus | 8 (32) | 21 (35) | 0.79 |
| Current smoker | 8 (32) | 22 (36.7) | 0.68 |
| Hypertension | 15 (60) | 38 (63.3) | 0.77 |
| Hypercholesterolaemia | 13 (52) | 36 (60) | 0.50 |
| Previous MI | 5 (20) | 12 (20) | 1.0 |
| Previous CABG | 1 (4) | 1 (1.7) | 0.5† |
| Multivessel disease | 9 (36) | 16 (26.7) | 0.39 |
| Mean (SD) LVEF (%) | 48.9 (8.8) | 52.8 (9.1) | 0.087* |
| Target coronary artery | 0.28 | ||
| LM | 3 (10.7) | 6 (9.1) | 1.0† |
| LAD | 5 (17.9) | 24 (36.4) | 0.09† |
| LCX | 5 (17.9) | 12 (18.2) | 0.97 |
| RCA | 15 (53.6) | 24 (36.4) | 0.12 |
CABG, coronary artery bypass graft; LAD, left anterior descending; LCX, left circumflex; LM, left main; LVEF, left ventricular ejection fraction; MI, myocardial infarction; RCA, right coronary artery.
Data are expressed as n (%) unless otherwise specified.
*Wilcoxon/Kruskal–Wallis test.
†Fisher's exact test.
Greyscale IVUS data of lesions with positive versus intermediate/negative remodelling
At the MLD site of lesions with positive remodelling, there was significantly greater external elastic membrane CSA (mean (SD) 20.61 (5.83) vs 16.20 (4.16) mm2, p<0.001), plaque and media CSA (16.46 (5.80) vs 12.16 (3.88) mm2, p<0.001) and plaque burden (78.3 (6.3) vs 73.2 (6.8)%, p = 0.001; non‐parametric test), but there was no difference in lumen CSA (4.36 (0.98) vs 4.19 (1.00) mm2, p = 0.25; non‐parametric test). The rate of echo‐lucent plaque was similar between both groups (14.3% vs 11.9%; p = 0.74). Echo‐lucent plaques were more frequent in ACS than in SAP lesions (23.3% vs 7.7%, p = 0.04).
VH data of lesions with positive versus intermediate/negative remodelling
Quantitative data
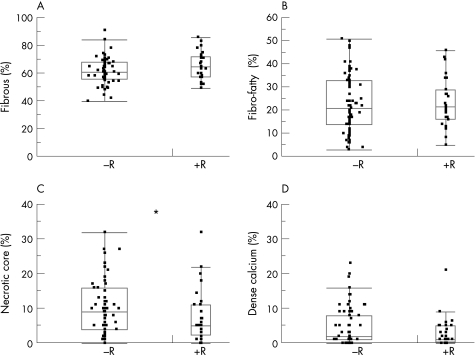
Plaque components are given as percent plaque area at the MLD, as well as for the entire lesion length (table 2, fig 2).
Table 2 Plaque components in positive and intermediate/negative remodelling index.
| RI ⩾1.05 (n = 28) | RI <1.05 (n = 67) | p Value | |||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| MLD | |||||
| Fibrous % | 65.73 (9.73) | 64.5 (57.2‐71.8) | 61.68 (9.51) | 61.0 (56.0–68.0) | 0.064 |
| Fibro‐fatty % | 23.36 (10.43) | 21.5 (16.2–29.0) | 23.13 (12.25) | 21.0 (14.0–33.0) | 0.93 |
| Necrotic core % | 7.62 (7.68) | 5.0 (2.2–11.0) | 10.54 (7.80) | 9.0 (4.0–16.0) | 0.048* |
| Dense calcium % | 3.01 (4.28) | 1.0 (0.3–5.0) | 4.61 (5.53) | 2.0 (1.0–8.0) | 0.26* |
| Entire lesion length | |||||
| Fibrous % | 64.57 (8.11) | 63.5 (59.9–68.3) | 64.98 (7.48) | 66.0 (60.3–70.1) | 0.82 |
| Fibro‐fatty % | 21.56 (7.12) | 22.5 (18.4–25.7) | 22.18 (9.90) | 21.9 (18.0– 26.5) | 0.94* |
| Necrotic core % | 8.89 (7.54) | 7.0 (3.7–13.0) | 9.43 (5.91) | 8.3 (5.1–12.8) | 0.34* |
| Dense calcium % | 4.94 (6.60) | 3.1 (1.5–6.1) | 4.11 (3.53) | 3.7 (1.4–5.4) | 0.89* |
MLD, minimal lumen diameter; RI, remodelling index.
Data are expressed as mean (SD) and as median with 25th and 75th interquartile range (IQR). Student's t‐test was performed for normally distributed data.
*Wilcoxon/Kruskal–Wallis tests for non‐normally distributed data.
Figure 2 Point representation (jittered display) with superimposed box plot of the four virtual histology components at the minimum lumen diameter site expressed as relative values. Although statistical significance is achieved for necrotic core, a large overlap of the individual values can be observed. +R, positive remodelling lesions; −R, negative/intermediate remodelling lesions. *p<0.05.
At the minimal lumen site, necrotic core was significantly smaller in lesions with positive remodelling (median (IQR) 5.0% (2.2–11.0%)) than in lesions with intermediate/negative remodelling (median (IQR) 9.0% (4.0–16.0%), p = 0.048). There were no statistical differences for the other plaque components at the MLD. For the entire lesion length, there were no significant statistical differences for the four plaque components. Linear regression for the RI with plaque components expressed as percentage at the MLD showed no significant correlation for any of the four components (fibrous: r = 0.15, p = 0.14; fibro‐fatty: r = −0.01, p = 0.91; necrotic core: r = −0.09, p = 0.37; dense calcium: r = −0.11, p = 0.27).
Subanalysis for greyscale echo‐lucent plaques
There were no significant differences for any of the four plaque components between echo‐lucent and non‐echo‐lucent plaques at the MLD (fibrous: 61.3 (8.9)% vs 63.1 (9.8)%, p = 0.56; fibro‐fatty: 28.8 (3.3)% vs 22.4 (1.3)%, p = 0.08; necrotic core: 6.7 (2.3)% vs 10.1 (0.9)%, p = 0.15; dense calcium: 2.8 (1.5)% vs 4.3 (0.6)%, p = 0.36) or over the entire lesion length (fibrous: 61.9 (2.2)% vs 65.3 (0.8)%, p = 0.15; fibro‐fatty: 24.9 (2.6)% vs 21.6 (1.0)%, p = 0.24; necrotic core: 7.7 (5.3)% vs 9.5 (0.7)%, p = 0.37; dense calcium: 4.4 (1.3) vs 4.3 (0.5)%, p = 0.96).
Qualitative data
No differences were observed in rate of TCFA (7.1% vs 16.4%, p = 0.33) or for the presence of multiple necrotic core layers (28.6% vs 19.4%, p = 0.33).
Discussion
This study shows a relationship between plaque morphology assessed by VH IVUS and the extent of arterial remodelling, with significantly less necrotic core percent area at the MLD site of lesions with positive remodelling.
Morphometric data
In line with previously published greyscale IVUS data, positive remodelling was observed with an increased frequency in patients with ACS.6,16,17 Lesions with positive remodelling have been shown to have an increased plaque burden, which is similar to our data.6,18,19,20
Plaque composition according to RI: knowledge from autopsy, greyscale IVUS and angioscopy studies
Although conventional greyscale IVUS is limited in its ability to differentiate plaque composition accurately, many studies investigating this topic have been published. Several greyscale IVUS published data have focused on plaque composition according to the clinical presentation, but only a few have described the association between plaque composition and RI. Reported data on plaque composition by greyscale IVUS show an increase in frequency of soft plaques and a decrease in calcium content in lesions with positive remodelling compared with negative remodelling.21,22 Plaque composition according to the clinical presentation has shown either an increase or no differences in frequency of soft plaques in patients with ACS.6,18,20,23 Calcium content is also decreased in patients with ACS.23 Some insights about plaque composition can also be obtained by angioscopy. Angioscopically, yellow plaques have been described to represent lipid‐rich plaque with a thin fibrous cap, and have been reported to be more frequent in lesions with positive remodelling as well as in patients with ACS.18 In this same study, the frequency of echo‐lucent plaques between the yellow and white plaques was not significantly different. Our results show an increased frequency of echo‐lucent plaques in patients with ACS compared with SAP lesions, but no differences between lesions with positive versus negative/intermediate RI. Those data have to be taken with caution, as greyscale IVUS is not an adequate method for plaque characterisation. The American College of Cardiology task force on IVUS has concluded that ultrasound images are fundamentally different from histology, and that greyscale IVUS cannot be used to detect and quantify specific histological contents.24
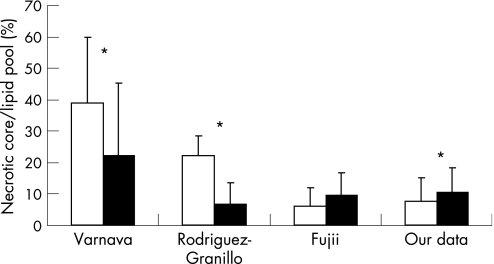
Two large pathological studies have investigated the correlation between coronary artery remodelling and plaque composition. In a study including mild‐to‐severe lesions (108 plaques) of 88 patients who died suddenly due to coronary artery disease (CAD), Varnava et al7 analysed the lesion site having the maximum plaque size. They observed a higher lipid core (expressed as a percentage of the total plaque size) in lesions with positive remodelling (defined in this study as an RI ⩾1.0) than in those with negative remodelling (mean (SD) 39.0 (21.0)% vs 22.3 (23.1)%, p<0.001).7 In the second study including 36 patients who died of severe coronary artery disease (CAD), Burke et al3 serially sectioned the coronary vessels at 3–4 mm intervals and analysed 1318 segments with atheromatous plaques. Their results showed that segments with positive remodelling had an increase in large lipid core (defined as >10% of the plaque area), large calcified area (defined as >10% of the plaque area), TCFA, thick FA and intraplaque haemorrhage; but less percent fibrous area.
Plaque composition according to RI: VH‐IVUS data
Frequency‐domain analysis of IVUS RF data has been shown to provide information on plaque composition. After entering several parameters in a classification tree, each region of interest has to be given one of the four defined component types. Each component type is given a different colour: red represents a highly lipidic necrotic region (named either necrotic core or lipid pool); yellow represents loosely packed bundles of collagen fibres with regions of lipid deposition. These areas are cellular and no cholesterol clefts or necrosis are present (named fibro‐fatty); green represents densely packed bundles of collagen fibres with no evidence of interfibre lipid accumulation (named fibrous); white represents the focal area of dense calcium (named dense calcium).
Our in vivo VH‐IVUS study shows that the lesions with positive remodelling had significantly less necrotic core percentage CSA at the MLD site compared with the lesions with intermediate/negative remodelling, although a large overlap of individual values can be observed. Those results are in line with the data of Fujii et al,12 but are contrary to the data of Rodriguez‐Granillo et al.13 In addition, our results also contradict the pathological data (fig 3).3,7 Not only the absolute or relative value for each component but also the distribution/location is linked to vulnerability. In our study, there were no differences in the frequency of TCFA or multiple necrotic core layers between the two groups. These observations are again contrary to pathological data. The resolution of VH‐IVUS is about 100–200 μm, which is above the histopathological definition of a thin fibrous cap (<65 m). It is therefore not certain that the virtual histology definition used for the detection of TCFA can be applied for this purpose, as it has not been validated in histopathological studies. The discrepancy between our data and those in the pathological study or in the study of Rodriguez‐Granillo et al13 might have resulted from differences in the patient/lesion population or in methods applied. In our study, culprit lesions were divided into two groups: a positive remodelling group and an intermediate/negative group with a cut‐off value for RI of 1.05. Fujii et al12 divided the lesions into two similar groups, but used a cut‐off value for RI of 1.00. Rodriguez‐Granillo et al13 studied non‐culprit lesions and divided them into three groups with an intermediate remodelling group (including only three patients). In addition, RI index does not take into account the dynamics of changes in plaque volumes. Some lesions are young and growing, some are matured and some are on their way to shrinkage. Therefore, a similar degree of RI does not necessarily correspond to a similar pathological finding. Furthermore, the IVUS catheter that we used in our study (20 MHz, phased array; Eagle Eye, Volcano Therapeutics) was different from the one used in the studies by Rodriguez‐Granillo et al13 and Fujii et al12 (30 MHz, mechanical; Boston Scientific), although our results are comparable to those of Fujii et al12.
Figure 3 Published data on the association between necrotic core and lipid pool expressed as relative values at the minimum lumen diameter (MLD) site with positive versus negative remodelling. Varnava et al,7 pathological study; Rodriguez‐Granillo et al,13 VH data; and Fujii et al,12 VH data. Positive remodelling (white box), negative remodelling (black box), *p<0.05.
Another limitation may lie in the VH validation itself. Ex vivo VH validation studies have reported a high accuracy for the classification of plaque components. Those studies analysed the selected region of interest representing homogeneous plaque components on the histology specimen. However, validation studies for VH tissue maps of entire plaque cross‐sections, in terms of absolute or relative values, have not yet been published.
As plaque and media CSA, and plaque burden are increased in lesions with positive RI, absolute values (mm2) for plaque components would consequently be higher in this subset of lesions. Therefore, we reported the data as relative values at the MLD site and for the entire lesion length. It is difficult to know which way of reporting the data is clinically the most appropriate. Although atherosclerosis is a pancoronary syndrome, coronary thrombosis occurs at focal sites of increased vulnerability (eg, plaque rupture site). In addition, drastic changes can be observed in plaque composition within a single lesion over only a few millimetres.3 Consequently, we feel that analysis at the MLD, or maybe the “worst looking three consecutive frames within a lesion”, would be the most appropriate.
Our study presents some limitations. This is a single‐centre retrospective study. There are no classifications for thrombus or blood on VH‐IVUS. Consequently, ruptured plaques filled with blood or thrombus, or luminal thrombus, are assigned to one of the current four plaque classifications, usually green (fibrous). This is, particularly, relevant to patients with ACS, and might have influenced the results.
Abbreviations
ACS - acute coronary syndrome
CSA - cross‐sectional area
IVUS - intravascular ultrasound
MLD - minimum lumen diameter
RF - radiofrequency
RI - remodelling index
SAP - stable angina pectoris
TCFA - thin‐cap fibroatheroma
VH - virtual histology
Footnotes
Competing interests: None declared.
References
- 1.Glagov S, Weisenberg E, Zarins C K.et al Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 19873161371–1375. [DOI] [PubMed] [Google Scholar]
- 2.Nishioka T, Luo H, Eigler N L.et al Contribution of inadequate compensatory enlargement to development of human coronary artery stenosis: an in vivo intravascular ultrasound study. J Am Coll Cardiol 1996271571–1576. [DOI] [PubMed] [Google Scholar]
- 3.Burke A P, Kolodgie F D, Farb A.et al Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002105297–303. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Nishikawa H, Mukai S.et al Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol 20013763–69. [DOI] [PubMed] [Google Scholar]
- 5.Pasterkamp G, Schoneveld A H, van der Wal A C.et al Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: the remodeling paradox. J Am Coll Cardiol 199832655–662. [DOI] [PubMed] [Google Scholar]
- 6.Schoenhagen P, Ziada K M, Kapadia S R.et al Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000101598–603. [DOI] [PubMed] [Google Scholar]
- 7.Varnava A M, Mills P G, Davies M J. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002105939–943. [DOI] [PubMed] [Google Scholar]
- 8.Potkin B N, Bartorelli A L, Gessert J M.et al Coronary artery imaging with intravascular high‐frequency ultrasound. Circulation 1990811575–1585. [DOI] [PubMed] [Google Scholar]
- 9.Yock P G, Linker D T. Intravascular ultrasound. Looking below the surface of vascular disease. Circulation 1990811715–1718. [DOI] [PubMed] [Google Scholar]
- 10.Nair A, Kuban B D, Obuchowski N.et al Assessing spectral algorithms to predict atherosclerotic plaque composition with normalized and raw intravascular ultrasound data. Ultrasound Med Biol 2001271319–1331. [DOI] [PubMed] [Google Scholar]
- 11.Nair A, Kuban B D, Tuzcu E M.et al Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation 20021062200–2206. [DOI] [PubMed] [Google Scholar]
- 12.Fujii K, Carlier S G, Mintz G S.et al Association of plaque characterization by intravascular ultrasound virtual histology and arterial remodeling. Am J Cardiol 2005961476–1483. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez‐Granillo G A, Serruys P W, Garcia‐Garcia H M.et al Coronary artery remodelling is related to plaque composition. Heart 200692388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez‐Granillo G A, Garcia‐Garcia H M, Mc Fadden E P.et al In vivo intravascular ultrasound‐derived thin‐cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol 2005462038–2042. [DOI] [PubMed] [Google Scholar]
- 15.Virmani R, Burke A P, Kolodgie F D.et al Vulnerable plaque: the pathology of unstable coronary lesions. J Intervent Cardiol 200215439–446. [DOI] [PubMed] [Google Scholar]
- 16.Hong M K, Park S W, Lee C W.et al Prospective comparison of coronary artery remodeling between acute coronary syndrome and stable angina in single‐vessel disease: correlation between C‐reactive protein and extent of arterial remodeling. Clin Cardiol 200326169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Birgelen C, Klinkhart W, Mintz G S.et al Plaque distribution and vascular remodeling of ruptured and nonruptured coronary plaques in the same vessel: an intravascular ultrasound study in vivo. J Am Coll Cardiol 2001371864–1870. [DOI] [PubMed] [Google Scholar]
- 18.Takano M, Mizuno K, Okamatsu K.et al Mechanical and structural characteristics of vulnerable plaques: analysis by coronary angioscopy and intravascular ultrasound. J Am Coll Cardiol 20013899–104. [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi M, Terashima M, Awano K.et al Morphology of vulnerable coronary plaque: insights from follow‐up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol 200035106–111. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Shen W, Zhang D. Relationship between coronary arterial remodeling and clinical presentation. Chin Med J (Engl) 2003116263–266. [PubMed] [Google Scholar]
- 21.Fuessl R T, Kranenberg E, Kiausch U.et al Vascular remodeling in atherosclerotic coronary arteries is affected by plaque composition. Coron Artery Dis 20011291–97. [DOI] [PubMed] [Google Scholar]
- 22.Sabate M, Kay I P, de Feyter P J.et al Remodeling of atherosclerotic coronary arteries varies in relation to location and composition of plaque. Am J Cardiol 199984135–140. [DOI] [PubMed] [Google Scholar]
- 23.Hodgson J M, Reddy K G, Suneja R.et al Intracoronary ultrasound imaging: correlation of plaque morphology with angiography, clinical syndrome and procedural results in patients undergoing coronary angioplasty. J Am Coll Cardiol 19932135–44. [DOI] [PubMed] [Google Scholar]
- 24.Mintz G S, Nissen S E, Anderson W D.et al American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2001371478–1492. [DOI] [PubMed] [Google Scholar]