Abstract
Extensive studies on protective immunity to rodent malaria provided the basis for the current experiments in which mice were immunized with recombinant (re) influenza and vaccinia viruses expressing selected sequences of the circumsporozoite (CS) protein of the human malaria parasite Plasmodium falciparum. Mice of different H-2 haplotypes immunized with re influenza viruses expressing the immunodominant B cell epitope of this CS protein produced high titers of antibodies to the parasite. A cytotoxic T lymphocyte epitope of the CS protein of P. falciparum, PF3, recognized by CD8+ T cells of H-2k mice, was expressed in a re vaccinia virus (VacPf) and a re influenza virus (FluPf). Immunization of mice with either FluPf or VacPf elicited a modest CS-specific CD8+ T cell response detected by interferon γ secretion of individual immune cells. Priming of mice with FluPf, followed by a booster with VacPf, resulted in a striking enhancement of this T cell response. The reverse protocol, i.e., priming with VacPf followed by a booster with FluPf, failed to enhance the primary response. VacPf also greatly enhanced the primary response of mice injected with P. falciparum sporozoites or with a lipopeptide containing PF3. A booster with FluPf also amplified the response of lipopeptide- or sporozoite-primed mice but less than a VacPf booster did. Although mice are not susceptible to infection by P. falciparum sporozoites, we demonstrated that administration of two distinct immunogens expressing PF3 elicited activated, extravasating CS-specific T cells that protected against an intracerebral VacPf challenge.
Malaria is the most deadly tropical disease, killing 2–3 million people/year. It is widespread, occurring in more than 90 countries and causing more than 300 million infections annually. Recent efforts to prevent the spread of this disease and control it through chemotherapy and spraying of residual insecticides largely have failed because of the development of widespread drug resistance of the parasites, insecticide resistance of the mosquito vectors, and various socioeconomic factors. For these reasons, the development of a vaccine as an additional tool for malaria control has become most urgent.
The feasibility of developing an effective malaria vaccine was demonstrated by the finding that immunization with irradiated sporozoites confers complete protection (sterile immunity) against this infection not only to rodents and monkeys but also to humans (1). Since then, several sporozoite antigens, shared or not with those of liver stages, were identified as potential targets of protective immune responses. One of these antigens—the circumsporozoite (CS) protein, a major sporozoite surface antigen and liver stage component—has been investigated extensively and shown to elicit neutralizing antibodies and cell-mediated immunity (1, 2). In two experimental rodent malaria systems (Plasmodium berghei and Plasmodium yoelii), the transfer of either anti-CS antibodies or T cells to naive mice conferred full protection against challenge with sporozoites of the corresponding malaria species (3, 4).
Various approaches involving several synthetic constructs and recombinant (re) proteins combined with several adjuvants have been used to elicit maximal antibody titers against the malaria CS protein. More recently, T cell-mediated responses against the liver stages of malaria parasites have been elicited by immunizing mice with re viruses or bacteria expressing selected plasmodial antigens. These vectors were selected as delivery systems for the presentation and processing of immunogens by the class I pathway. By using this approach, we demonstrated that inoculating mice with a re influenza virus expressing either the immunodominant B and/or a cytotoxic T lymphocyte (CTL) epitope of the CS of P. yoelii, followed by the inoculation of a re vaccinia virus expressing the same plasmodial sequences, results in extensive protection against this infection (5).
In the current study, we have extended this experimental approach to Plasmodium falciparum, the most pathogenic human malaria parasite, with the aim of generating an experimental basis for the development of a vaccine against this life-threatening disease. In the course of these experiments, we found that sporozoites of P. falciparum can prime as well as boost the protective response elicited by re viruses expressing sequences of the CS protein. Based on this observation, it is hoped that infected mosquito bites may, in the future, reinforce and prolong the protective effect of vaccination of individuals living in malaria endemic areas.
MATERIALS AND METHODS
Engineering re Influenza Viruses Expressing a CTL or B Cell Epitope of the CS Protein of P. falciparum.
The re influenza virus expressing the B cell epitope of the CS protein of P. falciparum (WSN/PfB1) was constructed by inserting the plasmodial amino acid sequence (NANP)3 into antigenic site B of the WSN/hemagglutinin. The FluPf virus was made by inserting the ORF encoding the plasmodial peptide KPKDELDYENDIEKKICKMEKCS after a leader peptide sequence in a bicistronic neuraminidase gene from WSN virus. Transfection of in vitro-synthesized RNA into Madin–Darby bovine kidney cells and rescue of infectious re influenza virus were done as described (6). The nucleotide sequence encoding CTL or B cell epitope has been confirmed by direct sequencing of purified viral RNA, and these re influenza viruses were designated FluPf or WSN/PfB1, respectively.
Construction of a re Vaccinia Virus Expressing the Entire P. falciparum CS Protein.
An EcoRI fragment containing the gene of the P. falciparum CS protein of the NF54 strain was isolated from the plasmid pA13 (7). This DNA fragment was blunt-ended by treatment with a large fragment of Escherichia coli DNA polymerase (Klenow), purified by agarose gel electrophoresis, and cloned into the SmaI site of the vaccinia virus insertion vector pSC11. As a result of this cloning strategy, we isolated a plasmid, pJRpfCS, containing the P. falciparum CS gene under control of the vaccinia virus early–late promoter p7.5, the β-galactosidase gene lacZ under control of the vaccinia virus late promoter p11, and flanking regions from the vaccinia virus thymidine kinase gene. To generate vaccinia P. falciparum CS re, the insertion vector pJRpfCS was introduced by homologous recombination into the thymidine kinase locus of the vaccinia virus genome. The re virus was selected by β-galactosidase expression and purified. The re vaccinia virus expressing the CS gene from the 7G8 strain of P. falciparum has been described (8).
Animals.
Female (5–8 weeks old) BALB/c (H-2d), C3H/HeOuJ (C3H) (H-2k), and C57BL/10SnJ (B10) (H-2b) mice were immunized with WSN/PfB1 for determination of their antibody response. For CD8+ T cell induction, B10BR and C3H mice (both H-2k) were used. Mice were purchased from The Jackson Laboratory or the National Institutes of Health, Frederick, MD.
Cells and Cell Culture Media.
LM1 cells (H-2k) were used as antigen- presenting cells or as target cells in CD8+ T cell assays (9). They were cultured in high glucose DMEM (Life Technologies, Grand Island, NY) supplemented as described. The medium used in the enzyme-linked immunospot (ELISPOT) assay and for the culture of immune lymphocytes was high glucose DMEM supplemented with 10% fetal calf serum, 10 mM Hepes, 1% EL-4 cell supernatant, 5 × 10−5 M 2-mercaptoethanol, and 2 mM l-glutamine (DMEM-EL4 medium).
Peptides and Lipopeptide.
A 23-mer peptide, KPKDELDYENDIEKKICKMEKCS, designated as P1, kindly was provided to us by A. Lal, Centers for Disease Control. This peptide contains the previously described CTL epitope of the CS protein of the 7G8 strain of P. falciparum recognized by B10.BR mice (10). Five overlapping 10-mer peptides were synthesized based on the sequence of this 23-mer, namely: PF1 = KPKDELDYEN; PF2 = DELDYENDIE; PF3 = DYENDIEKKI; PF4 = NDIEKKICKM; and PF5 = EKKICKMEKC.
The strain specificity of this epitope was determined by using a 22-mer peptide that has the amino acid sequence of the corresponding region of the NF54 strain of the CS protein of P. falciparum, KPKDELDYANDIEKKICKMKCS. An additional peptide, CSB3 = NDDSYIPSAEKI, which contains a T helper epitope recognized by H-2k mice, was used in some experiments aimed at inducing CD8+ T cells (11). We also used the peptide SDYEGRLI containing a known CTL epitope of the influenza virus nucleoprotein (12). The lipopeptide used in this study was generated as described (13) by linking two palmitic acid molecules to the NH2 terminus of the KSS-elongated PF3 peptide (Palm)2-KSS-DYENDIEKKI.
Sporozoites.
P. falciparum sporozoites of the 7G8 strain were obtained by salivary gland dissection of infected mosquitoes, kindly provided to us by I. Schneider of the Walter Reed Army Institute of Research. For the immunization of mice, 105 sporozoites were injected i.v. as an initial immunizing dose as well as a booster.
Routes of Immunizations, Schedules, and Dosages.
We used several immunization protocols as stated in the respective figure legends. For the induction of immune T cells, each mouse was injected with 2.5 nmol or 20 μg of the corresponding peptide plus 10 nmol of the CSB3 peptide emulsified in incomplete Freund’s adjuvant (IFA). The emulsion was injected s.c. at the base of the tail and into the foot pads. The mice were killed 10 days later, their spleens and inguinal and periaortic lymph nodes were removed, and the CS-specific CD8+ T cells of these organs were quantified by using an ELISPOT assay. Immunization with the lipopeptide also was done by s.c. injection by using a single dose of 120 μg. No other adjuvants were used in this immunization protocol. The combined virus immunization was done as described in the legend of Fig. 3 A and B.
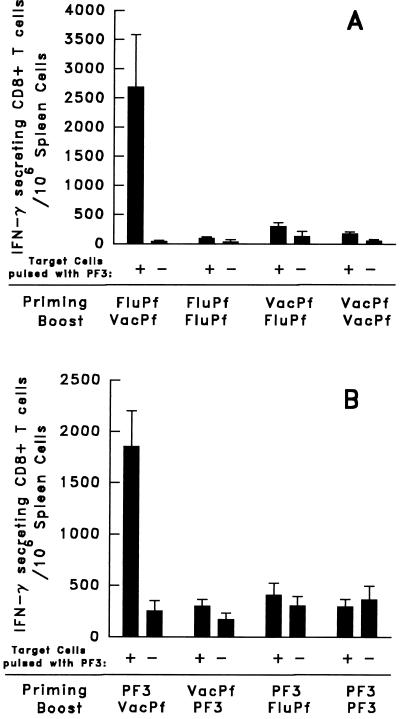
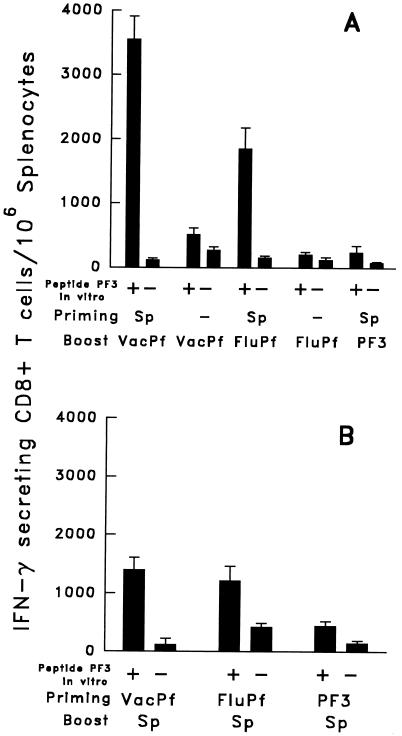
Figure 3.
CS-specific CD8+ spleen T cell response of C3H mice primed and/or boosted with FluPf, VacPf, or PF3. (A) Groups of three mice primed and/or boosted i.n. with 104 pfu of FluPf or 107 pfu of VacPf, injected i.p.. Boosters were given 21 days after priming using the same doses and routes of immunization. The number of specific IFN-γ- secreting CD8+ T cells in the spleen was determined by an ELISPOT assay 10 days after the booster. (B) Groups of three mice were primed and/or boosted with the peptide PF3 administered s.c. in IFA. Other mice were primed or boosted with VacPf or FluPf. (Bar = mean number of IFN-γ- producing cells/106 immune spleen T cells ± SE of duplicate cultures.)
Quantification of the CS-Specific CD8+ T Cells by an ELISPOT Assay.
The peptide PF3 represents an epitope not recognized by CD4+ but exclusively by CD8+ T cells secreting interferon (IFN) γ on in vitro restimulation by PF3-peptide pulsed target cells. None of these T cells secreted interleukin-4 or interleukin-5 Th-2- type lymphokines. We therefore used IFN-γ secretion by individual cells as an indicator of the presence of activated, specific CD8+ T cells. The standard protocol for the CD8+ ELISPOT assay was described (9, 5). The number of antigen-specific CD8+ IFN-γ- producing cells was determined by using freshly isolated spleens 24–28 hr after the splenocytes were incubated with peptide pulsed target cells (immediate ELISPOT assay) and also 6 days after in vitro stimulation of the splenocytes with peptide PF3 (expanded ELISPOT assay).
Antibody Detection by Indirect Immunofluorescence (IF) and ELISA.
The IF assay was performed on air-dried P. falciparum sporozoites as described (1). For the ELISA, we used a tetrabranched multiple antigen peptide based on the repeat of the P. falciparum CS protein [(NANP)3]4, designated (B)4, as a highly sensitive antigen for antibody detection (14). The titers correspond to the highest serum dilution giving an OD >2 × the mean OD obtained with normal BALB/c sera.
Immunization and Intracerebral (i.c.) Challenge with VacPf.
Female C3H mice were immunized first with the lipopeptide and then with 5 × 103 plaque-forming units (pfu) of FluPf as described in the legend of Fig. 6. Some immunized and naive control mice were killed 8 days later, and the number of CS-specific, IFN-γ-secreting CD8+ T cells in their spleens was determined. The remaining five mice from each group were challenged i.c. by the injection of 103 pfu of VacPf. To prevent bacterial infection, 1 mg/ml tetracycline hydrochloride was added to the drinking water throughout the study. All mice were weighed daily from the day of challenge to the day of death.
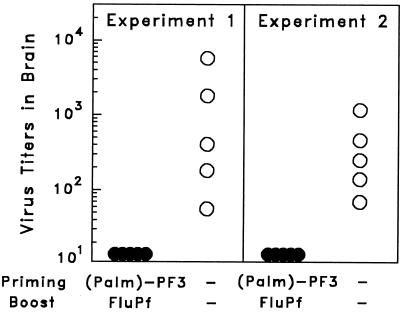
Figure 6.
Lipopeptide (120 μg) priming followed 2 weeks later by boosting with FluPf 5 × 103 pfu of C3H mice resulted in protection, manifested as a delay in vaccinia virus replication in the brain of immunized mice challenged with 103 pfu of VacPf. The number of vaccinia pfu in the brain of challenged mice (immunized and controls) was determined as described in Materials and Methods. The results of two identical experiments are shown.
Virus Replication in the Brain.
The level of virus replication in the brain of individual mice was determined 5 days after their i.c. challenge. Individual brains were weighed and homogenized in known volumes of Hanks’ balanced salt solution (Sigma). They then were frozen, thawed, sonicated, and centrifuged at 3,000 × g for 30 min. The supernatants kept on ice were diluted serially and placed on confluent CV-1 cells and overlaid with 1 ml of DMEM containing 2.5% fetal calf serum. After 2 days in a CO2 incubator at 37°C, the cells were stained with 0.1% crystal violet in 20% ethanol, and the number of virus plaques in the brain of individual mice was determined.
RESULTS
Induction and Persistence of Anti-Sporozoite Antibodies in Mice Immunized with WSN/PfB1.
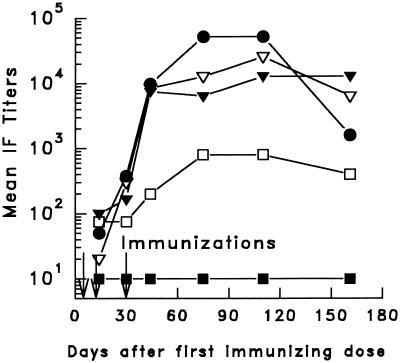
The immunogenicity of WSN/PfB1, expressing (NANP)3, was determined in BALB/c (H-2d), C3H (H-2k), and B10 (H-2b) mice. After repeated immunization, the mice of all three strains developed relatively high levels of anti-sporozoite antibodies, reaching or surpassing immunofluorescence titers of 1:12800 in several animals (data not shown). These titers were comparable to or greater than those obtained by repeated immunization with irradiated sporozoites. Exposing mice to three doses of WSN/PfB1 resulted in antibodies to P. falciparum sporozoites that persisted at high levels for the 5-month observation period (Fig. 1). Anti-peptide reactivity determined by ELISA, in the same sera, also showed high titers in mice of all three strains.
Figure 1.
Mean antisporozoite antibody titers of C3H, B6, and BALB/c mice immunized with WSN/PfB1. Immunofluorescence titers of the pooled sera of each group of mice were determined at the times indicated. After receiving the immunizing doses (indicated by arrows), the mice were bled monthly. Most mice received a first i.n. dose of 3 × 105 pfu followed 2 weeks later by the same i.n. dose and a third i.p. injection of 10 μg of the purified virus (C3H mice, •; B6, ▿; BALB/c, ▾). Two BALB/c mice received a single immunizing dose of WSN/PfB1 (□). B6 control mice received a single immunizing dose of WSN ▪.
Sequence of the CTL Epitope of the P. falciparum CS Protein Recognized by Murine CD8+ T Cells: Its Immunogenicity and Strain Specificity.
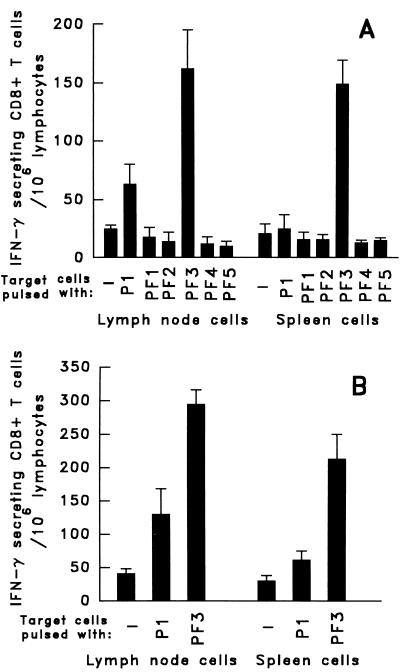
A 23-aa peptide originally was reported to contain the CTL epitope of the CS protein of P. falciparum (7G8 strain) recognized by murine CD8+ H-2k T cells (10). Because the optimal length for peptide binding to major histocompatibility complex class I molecules is usually between 8 and 10 aa, we decided to identify the precise sequence representing this epitope. Five overlapping 10-mer peptides (PF1– PF5) were synthesized corresponding to this 23-mer sequence. B10BR(H-2k) mice were immunized with a pool of these five peptides emulsified in IFA, adding the peptide CSB3, a universal T helper epitope of the CS protein. The spleens and lymph nodes of the mice were removed 10 days after s.c. immunization, and the T cells were incubated with target cells pulsed with the individual 10-mer peptides. A potent T cell response, revealed by secretion of IFN-γ, occurred only on incubation with the target cells pulsed with peptide PF3 (Fig. 2A), whereas the 23-mer peptide P1 gave a barely detectable response. A recent publication reached basically the same conclusion, namely that a nonamer peptide, differing only by the absence of the first N-terminal amino acid of PF3, contains this CTL epitope (15).
Figure 2.
Definition of the sequence and immunogenicity of the peptide containing the CTL epitope recognized by CS-specific CD8+ T cells. (A) Mice immunized once, s.c., with the peptide pool PF1–PF5 in IFA. Immune spleen and lymph node cells, obtained 10 days later, were restimulated in vitro with each of the 10-mer peptides and with the 23-mer P1. (B) Mice immunized s.c. with peptide PF3 in IFA. CD8+ immune spleen and lymph node cells restimulated in vitro with PF3 or P1 and the number of IFN-γ- secreting T cells determined.
The immunogenicity of peptide PF3 was determined by immunizing B10BR mice with this peptide emulsified in IFA. The ELISPOT assay performed on lymph nodes and splenic T cells of the immunized mice revealed close to 300 PF3-specific CD8+ T cells/106 lymph node cells and ≈200 PF3-specific cells/106 splenocytes (Fig. 2B), whereas target cells pulsed with the 23-mer peptide resulted in much smaller numbers of IFN-γ- secreting cells. This established the immunogenicity of peptide PF3, which contains a CTL epitope of the CS protein. This peptide was used in all subsequent immunization attempts as well as for the in vitro stimulation of immune CD8+ T cells.
The PF3 peptide used in our experiments, which contains the CTL epitope of the CS protein of the 7G8 strain of P. falciparum, differs in only two amino acids from the sequence of the corresponding region of the CS protein of the NF54 strain of this parasite. Mice were immunized with a vaccinia virus expressing the CS protein of the NF54 strain of P. falciparum to determine whether they elicited CD8+ T cells capable of recognizing PF3-pulsed target cells. Because this recognition failed to occur, it indicates that the two amino acid residues that differ in the PF3 domain of the CS protein of these two P. falciparum strains play a key role in epitope recognition (data not shown).
A Single Immunizing Dose of FluPf or VacPf Induces Low Levels of PF3-Specific CD8+ T Cells.
An immediate ELISPOT assay failed to detect PF3-specific CD8+ T cells in the spleens of B10BR mice 10 days after intranasal (i.n.) exposure to 103, 104, or 105 pfu of FluPf. However, after 6 days of in vitro stimulation of these cells with PF3-pulsed LM1 target cells, CS-specific CD8+ T cells were detected in these FluPf immunized mice. The largest response occurred in mice immunized with 103 pfu of FluPf, which displayed ≈3.4 × 104 IFN-γ-secreting cells/106 spleen cells (data not shown).
In view of these results, we considered the possibility that inoculation of the larger doses of FluPf (104 or 105 pfu) might have been immunosuppressive, decreasing the capacity of these animals to generate immune T cell responses. We therefore measured the response of splenic T cells of these same animals to peptide SDYEGRLI, a sequence of FluPf, which contains a CD8+ CTL epitope of the nucleoprotein of this virus. We detected 900 IFN-γ- secreting cells/106 spleen cells. Because the level of this response was comparable to that induced by immunization with the wild- type influenza virus, it indicated that the administration of 104 or 105 pfu of FluPf had not inhibited all CD8+ T cell responses.
As in the case of immunization with FluPf, immunization of mice with 105, 106, or 107 pfu of VacPf failed to elicit a PF3 specific CD8+ T cell response detectable by an immediate ELISPOT assay. However, 6 days after in vitro incubation of these immune cells with PF3- pulsed target cells, PF3-specific CD8+ T cells were detected among the splenocytes of these mice. The largest response was observed in mice immunized with 106 pfu of VacPf, which displayed ≈8 × 103 IFN-γ- secreting cells/106 spleen cells.
A Booster with VacPf Greatly Enhances the Primary PF3-Specific CD8+ T Cell Response Elicited by Immunization with FluPf or Peptide PF3.
Mice initially immunized with FluPf and later boosted with VacPf developed a PF3-specific CD8+ T cell response, which surpassed 2,500 IFN-γ-secreting T cells/106 freshly isolated spleen cells (immediate ELISPOT) (Fig. 3A). This response demonstrated that the very limited primary CD8+ T cell response to PF3, induced by immunization with FluPf, can be enhanced greatly by a subsequent inoculation of these mice with VacPf. These results, obtained in B10BR mice, were confirmed in mice of a different background, the C3H strain that shares the H-2k haplotype. Exposing C3H mice first to FluPf, followed 2 weeks later by an immunizing dose of VacPf, also resulted in a significant increase in the number of PF3- specific CD8+ T cells. It was noteworthy that reversing the order of administration of these re viruses, i.e., priming mice with VacPf followed by a second immunizing dose of FluPf, failed to increase the level of PF3-specific CD8+ T cells beyond that observed after the initial dose of VacPf (Fig. 3A).
The CD8+ T cell responses of mice primed with the PF3 peptide also was greatly enhanced when it was followed by the administration of VacPf. This type of combined immunization resulted in ≈2 × 103 PF3-specific CD8+ T cells/106 spleen cells (Fig. 3B). When we used the reverse schedule of immunization, the PF3 peptide failed to booster the response of VacPf-primed mice. The administration of two immunizing doses of the same re virus, or of peptide, i.e., VacPf, FluPf, or PF3 administered in IFA, failed completely to amplify the primary CD8+ T cell responses (Fig. 3 A and B).
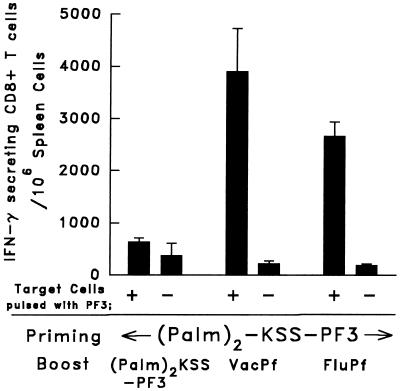
Immunogenicity of a Lipopeptide Based on the PF3 Sequence and Its Enhancement by VacPf or FluPf Booster.
Immunization with lipopeptides recently was shown to induce CD8+ T cell- mediated immune responses not only in mice but also in humans. Their administration also was shown to be safe as well as effective (16). These demonstrations led us to attempt to determine whether lipopeptides based on the PF3 sequence could induce specific CD8+ T cells. C3H mice were immunized s.c. with 120 μg of (Palm)2 -KSS-DYENDIEKKI, to which 10 nmol of the T helper epitope CSB3 was added. The spleen cells of mice immunized with 120 μg of this lipopeptide and examined 12 days later by the ELISPOT assay displayed ≈560 PF3-specific CD8+ T cells/10 6 spleen cells. When this immunization was followed by the administration of either VacPf or FluPf, the PF3-specific CD8+ T cell response was increased greatly. Thus, when VacPf was used as the second immunizing dose, we detected 3,900 IFN-γ- secreting, PF3-specific CD8+ T cells/106 spleen cells (Fig. 4). The administration of FluPf also considerably enhanced the peptide-specific CD8+ response elicited by the lipopeptide, reaching 2,700 PF3-specific CD8+ T cells/106 spleen cells (Fig. 4). A second injection of lipopeptide failed completely to boost the response elicited by the first dose. The administration of wild-type influenza or vaccinia viruses to lipopeptide-primed animals failed to enhance their primary response.
Figure 4.
Enhancement of the PF3- specific CD8+ T cell response in the spleen of C3H mice primed s.c. with 120 μg of the lipopeptide (Palm)2-KSS-PF3 and boosted 21 days later with 105 pfu of VacPf i.p., 104 pfu of FluPf i.n., or 80 μg of lipopeptide plus 10 nmol of CSB3, injected s.c. The freshly prepared T cells were incubated with PF3-pulsed irradiated LM1 cells, the number of IFN-γ-secreting T cells was determined. The spleens were removed 10 days after immunization.
Priming with P. falciparum Sporozoites Greatly Increases the PF3-Specific T Cell Responses to a Subsequent Immunization with FluPf or VacPf.
We found that an initial exposure of mice to sporozoites subsequently immunized with VacPf or FluPf resulted in considerable enhancement of their PF3-specific CD8+ T cell response. Particularly striking was the booster effect of VacPf on the CS-specific T cell response of sporozoite-primed mice, resulting in 3,600 PF3-specific CD8+ T cells/106 splenocytes (Fig. 5A). FluPf also boosted the sporozoite-induced PF3-specific CD8+ T cell response, but to a lesser degree, whereas a booster with the PF3 peptide failed to enhance the sporozoite-induced response.
Figure 5.
Administration of P. falciparum sporozoites increased the number of PF3-specific CD8+ T cells elicited by VacPf or FluPf. (A) C3H mice primed by the i.v. injection of 105 P. falciparum sporozoites were boosted i.p. with 107 pfu of VacPf, 104 pfu of FluPf i.n., or 120 μg of PF3 in IFA injected s.c. (B) Sporozoites (105 ) were injected i.v. 1 month after priming the mice with FluPf, VacPf, or PF3. Spleens were removed 10 days later, and the cells were examined by the ELISPOT assay.
We also used the reverse protocol to determine the effect that exposure to P. falciparum sporozoites might have on the PF3-specific T cell response of mice primed by an earlier inoculation of FluPf or VacPf and exposed 1 month later to an i.v. injection of 105 sporozoites of P. falciparum. The ELISPOT assay showed a PF3-specific splenic T cell response that was enhanced by the sporozoite booster (Fig. 5B). However, an i.v. injection of sporozoites enhanced only minimally the PF3- specific T cell response induced by priming with this peptide.
In Vivo Activation, Migration, and Protective Effect of PF3-Specific CD8+ T Cells.
Our next set of experiments, aimed to determine whether priming and boosting of mice with two distinct immunogens (both expressing the plasmodial CTL sequence) would generate functionally active PF3-specific T cells, capable of extravasating into internal organs where they could exert a protective role. The fact that mice are not susceptible to infection by sporozoites of the human malaria parasite P. falciparum makes it impossible to demonstrate directly, in vivo, the presence of protective malaria-specific T cells. We therefore used an indirect approach, developed years ago by Doherty et al.(17), for the detection of functionally active protective T cells in mice immunized against a pathogen that is not infective for mice.
Mice were immunized by an initial administration of lipopeptide (Palm)2 -PF3 followed by a booster with FluPf, also expressing the PF3 sequence. The challenge of the immunized and control animals was done by i.c. inoculation of VacPf, which by this route is highly pathogenic, replicating very rapidly. We found that the i.c. replication of VacPf was delayed considerably in the immunized mice compared with nonimmunized controls. In fact, in repeated experiments, VacPf was undetectable 5 days after challenge in the brains of the immunized animals at a time when it was detected in all control mice (Fig. 6). Weight loss due to viral infection occurred considerably later in immunized mice than in control mice.
It is noteworthy that, in these experiments, PF3 was the only sequence shared between the two immunogens used for priming and boosting the mice (lipopeptide and FluPf) and VacPf used for the i.c. challenge. These results indicate that the partial inhibition of re vaccinia virus replication in the immunized compared with control mice was mediated by the migration of PF3-specific activated protective CD8+ T cells to the brain.
DISCUSSION
The experiments presented in this manuscript were based on earlier observations indicating that re viruses that express sequences of foreign antigens can elicit potent cellular and antibody responses against these sequences, particularly if they represent internal microbial antigens. Moreover, the current experiments are the logical follow-up of our success in eliciting extensive protection in mice immunized by successive inoculation of two distinct re viruses (influenza and vaccinia) expressing sequences of the CS protein of P. yoelii (5).
The key question addressed by the current experiments was whether this type of immunization could be applied successfully to antigens of human malaria parasites such as the CS of P. falciparum. We characterized the humoral and cellular responses elicited by immunizing mice with re viruses and other constructs expressing selected sequences of the CS protein, a major P. falciparum antigen. Because mice are not susceptible to infection by this parasite, protection against challenge could not be determined directly. This limitation was circumvented by using a modification of an approach originally described by P. Doherty (17) that provides the opportunity to detect the presence of in vivo- activated, extravasating, protective, CS-specific CD8+ T cells in immunized mice. By using this approach, we found that the replication of i.c. inoculated VacPf was remarkably reduced in mice primed with a lipopeptide containing the CS-specific CD8+ T cell epitope PF3 and boosted with FluPf expressing the same peptide sequence. The observed anti-viral effect was most likely caused by the recognition by activated, extravasating CD8+ T cells of PF3 expressed by VacPf used for challenge. In fact, PF3 was the only sequence shared between the two immunogens (lipopeptide and FluPf) and the i.c. VacPf challenge.
As for the humoral immune response, we found that mice of three different H-2 haplotypes immunized with WSN/PfB1 produced relatively high titers of antibodies that reacted with P. falciparum sporozoites. These antibody titers decreased only slightly during the 5-month observation period.
With regard to the T cell response, we defined a 10-aa peptide, PF3, that contains an epitope of the P. falciparum CS protein recognized very effectively by CS-specific CD8+ T cells. This 10-mer was contained within a 23-aa sequence originally described as containing a CD8+ T cell epitope of this antigen. Immunization of B10/BR or C3H (H-2k) mice with re influenza or vaccinia viruses expressing PF3 failed to elicit a CS-specific T cell response detectable by an Immediate ELISPOT assay. However, after 6 days of in vitro stimulation with PF3- pulsed target cells, CS-specific, IFN-γ-secreting CD8+ T cells were detected in the spleen of both FluPf as well as VacPf immunized mice.
Noteworthy and important from the perspective of vaccine development was our finding that a first immunizing dose of FluPf, followed 2 weeks later by an inoculation of VacPf, resulted in a striking increase in the number of CS-specific CD8+ T cells. Under these conditions, an immediate ELISPOT revealed 2,500 IFN-γ/106 spleen cells. Neither the reverse order of immunization, VacPf followed by an inoculation of FluPf, nor two successive immunizing doses of the same re virus had any booster effect. Priming with another immunogen based on the PF3 sequence, such as the corresponding lipopeptide, followed days later by an immunizing dose of VacPf also resulted in a considerable enhancement of the primary CS-specific CD8+ T cell response.
Plausible explanations for the lack of adequate CS-specific priming by VacPf and for the highly effective booster it elicits might reside in the strong vaccinia-specific cellular response induced by an initial dose of this vector, competing and interfering with the T cell response to the foreign antigen. Priming with a different immunogen expressing PF3 circumvents this problem so that a booster with VacPf expressing PF3 elicits a great amplification of the earlier activated PF3-specific CD8+ T cells.
Probably most relevant to the situation in malaria endemic areas is our finding that the cellular response of mice to a single immunizing dose of sporozoites is enhanced greatly when it is followed by an inoculation of VacPf. This might indicate that the administration of a vaccine based on a viral vector, particularly vaccinia, expressing sporozoites and/or liver stage antigens of malaria parasites, could enhance greatly the usually low, inadequate immune response of most individuals exposed to multiple infected mosquito bites.
An HLA-based approach, termed “reverse immunogenetics”, led to the identification of peptides of CS and other sporozoite and/or liver stage antigens recognized by the presence of low levels of CD8+ T cells in the peripheral blood of individuals living in malaria endemic areas (18). Whether these human T cells can exert an inhibitory effect on the development of intra-hepatocytic stages of the malaria parasite remains uncertain.
In humans as in animals used in experiments, it is well established that high levels of anti-CS antibodies, which recognize the sporozoite surface, contribute to protection. This was demonstrated clearly by the complete resistance to sporozoite challenge of some and the delayed patency of other vaccinees immunized with (NANP)3 conjugated to tetanus toxoid (19) or other carriers. Recently, there was further indication of the protective role of anti-CS antibodies based on the very high titers of antibodies to P. falciparum sporozoites, detected in the sera of six protected individuals in a group of seven sporozoite- challenged vaccinees (20). These individuals were immunized with a fusion protein containing most of the repeats and the C terminal region of the CS of P. falciparum plus the surface antigen of hepatitis B virus combined with several different adjuvants. The possibility that cell-mediated immunity contributed to this protection is supported by the finding that appreciable CS-specific T cell responses were detected on in vitro restimulation of the peripheral blood lymphocytes of these vaccinees (R. Ballou, personal communication). The fine epitope specificity and the subset of these T cells currently is being defined. However, it remains to be ascertained whether these T cells contributed to the observed protection.
The experimental data obtained by immunizing mice with VacPf, FluPf, and P. falciparum sporozoites by using attenuated re viral vectors expressing plasmodial antigens deserve to be investigated further as bases for a malaria vaccine. Cold-adapted influenza viruses (21) and the modified Ankara (MVA) strain of vaccinia (22) expressing plasmodial sequences would be vectors of choice, because they can be expected to be safe for human use.
Acknowledgments
This work was supported by National Institutes of Health grants to R.S.N (RO-AI 36526), P.P., and A.G.-S.
ABBREVIATIONS
- CS
circumsporozoite
- CTL
cytotoxic T lymphocyte
- IFA
incomplete Freund’s adjuvant
- re
recombinant
- i.c.
intracerebral
- i.n.
intranasal
- pfu
plaque-forming unit
- IFN
interferon
- ELISPOT
enzyme-linked immunospot
References
- 1.Nussenzweig V, Nussenzweig R S. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- 2.Nardin E H, Nussenzweig R S. Annu Rev Immunol. 1993;11:687–727. doi: 10.1146/annurev.iy.11.040193.003351. [DOI] [PubMed] [Google Scholar]
- 3.Potocnjak P, Yoshida N, Nussenzweig R S, Nussenzweig V. J Exp Med. 1980;151:1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodrigues M, Nussenzweig R S, Romero P, Zavala F. J Exp Med. 1992;175:895–905. doi: 10.1084/jem.175.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues M, Li S, Murata K, Rodriguez D, Rodriguez J R, Bacik I, Bennink J R, Yewdell J W, García-Sastre A, Nussenzweig R S, et al. J Immunol. 1994;153:4636–4648. [PubMed] [Google Scholar]
- 6.Palese, P., Zavala, F., Muster, T., Nussenzweig, R. S. & García-Sastre, A. (1997) J. Infect. Dis. 176, Suppl., S45–S49. [DOI] [PubMed]
- 7.Caspers P, Gentz R, Matile H, Pink J R, Sinigaglia F. Mol Biochem Parasitol. 1989;35:185–190. doi: 10.1016/0166-6851(89)90121-7. [DOI] [PubMed] [Google Scholar]
- 8.Smith, G. L., Cheng, K. C. & Moss, B. (1986) Parasitol. 92, Suppl, S109–S117. [DOI] [PubMed]
- 9.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodrigues M M, Zavala F. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Miller L, Quakyi I, Keister D, Houghten R, Maloy W, Moss B, Berzofsky J, Good M. Nature (London) 1988;334:258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- 11.Romero P, Tam J, Schlessinger D, Clavijo P, Gibson H, Barr P, Nussenzweig R, Nussenzweig V, Zavala F. Eur J Immunol. 1990;18:1951–1957. doi: 10.1002/eji.1830181213. [DOI] [PubMed] [Google Scholar]
- 12.Gould K G, Scotney H, Brownlee G G. J Virol. 1991;65:5401–5409. doi: 10.1128/jvi.65.10.5401-5409.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nardin, E. H., Calvo-Calle, J. M., Oliveira, G. A., Clavijo, P., Nussenzweig, R., Simon, R., Zeng, W. & Rose, K. (1998) Vaccine, in press. [DOI] [PubMed]
- 14.Nardin E, Oliveira G A, Calvo-Calle M, Nussenzweig R S. Adv Immunol. 1995;60:105–149. doi: 10.1016/s0065-2776(08)60585-4. [DOI] [PubMed] [Google Scholar]
- 15.Malik A, Houghten R, Corradin G, Buus S, Berzofsky J A, Hoffman S L. Infect Immun. 1995;63:1955–1959. doi: 10.1128/iai.63.5.1955-1959.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitiello A, Ishioka G, Grey H M, Rose R, Farness P, LaFond R, Yuan L, Chisari F V, Furze J, Bartholomeuz R, et al. J Clin Invest. 1995;95:341–349. doi: 10.1172/JCI117662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty P C, Allan W, Boyle D B, Coupar B E H, Andrew M E. J Infect Dis. 1989;159:1119–1122. doi: 10.1093/infdis/159.6.1119. [DOI] [PubMed] [Google Scholar]
- 18.Aidoo M, Lalvani A, Allsopp C E M, Plebanski M, Meisner S J, Krausa P, Browning M, Morris-Jones S, Gotch F, Fidock D A, et al. Lancet. 1995;345:1003–1007. doi: 10.1016/s0140-6736(95)90754-8. [DOI] [PubMed] [Google Scholar]
- 19.Herrington D A, Clyde D F, Losonsky G, Cortesia M, Murphy J R, Davis J, Baqar S, Felix A M, Heimer E P, Gillessen D, et al. Nature (London) 1987;328:257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- 20.Stoute J A, Slaoui M, Heppner G, Momin P, Kester K, Desmons P, Wellde B T, Garçon N, Krzych U, Marchand, et al. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 21.Karron R A, Steinhoff M C, Subbarao E K, Wilson M H, Macleod K, Clements M L, Fries L H, Murphy B R. Pediatr Infect Dis J. 1995;14:10–16. doi: 10.1097/00006454-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sutter G, Wyatt L S, Foley P L, Bennink J R, Moss B. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]