Abstract
Background
Intensive statin therapy has been shown to improve prognosis in patients with coronary heart disease (CHD). It is unknown whether such benefit is mediated through the reduction of atherosclerotic plaque burden.
Aim
To examine the efficacy of high‐dose atorvastatin in the reduction of carotid intimal–medial thickness (IMT) and inflammatory markers in patients with CHD.
Design
Randomised trial.
Setting
Single centre.
Patients
112 patients with angiographic evidence of CHD.
Interventions
A high dose (80 mg daily) or low dose (10 mg daily) of atorvastatin was given for 26 weeks.
Main outcome measures
Carotid IMT, C‐reactive protein (CRP) and proinflammatory cytokine levels were assessed before and after therapy.
Results
The carotid IMT was reduced significantly in the high‐dose group (left: mean (SD), 1.24 (0.48) vs 1.15 (0.35) mm, p = 0.02; right: 1.12 (0.41) vs 1.01 (0.26) mm, p = 0.01), but was unchanged in the low‐dose group (left: 1.25 (0.55) vs 1.20 (0.51) mm, p = NS; right: 1.18 (0.54) vs 1.15 (0.41) mm, p = NS). The CRP levels were reduced only in the high‐dose group (from 3.92 (6.59) to 1.35 (1.83) mg/l, p = 0.01), but not in the low‐dose group (from 2.25 (1.84) to 3.36 (6.15) mg/l, p = NS). A modest correlation was observed between the changes in carotid IMT and CRP (r = 0.21, p = 0.03).
Conclusions
In patients with CHD, intensive atorvastatin therapy results in regression of carotid atherosclerotic disease, which is associated with reduction in CRP levels. On the other hand, a low‐dose regimen only prevents progression of the disease.
Management of patients with coronary heart disease (CHD) and hypercholesterolaemia by statins is one of the key components in the cocktail of medications in the current era.1 Although improvement in cardiovascular events by statins has been demonstrated in large, multicentre trials,1,2 current challenges focus on the approach of intensive statin therapy and on its benefits other than lipid lowering, such as a pleotrophic effect and modification of atherosclerosis plaque burden. In fact, recent clinical trials have demonstrated that high‐dose atorvastatin therapy in patients with CHD reduced the composite end points of mortality and major cardiovascular events.3,4 Intriguingly, such benefit was believed to be explained by the modification of the inflammatory reaction of atherosclerotic plaque, as reflected by the reduction in the high‐sensitivity C‐reactive protein (CRP) level.5 It is postulated that this plaque‐stabilisation effect may prevent the progression or may even promote the regression of atherosclerotic plaques in the arteries. In the REVERSAL study, high‐dose (80 mg daily) atorvastatin therapy prevented the increase in plaque volume in the coronary arteries, which was associated with a favourable decrease in CRP levels, in addition to low‐density lipoprotein (LDL) cholesterol reduction.6,7 With the use of non‐invasive imaging techniques, it is possible to monitor the effect of medical therapy in the modification of atherosclerotic plaque in various major arteries—in particular, the measurement of intimal–medial thickness (IMT) of the carotid arteries.8,9,10 For example, regression of carotid IMT by statin therapy was demonstrated in patients with familial hypercholesterolaemia,11,12 while comparison of IMT was considered as an important end point in drug trials of antihypertensive therapy, such as the Multicenter Isradipine Diuretic Atherosclerosis Study and Implementing New Strategies with Insulin Glargine for Hyperglycemia Therapy.13,14 In patients with CHD, however, it is less clear whether statin therapy can modify carotid IMT. Therefore, the objectives of this study were to examine whether intensive atorvastatin therapy regresses atherosclerosis by assessing carotid IMT, and to determine whether this is related to the reduction in inflammatory response, as reflected by CRP and inflammatory cytokines.
Methods
Patients
In total, 112 patients (mean (SD) age 66 (10) years, 82% men) with CHD were enrolled in the study. Patients were enrolled from July 2002 to June 2003, and were followed up for 6 months. In all patients, CHD was confirmed by angiographic evidence of coronary stenosis, with clinical evidence of previous myocardial infarction (46%), percutaneous coronary intervention (24%) or angina pectoris. These patients had hypercholesterolaemia, and so had already been treated with statin therapy before enrolment in the study (83%), or were newly diagnosed as having hypercholesterolaemia, with LDL cholesterol >2.6 mmol/l (or >100 mg/dl), or were determined by the in‐charge physicians to be suitable for statin therapy. Exclusion criteria included patients with significant systemic illness, infection, malignancy or any other terminal illness who were unlikely to survive for more than 6 months, patients with known contraindication to statin therapy and those who were unable to participate in the study. Concurrent medications are listed in table 1. The study was approved and conducted in compliance with the regulations of the ethics committee of the institution. Written informed consent was obtained from all patients.
Table 1 Comparison and clinical and demographic characteristics in low‐dose and high‐dose atorvastatin treatment groups (n = 53).
| Low dose (n = 55) | High dose (n = 57) | ||
|---|---|---|---|
| Age (years), mean (SD) | 66 (10) | 66 (9) | — |
| Sex, male:female | 40 (78%):11 (22%) | 52 (85%):9 (15%) | χ2 = 0.88 |
| Previous AMI | 22 (43.1%) | 29 (47.5%) | χ2 = 1.49 |
| Previous PCI | 9 (17.6%) | 17 (29.3%) | χ2 = 2.03 |
| Severity of CHD | |||
| One‐vessel disease | 20 (39.3%) | 36 (59%) | |
| Two‐vessel disease | 21 (41.2%) | 14 (23%) | χ2 = 3.96 |
| Triple‐vessel disease | 10 (19.6%) | 11 (18%) | |
| Heart failure | 8 (15.7%) | 11 (18%) | χ2 = 0.11 |
| Hypertension | 24 (47%) | 33 (54%) | χ2 = 1.13 |
| Diabetes | 13 (25%) | 18 (29.5%) | χ2 = 0.22 |
| Smoking | 26 (51.0%) | 23 (37.7%) | χ2 = 1.73 |
| LDL cholesterol, mol/l (or mg/dl), mean (SD) | 2.99 (1.02) (or 116 (39)) | 2.72 (0.85) (or 105 (33)) | — |
| Triglyceride, mmol/l (or mg/dl), mean (SD) | 1.77 (1.07) (or 156 (95)) | 1.81 (1.40) (or 160 (150)) | — |
| Medications | |||
| Antiplatelet | 50 (98.0%) | 59 (96.7%) | χ2 = 1.12 |
| Oral nitrates | 25 (49.0%) | 29 (47.5%) | χ2 = 0.02 |
| β‐Blockers | 34 (66.7%) | 48 (78.7%) | χ2 = 2.05 |
| Calcium channel blockers | 7 (13.7%) | 11 (18.0%) | χ2 = 0.38 |
| ACEI or ARB | 32 (62.7%) | 41 (67.2%) | χ2 = 0.24 |
| Diuretics | 4 (7.8%) | 4 (6.6%) | χ2 = 0.07 |
| Statins | 43 (84.3%) | 50 (82.0%) | χ2 = 1.34 |
ACEI, angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CHD, coronary heart disease; LDL, low‐density lipoprotein; PCI, percutaneous coronary intervention.
Values are n (%) unless otherwise specified. p = NS.
Study design
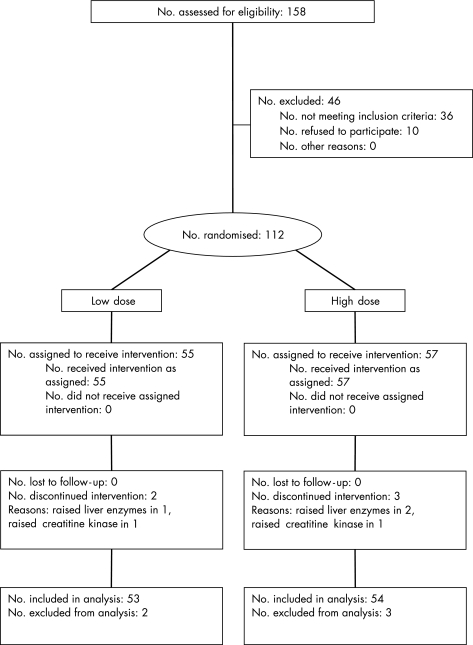
This is a double‐blind, randomised, controlled study to compare the efficacies of high‐ and low‐dose atorvastatin therapy (fig 1). All patients entered the washout phase, which lasted for 1 week. Any pre‐existing lipid‐lowering drugs were stopped during this period. Among these patients, 15 were statin naïve. All the other patients had previously received a relatively low dosage of statin, which included atorvastatin (5 mg in 6, 10 mg in 30 and 20 mg in 7 patients), simvastatin (5 mg in 3, 10 mg in 18, 20 mg in 27 and 40 mg in 2 patients) and pravastatin (10 mg in 2 patients and 20 mg in 2 patients). At the end of the washout phase and before randomisation, the IMT in both carotid arteries was measured by B‐mode ultrasound, and blood samples were collected for lipid profile, CRP, tumour necrosis factor‐α (TNF‐α), interleukin (IL)‐6, 8 and 18, as well as for other routine biochemical tests. Patients were then randomised to either low‐dose (10 mg daily) or high‐dose (80 mg daily) atorvastatin therapy for 6 months. The randomisation was allocated by means of numbered containers. The patients and all study personnel were blinded to treatment assignment. No other restrictions were used in the randomisation process. In the high‐dose group, atorvastatin was started from 20 mg daily for 2 weeks, then increased to 40 mg daily for another 2 weeks, and eventually reached the full dose starting from week 5. The number of tablets and medication bottles were designed to be equal to ensure blinding of randomisation. Patients were followed up regularly at 2, 4, 6, 14 and 26 weeks, when blood tests were repeated. At week 26, B‐mode ultrasound for carotid IMT was repeated. Any adverse event during the study period was reported promptly to the investigators and confirmed by hospital record. The primary end point of the study was the improvement in carotid IMT at week 26. The secondary end point was the reduction in CRP level and proinflammatory cytokines at week 26.
Figure 1 Flow diagram of the study.
Measurement of carotid IMT
Longitudinal images of the left and right carotid arteries were acquired by experienced cardiologists using a 10 MHz linear vascular probe (Vivid 5 or Vivid 7, GE Vingmed Ultrasound, Horten, Norway). Patients lay in the supine position during the examination. The common carotid artery segment was defined as the arterial segment 1 cm proximal to the carotid bulb/dilation.15,16 Minimal gain was adjusted to visualise the lumen–intimal and medial–adventitial interfaces defining IMT in the far wall. Digital images of three cardiac cycles were saved using ECG signals to optical discs. Carotid IMT was measured offline using a dedicated software (Carotid Analyzer, Medical Imaging Applications, Coralville, Iowa, USA) by an investigator blinded to randomisation and all clinical information. Measurements were performed automatically by the software while the region of interest was placed on the far wall of the common carotid artery segment along 1 cm length at end‐diastole.15,16 The maximal IMT was taken, not including plaques, for the following calculation. Plaque was defined as a localised thickening >1.2 mm that did not uniformly involve the whole artery. The mean of the maximal IMT values over three cardiac cycles was calculated for the left and right carotid arteries.
CRP and proinflammatory cytokine levels
Serum high‐sensitivity CRP concentration was measured by chemiluminescence immunoassay (Immulite Analyzer, Diagnostic Products, California, USA), with a detection limit of 0.1 mg/l. Serum IL‐6, IL‐8, IL‐18 and TNF‐α levels were measured by enzyme‐linked immunosorbent assay (BioSource International, California, USA), with detection limits of 0.2, 12.5, 2.0 and 0.09 μg/l, respectively.
Statistics
The parametric variables before and after atorvastatin therapy in each group were compared by paired sample t test. The comparison of parametric variables between the two groups was performed by unpaired t test. Pearson's correlation analysis was used to examine the relationship between the changes in CRP levels, LDL cholesterol levels and carotid IMT. In order to detect a 0.1 mm or 10% reduction in carotid IMT, given an SD of about 0.3 mm and a statistical power of 80%, a sample size of 50 patients in each arm was needed to allow for two‐sided α levels of 0.05. All data were expressed as mean (SD). A p value <0.05 was considered significant.
Results
The baseline clinical characteristics of the high‐dose and low‐dose groups are listed in table 1. There was no difference in patient demographics, severity of CHD by clinical diagnosis or by coronary angiography as well as medications (table 1). No patient was lost to follow‐up during the study period.
Changes in plasma lipid profiles after statin therapy
With respect to the baseline plasma lipid profiles, there was no significant difference between the two groups for total, high‐density lipoprotein (HDL) and LDL cholesterol as well as triglyceride values. The pre‐randomisation LDL cholesterol was 2.99 (1.02) mmol/l in the low‐dose group and 2.72 (0.85) mmol/l in the high‐dose group (p = NS; table 1). At the end of 26 weeks, this was reduced by 21.3% in the low‐dose group (p<0.001 vs baseline) and by 37.2% (p<0.001 vs baseline) in the high‐dose group (tables 2 and 3). The improvement was significantly greater in the high‐dose than in the low‐dose group (p = 0.001). Reduction of total cholesterol and triglyceride levels was also observed in both groups, with greater magnitude in the high‐dose group (−16.8% vs −23.6% for total cholesterol and −5.3% vs −18.5% for triglyceride). The HDL cholesterol levels were unchanged in both groups (tables 2 and 3).
Table 2 Changes in lipid profile, C‐reactive protein and other proinflammatory cytokine levels in the low‐dose atorvastatin group.
| Baseline | 26 weeks | p Value | |
|---|---|---|---|
| Total cholesterol, mmol/l (or mg/dl) | 5.08 (1.28) (or 197 (50)) | 4.13 (0.98) (or 160 (38)) | <0.001 |
| LDL cholesterol, mmol/l (or mg/dl) | 2.99 (1.02) (or 116 (39)) | 2.24 (0.68) (or 87 (26)) | <0.001 |
| HDL cholesterol, mmol/l (or mg/dl) | 1.29 (0.67) (or 50 (26)) | 1.30 (0.34) (or 50 (13)) | NS |
| Triglyceride, mmol/l (or mg/dl) | 1.77 (1.07) (or 157 (95)) | 1.49 (0.80) (or 132 (71)) | 0.002 |
| CRP (mg/l) | 2.25 (1.84) | 3.36 (6.15) | NS |
| IL‐6, pg/l | 4.32 (2.44) | 3.69 (3.43) | NS |
| IL‐8, pg/l | 4.57 (3.22) | 3.46 (1.19) | 0.01 |
| IL‐18, pg/l | 280 (100) | 244 (93) | <0.001 |
| TNF‐α, pg/l | 3.21 (1.43) | 1.94 (1.13) | <0.001 |
| WBC, ×109/l | 6.99 (2.12) | 7.03 (1.98) | NS |
| ALT, mmol/l | 23.6 (9.4) | 28.5 (13.3) | NS |
| CPK, mmol/l | 120 (59) | 140 (86) | NS |
ALT, alanine aminotransferase; CHD, coronary heart disease; CPK, creatinine phosphokinase; CRP, C‐reactive protein; HDL, high‐density lipoprotein; IL, interleukin; LDL, low‐density lipoprotein; NS, non‐significant; TNF‐α, tumour necrosis factor‐α; WBC, white cell count.
Values are means (SD).
Table 3 Changes in lipid profile, C‐reactive protein and other proinflammatory cytokine levels in the high‐dose atorvastatin group.
| Baseline | 26 week | p Value | |
|---|---|---|---|
| Total cholesterol, mmol/l (or mg/dl) | 4.48 (1.15) (or 173 (45)) | 3.32 (0.75) (or 128 (29)) | <0.001 |
| LDL cholesterol, mmol/l (or mg/dl) | 2.72 (0.85) (or 105 (33)) | 1.67 (0.60) (or 65 (23)) | <0.001 |
| HDL cholesterol, mmol/l (or mg/dl) | 1.05 (0.33) (or 41 (13)) | 1.10 (0.31) (or 43 (12)) | NS |
| Triglyceride, mmol/l (or mg/dl) | 1.81 (1.40) (or 160 (124)) | 1.25 (0.65) (or 111 (58)) | <0.001 |
| CRP, mg/l | 3.92 (6.59) | 1.35 (1.83) | 0.01 |
| IL‐6, pg/l | 5.75 (5.75) | 4.42 (4.33) | 0.003 |
| IL‐8, pg/l | 4.42 (1.76) | 3.80 (2.30) | 0.02 |
| IL‐18, pg/l | 298 (109) | 273 (108) | 0.03 |
| TNF‐α, pg/l | 2.85 (1.51) | 1.99 (0.86) | <0.001 |
| WBC, ×109/l | 6.81 (1.50) | 7.01 (1.72) | NS |
| ALT, mmol/l | 28.5 (17.8) | 34.7 (19.9) | NS |
| CPK, mmol/l | 116 (80) | 141 (68) | NS |
ALT, alanine aminotransferase; CPK, creatinine phosphokinase; CRP, C‐reactive protein; HDL, high‐density lipoprotein; IL, interleukin; LDL, low‐density lipoprotein; NS, non‐significant; TNF‐α, tumour necrosis factor‐α; WBC, white cell count.
Values are in means (SD).
Changes in carotid IMT after statin therapy
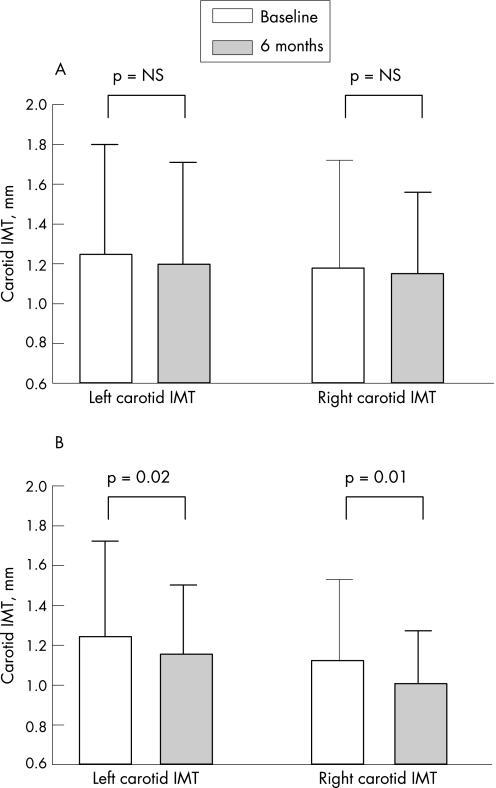
For the primary end point of carotid IMT, there was a trend, but insignificant, towards a reduction in the low‐dose group (left carotid: −4.0%, p = NS; right carotid: −2.5%, p = NS; tables 4 and 5 and figs 2 and 3). However, in the high‐dose group, 26‐week therapy resulted in a significant reduction in carotid IMT (left carotid: −7.3%, p = 0.02; right carotid: −9.8%, p = 0.01; tables 4 and 5 and figs 2 and 4). In both groups, there was a good correlation between baseline carotid IMT and the absolute reduction in IMT (low dose: r = −0.66, p<0.001; high dose: r = 0.78, p<0.001).
Table 4 Changes in carotid intimal–medial thickness in the low‐dose atorvastatin treatment group.
| Baseline | 6 month | p Value | |
|---|---|---|---|
| Left carotid IMT, mm | 1.25 (0.55) | 1.20 (0.51) | NS |
| Right carotid IMT, mm | 1.18 (0.54) | 1.15 (0.41) | NS |
IMT, intimal–medial thickness; NS, non significant.
Values are means (SD).
Table 5 Changes in carotid intimal–medial thickness (IMT) in the high‐dose atorvastatin treatment group.
| Baseline | 6 month | p Value | |
|---|---|---|---|
| Left carotid IMT, mm | 1.24 (0.48) | 1.15 (0.35) | 0.02 |
| Right carotid IMT, mm | 1.12 (0.41) | 1.01 (0.26) | 0.01 |
Values are means (SD).
Figure 2 Bar charts showing the changes in carotid intimal–medial thickness (IMT) between baseline and after 6 months of atorvastatin therapy in the low‐dose (A) and high‐dose groups (B).
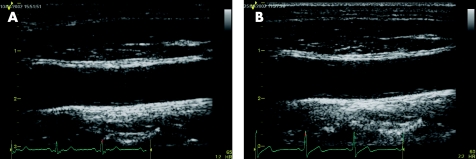
Figure 3 Carotid B‐mode ultrasound of a patient put on low‐dose atorvastatin (10 mg daily). There was no improvement in carotid intimal–medial thickness between baseline (A) and after treatment for 26 weeks (B).
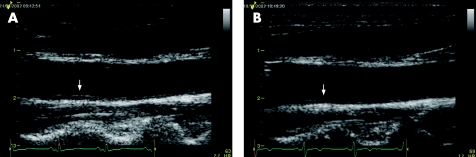
Figure 4 Carotid B‐mode ultrasound of a patient put on high‐dose atorvastatin (80 mg daily). The baseline (A) image showed an area of increased carotid intimal–medial thickness (arrow), which was significantly regressed after atorvastatin treatment for 26 weeks (B).
Changes in inflammatory markers
Serum CRP level showed a non‐significant trend to increase in the low‐dose group at 26 weeks. In contrast, there was a significant reduction in CRP level in the high‐dose group (p = 0.01). For proinflammatory cytokines, there was a significant reduction in IL‐8, IL‐18 and TNF‐α levels in the low‐dose group, but not in IL‐6 (table 2). However, all of the proinflammatory cytokines were significantly reduced in the high‐dose group (table 3). The white cell count remained unchanged in both groups.
There was no correlation between the changes in LDL cholesterol and CRP levels (r = 0.04, p = NS) or between changes in LDL cholesterol and carotid IMT (r = 0.06, p = NS). However, a modest but significant correlation was observed between the changes in carotid IMT and CRP levels (r = 0.21, p = 0.03).
Clinical follow‐up and discontinuation rate of atorvastatin
At the end of the study, no patient was lost to follow‐up and there was no death. There were six hospitalisations during the study period, with two in the low‐dose group and four in the high‐dose group (p = NS). The causes of hospitalisation included unstable angina in one patient and percutaneous coronary intervention for angina in another patient in the low‐dose group, and unstable angina in one patient, percutaneous coronary intervention in one patient, stroke in one and heart failure in another patient in the high‐dose group. Two patients discontinued atorvastatin therapy in the low‐dose group, and the reasons were elevated alanine aminotransferase in one and elevated muscle creatinine phosphokinase in another patient. In the high‐dose group, three patients discontinued atorvastatin. The reasons were elevated alanine aminotransferase in two patients and elevated creatinine phosphokinase in one patient.
Comment
This study is the first double‐blind, randomised, controlled study that demonstrates the regression of carotid IMT by intensive statin therapy (atorvastatin 80 mg daily) in patients with CHD. Such a benefit was not observed in the low‐dose (atorvastatin 10 mg daily) group. Reduction of CRP, an inflammatory marker for adverse outcome of CHD, was observed only in the high‐dose group. Therefore, secondary prevention by high‐dose atorvastatin therapy is able to reduce carotid atherosclerotic plaque burden, which is associated with the decrease in inflammatory activity of the plaque, as reflected by the reduced CRP and proinflammatory cytokine levels.
Regression of carotid IMT by intensive statin therapy
Modulation of atherosclerotic plaque burden is a new treatment target in patients with CHD. It has been shown that the progression of atherosclerotic plaque is an active disease process that involves a cascade of reactions, including the accumulation of lipid molecules, infiltration of macrophages to form foam cells, and eventually formation of lipid and fibrocellular components in the intimal–medial layer of the arterial wall. Atherosclerosis is not a focal disease, but is a “systemic” condition that also affects other major arteries of the body. While accurate quantification of atherosclerotic plaque volume in the coronary arteries is possible by invasive intravascular ultrasound, examination of carotid arteries for IMT is proven to be a reliable marker to reveal the severity of atherosclerosis and to predict the presence of CHD.8,9,10,17,18,19,20 Furthermore, assessment of carotid IMT is also shown to be a strong indicator for adverse cardiovascular events.10,21,22,23 Therefore, the current double‐blind, randomised, controlled study examined the efficacy of atorvastatin therapy in modifying atherosclerotic plaque burden in patients with CHD by assessing the IMT in both common carotid arteries. It was observed that high‐dose atorvastatin therapy not only prevented the progression of atherosclerotic plaque formation in the carotid arteries but also actually regressed the IMT by up to 10% after 26 weeks. This was in contrast to the low‐dose regimen that only prevented the progression of carotid disease.
Despite the high prevalence of CHD in many communities, this study is the only double‐blind, randomised, controlled study to demonstrate the superiority of high‐dose statin therapy in the modulation of carotid IMT in these patients. The role of intensive statin therapy in carotid IMT was examined in subjects with familial hypercholesterolaemia.11,12 In the ASAP study, 325 subjects with familial hypercholesterolaemia who had very high cholesterol levels (mean 10 mmol/l or 38.7 mg/dl) were randomised to atorvastatin 80 mg daily or simvastatin 40 mg daily for 24 months, and regression of carotid IMT was observed only in the atorvastatin group.12 In 18 subjects with asymptomatic familial hypercholesterolaemia with documented plaques, atherosclerotic plaque regression in the aorta and carotid arteries was also demonstrated by magnetic resonance imaging after treatment by simvastatin for 12 months.24 In the present study, the finding of regression of carotid IMT after high‐dose atorvastatin therapy in patients with CHD was in contrast to the REVERSAL study, where intravascular ultrasound demonstrated only the prevention of coronary atherosclerotic plaque progression by high‐dose atorvastatin therapy.6 The benefit of statin therapy has also been demonstrated by coronary angiography in a placebo controlled study which reported that simvastatin and niacin therapy markedly reduced coronary angiographic progression in patients with CHD and low HDL levels.25 Furthermore, in an open‐label, randomised trial, treatment with atorvastatin (mean dose of 32.5 mg) was found to prevent progression of plaque volume in coronary arteries in patients with CHD, when compared with usual care that comprised cholestyramine, fibrate and statin in half of the patients.26 The benefit of intensive atorvastatin therapy in our study could have been underestimated, as most of our patients had been treated with statin therapy which might have already exerted some beneficial effect and which also explains the relatively low baseline LDL cholesterol levels.
Improvement of inflammatory markers by intensive statin therapy
Recent large, randomised, multicentre trials have confirmed the superiority of high‐dose atorvastatin therapy (80 mg daily) in the reduction of cardiovascular events when compared with less intensive regimens, such as atorvastatin 10 mg daily or pravastatin 40 mg daily, in patients with CHD.3,4 This aggressive regimen commonly lowers LDL cholesterol to a level <80 mg/dl (or <2.07 mmol/l)3,4,6—that is, much lower than the target level of the current guideline of <100 mg/dl.27 The reason why intensive statin therapy is better than low‐ or moderate‐dose statin therapy is not entirely understood. It is now known that the amount of atherosclerotic plaque burden is a major determinant of risk of plaque rupture leading to cardiovascular events.28,29 Similarly, carotid IMT has been demonstrated to be a predictor of adverse cardiovascular events.21,22,23 Therefore, reduction in carotid IMT is likely to be a marker of reduced atherosclerotic plaque burden in the major arteries, which may explain why cardiovascular and cerebrovascular event rates were decreased by intensive atorvastatin therapy in recent multicentre trials.3,4 As most of the patients had been receiving statin therapy at a dose equivalent to the low‐dose regimen, this might have negated some of the potential early benefit of low‐dose statin on the progression of carotid IMT. However, this will not affect the comparison between high‐ and low‐dose regimen, as such an effect was present in both groups.
Recently, the benefit of high‐dose atorvastatin therapy in preventing the progression of atherosclerotic plaque in coronary arteries was explained by the favorable reduction of CRP rather than LDL cholesterol levels.6,7 In the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE‐IT) study, those patients who had improvement of both LDL cholesterol and CRP levels after statin therapy had the most favourable clinical outcome.5 Therefore, it appears that aggressive atorvastatin therapy is able to modify atherosclerotic plaque by reducing the inflammatory activity that may be associated with the reduction of CRP levels. It results in less uptake of oxidised LDL by macrophages and production of other proinflammatory cytokines and adhesion molecules, in addition to a further decrease in the LDL cholesterol by high‐dose therapy. In our present study, the CRP level was decreased by 65% in the high‐dose group, but showed a trend to increase in the low‐dose group. The favourable change in carotid IMT also correlated with the change in CRP level after statin therapy. All the proinflammatory cytokines tested were also significantly reduced in the high‐dose group, which included IL‐6, IL‐8 and IL‐18 as well as TNF‐α.30,31,32,33 The reduction of inflammatory activity in atherosclerotic plaques is likely to be an important mediator of plaque regression in the carotid arteries.
Acknowledgements
We thank Dr Raymond HW Chan, Dr MH Jim, Dr David CW Siu, Dr HH Ho and Dr Raymond Miu for their contributions to patient care in the study. This study is in part supported by a research fund from Pfizer. Hong Kong and a research grant from the Li Ka Shing Institute of Health Sciences.
Abbreviations
CHD - coronary heart disease
CRP - C‐reactive protein
HDL - high‐density lipoprotein
IMT - intimal–medial thickness
IL - interleukin
LDL - low‐density lipoprotein
TNF‐α - tumour necrosis factor α
References
- 1.Sacks F M, Pfeffer M A, Moye L A.et al The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 19963351001–1009. [DOI] [PubMed] [Google Scholar]
- 2.Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 19943441383–1389. [PubMed] [Google Scholar]
- 3.Cannon C P, Braunwald E, McCabe C H.et al Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 20043501495–1504. [DOI] [PubMed] [Google Scholar]
- 4.Larosa J C, Grundy S M, Waters D D.et al Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 20053521425–1435. [DOI] [PubMed] [Google Scholar]
- 5.Ridker P M, Cannon C P, Morrow D.et al C‐reactive protein levels and outcomes after statin therapy. N Engl J Med 200535220–28. [DOI] [PubMed] [Google Scholar]
- 6.Nissen S E, Tuzcu E M, Schoenhagen P.et al Effect of intensive compared with moderate lipid‐lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 20042911071–1080. [DOI] [PubMed] [Google Scholar]
- 7.Nissen S E, Tuzcu E M, Schoenhagen P.et al Statin therapy, LDL cholesterol, C‐reactive protein, and coronary artery disease. N Engl J Med 200535229–38. [DOI] [PubMed] [Google Scholar]
- 8.Crouse I I I, JR, Craven T E, Hagaman A P.et al Association of coronary disease with segment‐specific intimal–medial thickening of the extracranial carotid artery. Circulation 1995921141–1147. [DOI] [PubMed] [Google Scholar]
- 9.Geroulakos G, O'Gorman D J, Kalodiki E.et al The carotid intima‐media thickness as a marker of the presence of severe symptomatic coronary artery disease. Eur Heart J 199415781–785. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary D H, Polak J F, Kronmal R A.et al Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 199934014–22. [DOI] [PubMed] [Google Scholar]
- 11.Nolting P R, de Groot E, Zwinderman A H.et al Regression of carotid and femoral artery intima‐media thickness in familial hypercholesterolemia: treatment with simvastatin. Arch Intern Med 20031631837–1841. [DOI] [PubMed] [Google Scholar]
- 12.Smilde T J, van Wissen S, Wollersheim H.et al Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double‐blind trial. Lancet 2001357577–581. [DOI] [PubMed] [Google Scholar]
- 13.Borhani N O, Mercuri M, Borhani P A.et al Final outcome results of the Multicenter Isradipine Diuretic Atherosclerosis Study (MIDAS). A randomized controlled trial. JAMA 1996276785–791. [PubMed] [Google Scholar]
- 14.Simon A, Gariepy J, Moyse D.et al Differential effects of nifedipine and co‐amilozide on the progression of early carotid wall changes. Circulation 20011032949–2954. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell C K, Aeschlimann S E, Korcarz C E. Carotid intima‐media thickness testing: technical considerations. J Am Soc Echocardiogr 200417690–692. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, Yamane K, Fujikawa R.et al Westernization of lifestyle markedly increases carotid intima‐media wall thickness (IMT) in Japanese people. Atherosclerosis 200316667–72. [DOI] [PubMed] [Google Scholar]
- 17.Holaj R, Spacil J, Petrasek J.et al Intima‐media thickness of the common carotid artery is the significant predictor of angiographically proven coronary artery disease. Can J Cardiol 200319670–676. [PubMed] [Google Scholar]
- 18.Balbarini A, Buttitta F, Limbruno U.et al Usefulness of carotid intima‐media thickness measurement and peripheral B‐mode ultrasound scan in the clinical screening of patients with coronary artery disease. Angiology 200051269–279. [DOI] [PubMed] [Google Scholar]
- 19.Hallerstam S, Larsson P T, Zuber E.et al Carotid atherosclerosis is correlated with extent and severity of coronary artery disease evaluated by myocardial perfusion scintigraphy. Angiology 200455281–288. [DOI] [PubMed] [Google Scholar]
- 20.Kablak‐Ziembicka A, Tracz W, Przewlocki T.et al Association of increased carotid intima‐media thickness with the extent of coronary artery disease. Heart 2004901286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha A K, Eigenbrodt M, Mehta J L. Does carotid intima media thickness indicate coronary atherosclerosis? Curr Opin Cardiol 200217526–530. [DOI] [PubMed] [Google Scholar]
- 22.Hodis H N, Mack W J, LaBree L.et al The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med 1998128262–269. [DOI] [PubMed] [Google Scholar]
- 23.Chambless L E, Folsom A R, Clegg L X.et al Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 2000151478–487. [DOI] [PubMed] [Google Scholar]
- 24.Corti R, Fayad Z A, Fuster V.et al Effects of lipid‐lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high‐resolution, noninvasive magnetic resonance imaging. Circulation 2001104249–252. [DOI] [PubMed] [Google Scholar]
- 25.Brown B G, Zhao X Q, Chait A.et al Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 20013451583–1592. [DOI] [PubMed] [Google Scholar]
- 26.Schartl M, Bocksch W, Koschyk D H.et al Use of intravascular ultrasound to compare effects of different strategies of lipid‐lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation 2001104387–392. [DOI] [PubMed] [Google Scholar]
- 27.National Cholesterol Education Program (NCEP) Expert Panel Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 20012852486–2497. [DOI] [PubMed] [Google Scholar]
- 28.Fuster V, Stein B, Ambrose J A.et al Atherosclerotic plaque rupture and thrombosis. Evolving concepts. Circulation 199082II47–II59. [PubMed] [Google Scholar]
- 29.Little W C. Angiographic assessment of the culprit coronary artery lesion before acute myocardial infarction. Am J Cardiol 19906644G–47G. [DOI] [PubMed] [Google Scholar]
- 30.Biasucci L M, Vitelli A, Liuzzo G.et al Elevated levels of interleukin‐6 in unstable angina. Circulation 199694874–877. [DOI] [PubMed] [Google Scholar]
- 31.Blankenberg S, Tiret L, Bickel C.et al Interleukin‐18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation 200210624–30. [DOI] [PubMed] [Google Scholar]
- 32.Aukrust P, Berge R K, Ueland T.et al Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. J Am Coll Cardiol 200137485–491. [DOI] [PubMed] [Google Scholar]
- 33.Waehre T, Damas J K, Gullestad L.et al Hydroxymethylglutaryl coenzyme a reductase inhibitors down‐regulate chemokines and chemokine receptors in patients with coronary artery disease. J Am Coll Cardiol 2003411460–1467. [DOI] [PubMed] [Google Scholar]