Abstract
Background
β Blocker treatment may worsen glucose metabolism.
Objective
To study the development of new onset diabetes in a large cohort of patients with heart failure treated with either metoprolol or carvedilol.
Design
Prospective and retrospective analysis of a controlled clinical trial.
Setting
Multinational multicentre study.
Patients
3029 patients with chronic heart failure.
Interventions
Randomly assigned treatment with carvedilol (n = 1511, target dose 50 mg daily) or metoprolol tartrate (n = 1518, target dose 100 mg daily).
Results
Diabetic events (diabetic coma, peripheral gangrene, diabetic foot, decreased glucose tolerance or hyperglycaemia) and new onset diabetes (clinical diagnosis, repeated high random glucose level or glucose lowering drugs) were assessed in 2298 patients without diabetes at baseline. Diabetic events occurred in 122/1151 (10.6%) patients in the carvedilol group and 149/1147 (13.0%) patients in the metoprolol group (hazard ratio (HR) = 0.78; 95% confidence interval (CI) 0.61 to 0.99; p = 0.039). New onset diabetes was diagnosed in 119/1151 (10.3%) v 145/1147 (12.6%) cases in the carvedilol and metoprolol treatment groups (HR = 0.78, CI 0.61 to 0.997; p = 0.048), respectively. Patients with diabetes at baseline had an increased mortality compared with non‐diabetic subjects (45.3% v 33.9%; HR = 1.45, CI 1.28 to 1.65). Both diabetic and non‐diabetic subjects at baseline had a similar reduction in mortality with carvedilol compared with metoprolol (RR = 0.85; CI 0.69 to 1.06 and RR = 0.82; CI 0.71 to 0.94, respectively).
Conclusion
A high prevalence and incidence of diabetes is found in patients with heart failure over a course of 5 years. New onset diabetes is more likely to occur during treatment with metoprolol than during treatment with carvedilol.
Keywords: β adrenergic receptor antagonists, carvedilol, diabetes mellitus, heart failure, metoprolol
Diabetes affects 10–30% of patients with heart failure.1 When patients with prediabetic glucose abnormalities are also included the combined prevalence increases to 40%.1 The presence of diabetes increases the risk of death by 50% in patients with heart failure, in particular among women2 and patients with ischaemic cardiomyopathy.3,4,5 Diabetes in patients with heart failure is therefore of major concern. β Blockers are an important treatment for heart failure in patients whether or not they have diabetes,6 despite reported increases in blood glucose of up to 1.55 mmol/l,7 and HbA1c by as much as 1%.8 These changes may potentially be of clinical importance in patients with heart failure.
Carvedilol has been reported to have a more favourable effect on glucose metabolism than other β blockers, and it stabilised HbA1c, improved insulin sensitivity and slowed development of microalbuminuria compared with metoprolol in one large trial in hypertension.9 The Carvedilol Or Metoprolol European Trial (COMET)10,11 demonstrated that carvedilol at a target dose of 50 mg daily reduced mortality in patients with heart failure compared with metoprolol tartrate at a target dose of 100 mg daily. The main aim of this report was to determine whether carvedilol was associated with a different incidence of diabetes in new onset diabetes compared with metoprolol in patients with heart failure.
Methods
COMET was a randomised, double blind, parallel group trial comparing carvedilol with metoprolol tartrate for the treatment of chronic heart failure accompanied by left ventricular systolic dysfunction. The study design and primary results have been described in detail previously.10,11 A total of 3029 patients with chronic heart failure were randomised at 316 centres in 15 European countries. Patients were recruited between December 1996 and January 1999, and follow‐up was concluded in November 2002. The study was conducted according to the Declaration of Helsinki, was approved by relevant ethical boards and the patients gave informed consent to participation.
Patients
In brief, inclusion required the presence of symptomatic chronic heart failure (NYHA class II–IV), a need for diuretic treatment, at least one admission to hospital for a cardiovascular problem within the past 2 years, stable concomitant medication including ACE inhibitors (if tolerated) for at least 4 weeks and left ventricular ejection fraction below 35%. Major exclusion criteria were requirement for intravenous inotropic treatment, current treatment with calcium channel blockers (of the diltiazem or verapamil class), amiodarone (>200 mg/day) or class I antiarrhythmic drugs, or administration of any investigational drug within the preceding 30 days. We also excluded patients with a contraindication to a β blocker, acute coronary syndrome or symptomatic/sustained ventricular arrhythmia within the previous 2 months, uncontrolled hypertension, haemodynamically significant valve disease, known drug or alcohol abuse, poor compliance and, finally, any serious disease that might complicate treatment or reduce life expectancy.
Treatment
At randomisation patients were assigned to either 3.125 mg carvedilol twice daily or 5 mg metoprolol tartrate twice daily. The dose of each β blocker was to be doubled at 2‐week intervals towards a target dose of 25 mg carvedilol twice daily or 50 mg metoprolol tartrate twice daily.
End points: definitions
Diabetes was considered present at baseline if reported by the investigator as a concomitant disease. It included patient reporting and notes in hospital files.
The following definitions were used in the analyses: a diabetes related adverse event was a prespecified end point and was defined as the reporting, among patients without diabetes at baseline, of at least one of the following adverse events (diabetes, diabetic coma, peripheral gangrene/diabetic foot, decreased glucose tolerance or hyperglycaemia).
Because the diabetes related adverse events also included events (hyperglycaemia, decreased glucose tolerance) that were not necessarily diabetes, we subsequently defined a new end point retrospectively—new onset diabetes. This was considered present if:
A clinical diagnosis of diabetes was reported. If the investigator reported an adverse event coded as diabetes mellitus or diabetic coma, or if the patients had started chronic medical treatment with insulin or oral glucose lowering treatment.
If the patient had at least two random blood glucose readings above 11.1 mmol/l. Random glucose was measured four times during the first year and thereafter once a year. Random glucose was measured by the investigator using the local laboratory. Blood glucose was requested, but in some cases plasma glucose may have been reported. For this reason we used the conservative estimate of 11.1 mmol/l as the cut‐off point.
If adverse event reporting was unclear/contradictory from the original case report forms (such as the reporting of a single high blood glucose reading) an additional page was sent to the investigators requesting to review the patient file and confirm the existence of diabetes, give the date diabetes was diagnosed and tick the following possibilities: need for diabetic drug treatment, repeated high blood glucose results, a positive oral glucose tolerance test, repeated high fasting glucose. Only when a date (at least month/year) and at least one of the tick boxes was answered as “yes” was the patient classified as diabetic.
Statistical analyses
Differences between patients with or without diabetes at baseline were assessed using t tests for continuous variables and the χ2 test for categorical data. Kaplan–Meier mortality estimates were calculated by treatment for those with or without diabetes at baseline. Time to event analyses were performed using Cox proportional hazard models. When time to first diabetes related adverse event or new onset diabetes was examined patients were censored at the time of death. The multivariate model for new onset diabetes was produced using forward and backward stepwise procedures, with a threshold probability of 0.05. The relationship between mortality and diabetes (diabetes at baseline and new onset diabetes) was assessed using diabetes as a time dependent covariate. All significant (obtained using stepwise procedures) baseline prognostic factors were included in this model. Model assumptions (proportional hazard assumption, linearity of continuous variables and lack of interaction) were tested and found valid.
Results
Of the 3029 patients randomised in COMET, 731 (24.1%) had diabetes at baseline. Table 1 shows the baseline demographics of patients with and without diabetes. The patients with and without diabetes at baseline differed significantly from each other, but in both groups patients were well matched with respect to treatment allocation.
Table 1 Baseline characteristics.
| Characteristic | Diabetes at baseline (n = 731) | No diabetes at baseline (n = 2298) | p Value (diabetes versus no diabetes) | ||
|---|---|---|---|---|---|
| Carvedilol (n = 360) | Metoprolol (n = 371) | Carvedilol (n = 1151) | Metoprolol (n = 1147) | ||
| Age (years), mean (SD) | 63.4 (9.3) | 64.4 (9.7) | 61.1 (11.8) | 61.6 (11.9) | <0.0001 |
| Gender (% male) | 73.9 | 77.6 | 81.1 | 81.0 | 0.0019 |
| Race (% white) | 98.1 | 98.9 | 99.2 | 99.1 | 0.3324 |
| Body mass index (kg/m2), mean (SD) | 28.0 (4.5) | 27.4 (4.6) | 26.5 (4.4) | 26.6 (4.3) | <0.0001 |
| Systolic BP (mm Hg), mean (SD) | 128.8 (20.4) | 129.3 (20.1) | 125.1 (18.8) | 125.2 (19.4) | <0.0001 |
| Diastolic BP (mm Hg), mean (SD) | 77.1 (11.7) | 77.5 (10.8) | 77.1 (10.7) | 77.1 (10.9) | 0.7045 |
| Heart rate (bpm), mean (SD) | 82.9 (12.7) | 81.3 (13.7) | 80.6 (13.2) | 81.0 (13.5) | 0.0205 |
| NYHA class (%) | |||||
| II | 40.3 | 39.9 | 50.8 | 51.3 | <0.0001 |
| III | 55.8 | 55.0 | 46.1 | 44.6 | |
| IV | 3.9 | 5.1 | 3.0 | 4.1 | |
| Duration CHF (months), mean/median | 49.3/31.0 | 46.0/24.0 | 40.5/20.0 | 40.9/19.0 | 0.0024 |
| Aetiology CHF (%) | |||||
| Ischaemic heart disease | 59.2 | 65.5 | 48.9 | 49.9 | <0.0001 |
| Hypertension | 23.1 | 23.2 | 16.2 | 15.8 | <0.0001 |
| Dilated cardiomyopathy | 37.2 | 36.7 | 46.3 | 45.9 | <0.0001 |
| Previous valve surgery | 1.7 | 1.9 | 3.2 | 2.2 | 0.1634 |
| LVEF, mean (SD) | 26.5 (6.9) | 27.0 (6.9) | 25.5 (7.3) | 26.2 (7.2) | 0.0042 |
| NT‐proBNP (pg/ml), median | 1473 | 1290 | 1257 | 1158 | 0.7671 |
| Haemoglobin level (g/l), mean (SD) | 141 (16) | 140 (16 | 142 (15) | 142 (15) | 0.0076 |
| Serum creatinine (μmol/l), mean (SD) | 110.6 (43.3) | 110.4 (37.3) | 106.1 (40.2) | 106.1 (42.3) | 0.012 |
| Blood glucose (mmol/l), mean (SD) | 9.5 (4.2) | 9.6 (3.9) | 5.7 (1.5) | 5.6 (1.4) | <0.0001 |
| Previous MI (%) | 49.6 | 47.4 | 39.8 | 38.8 | <0.0001 |
| CAD (confirmed by angiography) (%) | 66.7 | 70.1 | 54.3 | 56.8 | <0.0001 |
| Current angina (%) | 23.5 | 27.6 | 20.6 | 20.2 | 0.0033 |
| Previous angioplasty (%) | 8.9 | 10.5 | 7.8 | 7.4 | 0.0721 |
| Previous CABG (%) | 18.6 | 24.5 | 14.4 | 16.7 | 0.0002 |
| Hypertension (%) | 50.3 | 48.6 | 33.5 | 32.3 | <0.0001 |
| Stroke (%) | 7.3 | 8.6 | 7.0 | 6.7 | 0.3026 |
| ECG findings (%) | |||||
| Sinus rhythm | 75.0 | 74.9 | 74.2 | 74.7 | 0.7828 |
| Atrial fibrillation/flutter | 21.7 | 18.9 | 20.1 | 19.3 | 0.7332 |
| Paced rhythm | 3.6 | 7.3 | 6.7 | 7.1 | 0.1811 |
| LBBB | 5.3 | 4.3 | 6.0 | 5.5 | 0.3239 |
| Diuretics (%) | 98.3 | 98.4 | 99.0 | 98.6 | 0.3297 |
| ACE inhibitors (%) | 91.7 | 91.6 | 91.5 | 91.0 | 0.7363 |
| Angiotensin receptor antagonists (%) | 5.0 | 6.5 | 6.6 | 7.0 | 0.3204 |
| Digitalis (%) | 65.6 | 57.1 | 59.1 | 58.6 | 0.2396 |
| Antiarrhythmics (%) | 10.8 | 12.9 | 13.0 | 11.4 | 0.8139 |
| Nitrates (%) | 40.6 | 41.8 | 30.2 | 29.9 | <0.0001 |
| Aldosterone antagonists (%) | 11.9 | 11.3 | 10.5 | 10.5 | 0.4051 |
| β Blockers (%) | 6.1 | 5.1 | 3.6 | 4.1 | 0.0437 |
| Anticoagulants (%) | 43.6 | 41.5 | 49.2 | 44.3 | 0.0475 |
| Aspirin (%) | 41.4 | 44.2 | 32.9 | 37.0 | 0.0001 |
| Lipid lowering agents (statins) (%) | 24.7 | 24.5 | 19.0 | 20.9 | 0.0073 |
CHF, chronic heart failure; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal prohormone brain natriuretic peptide.
New onset diabetes
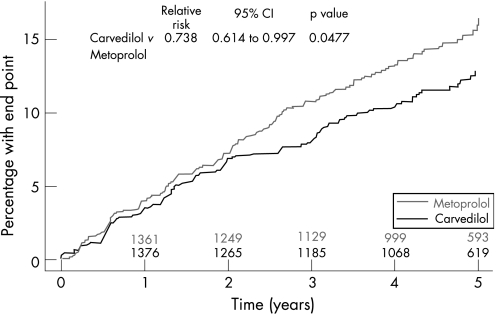
Analysis of new onset diabetes was performed only in patients without diabetes at baseline. Patients without diabetes at baseline had a follow‐up of 47–72 months. A diabetes related adverse event occurred in 271 patients and new onset diabetes was detected in 264 patients. There were significant differences in the rates of these events between the patients randomised to carvedilol and metoprolol. Thus, 122 patients receiving carvedilol and 149 patients receiving metoprolol had diabetes related adverse events (hazard ratio (HR) = 0.78; 95% confidence intervals (CI) 0.61 to 0.99; p = 0.039) and 119 patients receiving carvedilol and 145 patients receiving metoprolol developed new onset diabetes (HR = 0.78, 95% CI 0.61 to 0.997; p = 0.048, fig 1). The Kaplan–Meier estimates that 28 patients would be need to be treated for 5 years to avoid new onset diabetes.
Figure 1 Development of new onset diabetes.
Diabetes related adverse events included the development of diabetes in 100/1151 (8.7%) patients receiving carvedilol and in 116/1147 (10.1%) patients receiving metoprolol, decreased glucose tolerance or hyperglycaemia alone in 19/1151 (1.7%) and 33/1147 (2.9%) patients, and peripheral gangrene/diabetic foot alone in 3/1151 (0.3%) and 0/1147 (0.0%) patients receiving carvedilol and metoprolol, respectively. The diagnosis of new onset diabetes was based on a clinical diagnosis in 109/1151 (8.3%) patients receiving carvedilol and 130/1147 (11.3%) patients receiving metoprolol, on the addition of glucose lowering medical treatment alone in 3/1151 (0.3%) and 9/1147 (0.8%) patients and solely based on the finding of at least two random measurements of plasma glucose above 11.1 mmol/l in 7/1151 and 6/1147 patients receiving carvedilol and metoprolol, respectively.
Table 2 shows those factors that independently predicted the risk of developing diabetes in a multivariable model. New onset diabetes was more likely to occur in the patients with a high body mass index, a history of hypertension and NYHA class IV, and less likely to occur in the patients with NYHA class III and higher serum creatinine. Metoprolol remained independently associated with a greater likelihood of developing new onset diabetes in this analysis.
Table 2 Multivariable risk of new onset diabetes. All variables in table 1 were used in development of the model. Only significant variables were included in the final model.
| Relative risk (95% CI) | p Value | |
|---|---|---|
| Carvedilol v metoprolol | 0.756 (0.587 to 0.973) | 0.0300 |
| ↑ BMI (per unit) | 1.1 (1.075 to 1.125) | <0.0001 |
| NYHA III v II | 0.757 (0.582 to 0.986) | 0.0386 |
| NYHA IV v II | 1.956 (1.047 to 3.656) | 0.0354 |
| Digitalis | 2.113 (1.581 to 2.823) | <0.0001 |
| ↑ Haemoglobin (per g/l) | 1.145 (1.051 to 1.247) | 0.0019 |
| Creatinine (μmol/l) >90 v ⩽90 | 0.735 (0.545 to 0.991) | 0.0433 |
| Creatinine (μmol/l) >110 v ⩽90 | 0.735 (0.535 to 1.011) | 0.0588 |
| ↑ Glucose (per mmol/l) | 1.561 (1.479 to 1.648) | <0.0001 |
Blood glucose
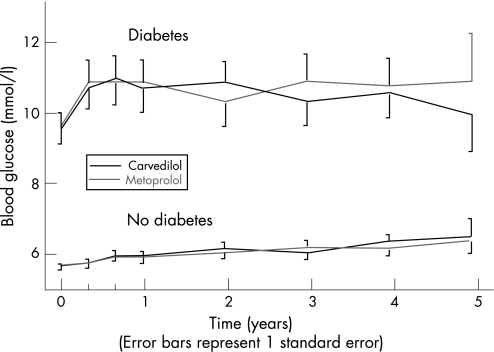
During the trial, blood glucose was measured every 4 months; fig 2 shows the blood glucose measurements during the study in the patients subdivided by treatment and by the presence of diabetes at baseline. There was no statistically significant difference between patients treated with carvedilol and metoprolol, but there was an overall significant trend for an increase in random blood glucose over the course of the trial in patients without diabetes at baseline (p<0.0001, with repeated measures analysis including only patients with 4 years complete laboratory follow‐up).
Figure 2 Values of random glucose measurements in patients with and without diabetes and treated with carvedilol and metoprolol. There was no significant difference between patients treated with carvedilol and metoprolol, but there was an overall significant trend for an increase in random blood glucose over the course of the trial in patients without diabetes at baseline (p<0.0001).
Mortality
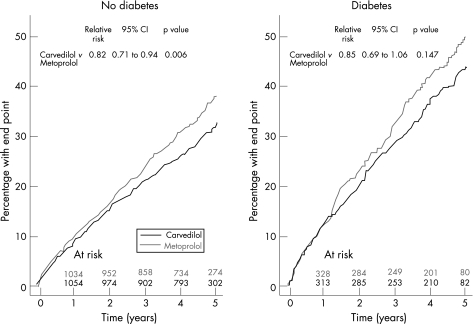
Patients with diabetes at baseline had an increased risk of death compared with patients without diabetes (45.3% v 33.9%; HR = 1.45; CI 1.28 to 1.65; p<0.0001). Figure 3 shows mortality curves from the whole COMET population subdivided by treatment allocation and the presence of diabetes at baseline. The 5 year mortality rate for patients without diabetes at baseline was 32.7% (95% CI 29.8% to 35.7%) for those receiving carvedilol and 38.1% (95% CI 35.1% to 41.2%) for those receiving metoprolol. In patients with diabetes at baseline the 5 year mortality rate was 44.0% (95% CI 38.7% to 49.7%) for those receiving carvedilol and 50.1% (95% CI 44.7% to 55.7%) for those receiving metoprolol. Both diabetic and non‐diabetic subjects at baseline had similar risk reductions for mortality in favour of carvedilol compared with metoprolol (RR = 0.85; CI 0.69 to 1.06; p = 0.147 for diabetic subjects and RR = 0.82; 95% CI 0.71 to 0.94; p = 0.006 for non‐diabetic subjects, respectively). There was no interaction between baseline diabetic status and treatment with either carvedilol or metoprolol (p = 0.772).
Figure 3 All‐cause mortality in patients with and without diabetes.
Both diabetes and treatment allocation were independently related to all‐cause mortality in a multivariate analysis that included both diabetes at baseline and new onset diabetes as time dependent variables (HR = 0.832; 95% CI, 0.739 to 0.936; p = 0.0022 for carvedilol versus metoprolol and HR = 1.298; 95% CI 1.147 to 1.469; p<0.0001 for diabetes (baseline and new onset) versus no diabetes). The prognostic value of these variables was maintained in a multivariate analysis including all the clinical variables independently associated with an increased mortality in COMET (table 3).
Table 3 Time dependent analysis of all‐cause mortality, including both diabetes at baseline and new onset diabetes as a time dependent factor. CAD removed, paced rhythm added and CAD removed, AF added.
| RR (95% CI) | p Value | |
|---|---|---|
| Carvedilol v metoprolol | 0.792 (0.698 to 0.899) | 0.0003 |
| Diabetes | 1.2 (1.046 to 1.377) | 0.0095 |
| ↑ Age (per year) | 1.031 (1.023 to 1.038) | <0.0001 |
| Female v male | 0.722 (0.603 to 0.863) | 0.0004 |
| ↑ Weight (per kg) | 0.992 (0.987 to 0.997) | 0.0033 |
| Systolic BP >120 mm Hg | 0.763 (0.667 to 0.873) | 0.0001 |
| NYHA III v II | 1.429 (1.246 to 1.639) | <0.0001 |
| NYHA IV v II | 1.722 (1.294 to 2.293) | 0.0002 |
| ↑ Duration of HF (per month) | 1.001 (1 to 1.002) | 0.0044 |
| ↑ LVEF (per%) | 0.976 (0.967 to 0.985) | <0.0001 |
| Previous MI | 1.344 (1.167 to 1.548) | <0.0001 |
| Stroke | 1.238 (0.997 to 1.538) | 0.0536 |
| AF | 0.988 (0.835 to 1.169) | 0.8901 |
| Digitalis | 1.45 (1.263 to 1.666) | <0.0001 |
| Antiarrhythmic drugs | 1.556 (1.308 to 1.851) | <0.0001 |
| Nitrates | 1.15 (1.003 to 1.318) | 0.0447 |
| Lipid lowering | 0.767 (0.647 to 0.908) | 0.0021 |
| ↑ Haemoglobin (g/l) | 0.924 (0.885 to 0.965) | 0.0003 |
| ↑ Sodium (mmol/l) | 0.949 (0.933 to 0.965) | <0.0001 |
| ↑ Creatinine (μmol/l) | 1.002 (1.001 to 1.003) | <0.0001 |
CAD, coronary artery disease; AF, atrial fibrillation.
Discussion
This study demonstrates that treatment with carvedilol is associated with less development of new onset diabetes in patients with heart failure compared with treatment with metoprolol tartrate. The study further demonstrates that not only is the prevalence of diabetes high in patients with heart failure but also the incidence is high, amounting to 10–15% over 5 years.
Chronic administration of β blockers is now mandatory in patients with chronic heart failure who do not have contraindications to their use.12 However, these agents are associated with a reduction in insulin sensitivity and with an increased risk of diabetic complications.7,8 Carvedilol has more favourable metabolic effects in diabetic patients than traditional β blockers9,13 and reduces mortality in patients with chronic heart failure more than metoprolol tartrate.10 It was therefore important to assess whether the specific pharmacological characteristics of carvedilol might influence the development of diabetic complications and new onset diabetes and determine its effects on mortality, compared with metoprolol, in COMET.
Our study shows that diabetes, whether chronic or new onset, increases mortality in patients with heart failure. Further, we have shown that, in addition to a high baseline prevalence of diabetes (24%), the incidence of diabetes is substantial (a further 10–15%) among patients with heart failure during 5 years of follow‐up, and among patients who have not been diagnosed with diabetes, there is a progressive increase in random blood glucose levels.
Diabetes is an important risk factor for the development of cardiovascular disease, including heart failure,14 and therefore the high prevalence of diabetes in our study is not surprising. The high risk of developing diabetes during the trial may reflect a high prevalence of prediabetic glucose abnormalities at baseline that developed into overt diabetes during the trial. However, it is also possible that heart failure is a risk factor for diabetes, rather than the opposite.15 The gradual increase in random glucose measurements over the course of the trial should be interpreted similarly.
In 1996 Jacob et al demonstrated improved insulin sensitivity when patients with hypertension were treated with carvedilol and poorer insulin sensitivity during treatment with metoprolol.13 This finding was the basis for a large hypertension trial in patients with diabetes,9 in which carvedilol stabilised HbA1c, improved insulin resistance, and slowed development of microalbuminuria, compared with metoprolol. The beneficial effect of carvedilol on insulin sensitivity, compared with metoprolol, is a possible explanation for the findings of the present study, although the mechanism of this effect is uncertain. However, both its α blocking and antioxidative properties16 may play a role. The antioxidative property might be important for preserving β cells.17
Despite the adverse effect of β blockers on insulin sensitivity, they appear effective in reducing major cardiovascular events and death in patients with diabetes. A retrospective analysis demonstrated reduced mortality in diabetic patients with myocardial infarction when treated with β blockers,18 and in heart failure studies the benefit of β blockade was also evident in the subgroup with diabetes.19,20 Finally, the United Kingdom Prospective Diabetes Study (UKPDS) demonstrated that β blockade can prevent heart failure in patients with diabetes.21
In COMET, new onset diabetes was more likely to occur in patients with concomitant cardiovascular risk factors (hypertension, higher body mass index) and NYHA class IV, but less likely to occur in the patients with NYHA class III and increased serum creatinine levels. These last results contrast with those of previous studies,5,15 which showed a higher prevalence of diabetes in the patients with more advanced heart failure. Although we cannot explain the differences, we note that the other studies examined prevalence, while we focused on incidence in this study
The incidence of diabetes as well as that of diabetes related adverse events is known to influence the outcome and resource utilisation of the patients. It has been suggested that ACE inhibitors and angiotensin receptor blockers reduce the incidence of new onset diabetes compared with placebo in randomised controlled trials.22 As far as we know, our study is the first trial showing that the choice of the β blocker can influence the incidence of this event, with a lower rate of diabetic related complications and new onset diabetes in patients assigned to carvedilol than in those receiving metoprolol.
COMET has been criticised for the administration of doses of metoprolol lower than in previous trials.23,24,25 However, this criticism is not applicable to the present study. The severity of heart failure as judged by New York Heart Association class and number of admissions to hospital was similar in the two treatment groups,26 and thus undertreatment of heart failure is an unlikely explanation for the findings. It is doubtful that the use of higher doses of metoprolol tartrate would have increased the likelihood of developing new onset diabetes and diabetic complications in this patient group.
There are some limitations to the study. This is a retrospective analysis relying on post hoc identification of the end point of new onset diabetes. A large proportion of patients in COMET had ischaemic heart disease, and in such a population diabetes, impaired glucose tolerance and insulin resistance are common.27 It is therefore likely that the new onset diabetes in this study is deterioration of pre‐existing disturbances in glucose metabolism.
The study relies on a definition of diabetes which is essentially a clinical diagnosis and does not rely on formal adherence to guidelines. This weakness is shared with most other studies on the epidemiology of diabetes in heart failure.
Conclusion
This study demonstrates both a high prevalence and incidence of diabetes in patients with heart failure over a course of 5 years and a steady increase in random measurements of blood glucose. New onset diabetes was more likely to occur during treatment with metoprolol than during treatment with carvedilol.
Acknowledgements
The COMET study was sponsored by Hoffman‐La Roche Pharmaceuticals and GlaxoSmithKline Pharmaceuticals.
Footnotes
Competing interests: Andrew Charlesworth and Phillip Spark are employees of Nottingham Clinical Research Group that performed the statistical analyses of the trial. Mary Ann Lukas and Armin Scherhaug are employees of the sponsors of the study. All other authors have competing interest in the form of paid lectures and participation in advisory boards for numerous pharmaceutical companies.
The COMET investigators are listed in a previous publication: Poole‐Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003;362:7–13.
Ethical approval: This study was approved by all relevant ethical committees before initiation.
References
- 1.Solang L, Malmberg K, Ryden L. Diabetes mellitus and congestive heart failure. Further knowledge needed. Eur Heart J 199920789–795. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson I, Brendorp B, Seibaek M.et al Influence of diabetes and diabetes‐gender interaction on the risk of death in patients hospitalized with congestive heart failure. J Am Coll Cardiol 200443771–777. [DOI] [PubMed] [Google Scholar]
- 3.Das S R, Drazner M H, Yancy C W.et al Effects of diabetes mellitus and ischemic heart disease on the progression from asymptomatic left ventricular dysfunction to symptomatic heart failure: a retrospective analysis from the Studies of Left Ventricular Dysfunction (SOLVD) Prevention Trial. Am Heart J 2004148883–888. [DOI] [PubMed] [Google Scholar]
- 4.Dries D L, Sweitzer N K, Drazner M H.et al Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol 200138421–428. [DOI] [PubMed] [Google Scholar]
- 5.Domanski M, Krause‐Steinrauf H, Deedwania P.et al The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol 200342914–922. [DOI] [PubMed] [Google Scholar]
- 6.Lopez‐Sendon J, Swedberg K, McMurray J.et al Expert consensus document on beta‐adrenergic receptor blockers. Eur Heart J 2004251341–1362. [DOI] [PubMed] [Google Scholar]
- 7.Dornhorst A, Powell S H, Pensky J. Aggravation by propranolol of hyperglycaemic effect of hydrochlorothiazide in type II diabetics without alteration of insulin secretion. Lancet 19851123–126. [DOI] [PubMed] [Google Scholar]
- 8.Holzgreve H, Nakov R, Beck K.et al Antihypertensive therapy with verapamil SR plus trandolapril versus atenolol plus chlorthalidone on glycemic control. Am J Hypertens 200316(5Pt 1)381–386. [DOI] [PubMed] [Google Scholar]
- 9.Bakris G L, Fonseca V, Katholi R E.et al Metabolic effects of carvedilol vs metoprolol in patients with type 2 diabetes mellitus and hypertension: a randomized controlled trial. JAMA 20042922227–2236. [DOI] [PubMed] [Google Scholar]
- 10.Poole‐Wilson P A, Swedberg K, Cleland J G.et al Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 20033627–13. [DOI] [PubMed] [Google Scholar]
- 11.Poole‐Wilson P A, Cleland J G, Di Lenarda A.et al Rationale and design of the Carvedilol Or Metoprolol European Trial in patients with chronic heart failure: COMET. Eur J Heart Fail 20024321–329. [DOI] [PubMed] [Google Scholar]
- 12.Swedberg K, Cleland J, Dargie H.et al Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005261115–1140. [DOI] [PubMed] [Google Scholar]
- 13.Jacob S, Rett K, Wicklmayr M.et al Differential effect of chronic treatment with two beta‐blocking agents on insulin sensitivity: the carvedilol‐metoprolol study. J Hypertens 199614489–494. [PubMed] [Google Scholar]
- 14.Kannel W B, McGee D L. Diabetes and cardiovascular disease. The Framingham study. JAMA 19792412035–2038. [DOI] [PubMed] [Google Scholar]
- 15.Suskin N, McKelvie R S, Burns R J.et al Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J 2000211368–1375. [DOI] [PubMed] [Google Scholar]
- 16.Foody J M, Farrell M H, Krumholz H M. β‐Blocker therapy in heart failure: scientific review. JAMA 2002287883–889. [DOI] [PubMed] [Google Scholar]
- 17.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 200424816–823. [DOI] [PubMed] [Google Scholar]
- 18.Malmberg K, Herlitz J, Hjalmarson A.et al Effects of metoprolol on mortality and late infarction in diabetics with suspected acute myocardial infarction. Retrospective data from two large studies. Eur Heart J 198910423–428. [DOI] [PubMed] [Google Scholar]
- 19.Packer M, Coats A J S, Fowler M B.et al Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 20013441651–1658. [DOI] [PubMed] [Google Scholar]
- 20.Deedwania P C, Giles T D, Klibaner M.et al Efficacy, safety and tolerability of metoprolol CR/XL in patients with diabetes and chronic heart failure: experiences from MERIT‐HF. Am Heart J 2005149159–167. [DOI] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study Group Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998317713–720. [PMC free article] [PubMed] [Google Scholar]
- 22.Abuissa H, Jones P G, Marso S P.et al Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes a meta‐analysis of randomized clinical trials. J Am Coll Cardiol 200546821–826. [DOI] [PubMed] [Google Scholar]
- 23.Anonymous Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Lancet 19993532001–2007. [PubMed] [Google Scholar]
- 24.Hjalmarson A, Waagstein F. COMET: a proposed mechanism of action to explain the results and concerns about dose. Lancet. 2003;362: 1077; author reply 1077–8, [DOI] [PubMed]
- 25.Metra M, Torp‐Pedersen C, Swedberg K.et al Influence of heart rate, blood pressure, and beta‐blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J 2005262259–2268. [DOI] [PubMed] [Google Scholar]
- 26.Torp‐Pedersen C, Poole‐Wilson P, Swedberg K.et al Effects of metoprolol and carvedilol on cause‐specific mortality and morbidity in patients with chronic heart failure ‐ COMET. Am Heart J 2005149370–376. [DOI] [PubMed] [Google Scholar]
- 27.Seibaek M, Sloth C, Vallebo L.et al Glucose tolerance status and severity of coronary artery disease in men referred to coronary arteriography. Am Heart J 1997133622–629. [DOI] [PubMed] [Google Scholar]