Abstract
Objectives
To examine N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) variability in plasma and urine samples of patients with stable heart failure (HF) during a 24‐month follow‐up.
Design
Prospective study.
Setting
Teaching hospital based study.
Patients
74 clinically and functionally stable patients (NYHA class 2±0.5) out of 114 patients diagnosed with HF were followed up, and NT‐proBNP plasma and urine levels were measured at baseline, 12 and 24 months.
Results
Significant differences in mean urinary levels (p<0.01) were found during follow‐up, but no changes were found in plasma. Bland–Altman plots showed few variations in plasma percentages in the three intervals (stage I–basal; stage II–stage I; stage II–basal) with a coefficient of reproducibility (CR) of 22%, 21% and 25%, respectively. Changes in NT‐proBNP urinary levels had a CR of 7.1%, 6.8% and 9.4% at the three intervals, respectively. A good correlation was found between plasma and urinary levels of NT‐proBNP (p<0.001) and between the different NT‐proBNP plasma (p<0.001) and urine measurements (p<0.001).
Conclusions
NT‐proBNP plasma and urine levels show good stability in a 24‐month follow‐up of patients with stable heart failure. Thus, assessment of urinary and plasma NT‐proBNP concentrations may be a useful tool for monitoring patients with HF during follow‐up. The results suggest that variations in peptide concentrations exceeding 22% in plasma and 7% in urine in a 12‐month follow‐up and 25% and 9% in a 24‐month follow‐up may indicate pathophysiological changes.
Keywords: natriuretic peptides, urine, plasma, stability, variability
Cardiac natriuretic peptides are a family of hormones secreted by cardiomyocytes, in response to ventricular volume expansion and pressure overload.1 They have potent diuretic, natriuretic and vascular smooth‐muscle‐relaxing effects and interact with the hormonal and nervous system.2,3
The potential clinical usefulness of assays for natriuretic peptides, especially B‐type natriuretic peptide (BNP) or the N‐terminal fragment of proBNP (NT‐proBNP), for screening of heart disease,4 for stratification of patients with congestive heart failure (HF),5 for detection of left ventricular systolic and/or diastolic dysfunction,6,7 and for differential diagnosis of dyspnoea,8 has been confirmed recently. Furthermore, the Task Force of the European Society of Cardiology for the Diagnosis and Treatment of chronic HF recommended that a natriuretic peptide assay should be included in the first step of the algorithm for the diagnosis of HF together with electrocardiography and chest x ray findings, on the basis of their strong negative predictive value.9
Previous studies have evaluated the biological variation of BNP and its related peptides in both healthy people10 and patients with chronic HF.11,12 Higher variability was found in BNP than in NT‐proBNP plasma levels, because of its pulsatile pattern of release and its rapid turnover.13 The diagnostic accuracy and prognostic relevance of the assay of cardiac natriuretic peptide was also evaluated in detail in all clinical settings, including their use as screening methods in asymptomatic subjects, as a diagnostic test in primary care, emergency department, coronary care unit, cardiological ambulatory and hospital care settings.14 Furthermore, urinary NT‐proBNP levels have been proposed as a valuable tool for diagnosis and prognosis.15 Although the follow‐up of change in NT‐proBNP levels is an established tool, studies determining simultaneously the changes in urine and plasma over time in patients with clinically stable HF have never been published. This would allow us to know the usefulness of this peptide in the clinical arena. We hypothesised that NT‐proBNP levels in plasma and urine may change over time even in patients with stable HF, limiting its diagnostic and prognostic power.
Therefore, the purpose of this study was to analyse simultaneously NT‐proBNP variability in plasma and urine samples during a 24‐month follow‐up, in a cohort of patients with clinically and functionally stable HF. We also investigated the plasma/urine ratio stability over time.
Patients and methods
Patients
The patients comprised 114 consecutive patients with HF (mean (SD) age 64 (13) years, 72% male) who had been diagnosed according to American Heart Association16 and European Society of Cardiology9 heart failure criteria. An electrocardiogram, chest x ray examination, modified Naughton protocol and echo‐Doppler study were performed. The cause of HF was multifactorial: 45% ischaemic cardiomyopathy, 40% dilated cardiomyopathy, 12% hypertensive cardiomyopathy and 3% valvulopathy. Patients in atrial fibrillation, with acute coronary syndromes, acute or chronic liver disease, and pulmonary and renal diseases were not eligible. Of the 114 patients, 40 were not included in the study: 17 had died, 21 had undergone non‐fatal cardiac events and 2 could not be located.
Seventy‐four patients met the criteria for inclusion and stability, mean (SD) age 65 (11) years (range 39–86), and 74% were male. The cause of HF was 49% ischaemic cardiomyopathy, 33% dilated cardiomyopathy, 14% hypertensive cardiomyopathy and 4% valvulopathy. All patients had been receiving stable medical treatment for at least 2 months before the study, with ACE inhibitors, diuretics, β blockers, aldosterone antagonists, digoxin, calcium channel blockers and angiotensin receptor antagonists II. The glomerular filtration rate was calculated using the modified diet in renal disease equation.17 All patients were followed up until the end of the study at month 24, with a three‐stage sample collection: basal, 12 months (stage I) and 24 months (stage II). Informed consent was obtained from each patient and the project was approved by the hospital ethics committee and conducted in accordance with the guidelines of the Declaration of Helsinki.
Sample collection
Samples were collected under standardised conditions to minimise sources of preanalytical variation. A venous blood specimen was taken by venepuncture between 08:00 and 10:00 with the subjects in a sitting position for at least 30 minutes. Subjects also provided a urine sample, the first urine of the day.18 Plasma samples were immediately centrifuged at 1300 rpm, 4°C, for 10 minutes, and the urine samples were centrifuged twice at 13 200 rpm, 4°C, for 30 minutes to avoid possible NT‐proBNP measurement interference produced by the precipitation of salts in urine. Both plasma and urine samples were aliquoted and stored in cryotubes at –80°C until further analysis.
Measurement of NT‐proBNP levels
NT‐proBNP plasma and urine levels were determined in duplicate using an electrochemiluminescence immunoassay (Elecsys®proBNP from Roche Diagnostics, Germany) based on the sandwich principle.19,20 The results are expressed as pg/ml (equivalent to ng/l, SI units). The lower detection limit was 5 pg/ml, and intra‐assay variation was 2.6%.
Echo‐Doppler study
The echo‐Doppler study was performed with standard echocardiographic systems equipped with a 2.5 MHz transducer used by the hospitals taking part in the study in their routine clinical practice. For each patient, four consecutive beats were measured and averaged for each Doppler variable. Standard apical and parasternal long‐axis views were recorded on videotape and analysed at a central laboratory, using a computerised system (Eco‐dat; Software Medicina SA, Madrid, Spain), blinded to the results of the NT‐proBNP assay.
The ejection fraction (EF) was determined by the area–length method and calculated as 100 × ((telediastolic volume − telesystolic volume)/telediastolic volume). Pulsed‐wave Doppler was used to measure peak flow velocity in early diastole (E wave) and during atrial contraction (A wave) at valve level; the E/A ratio was calculated, and the E wave deceleration time was also measured.
Statistical analysis
Continuous variables were expressed as mean (SD) and categorical variables as a number of patients or percentage. Results for each variable were tested for normality using the Kolmogorov–Smirnov method. NT‐proBNP plasma and urine concentrations exhibit a non‐normal distribution and were log transformed (and proved to be normalised) before parametric correlation analysis. Temporal changes in peptide levels and ventricular variables were analysed using the paired Student's t test, and categorical variable changes were compared using the McNemar test. To correlate temporal changes in NT‐proBNP urine and plasma levels with changes in EF, E/A, deceleration time and modified Naughton protocol, Pearson's coefficient was determined. Correlation between NT‐proBNP plasma and urine levels at baseline, stage I and stage II was also determined using Pearson's coefficient, and correlation with either plasma or urine levels at the different stages (basal, stage I and stage II).
To compare both NT‐proBNP plasma and urine levels over two different time intervals (stage I − basal; stage II – basal; stage II – stage I), we used the statistical method of Bland and Altman.21,22 In this graphical method the percentage of change of the averages ((NT‐proBNP stage I – NT‐proBNP basal)/average stage I + basal)) is plotted against the average of the two NT‐proBNP measurements. This expression is useful to normalise and compare the data without taking into account the magnitude of the NT‐proBNP measurement. Based on this approach, the limits of agreement were determined by the mean difference plus or minus the coefficient of reproducibility (CR), where CR was calculated as 1.96 × SD of the percentage of changes. In this case, a high CR indicates poor reproducibility. A p value <0.05 was considered significant for all measures. All statistical analyses were performed using the statistical package for social sciences (SPPS 10.1) software (SPSS Inc., Chicago, Illinois, USA).
Results
Table 1 summarises natriuretic peptide plasma and urine levels, clinical characteristics and left ventricular functional measures in the three stages for our 74 patients with stable HF. Statistical differences in systolic blood pressure, ejection fraction and urine NT‐proBNP were seen with respect to the basal stage (p<0.05, p<0.05 and p<0.01, respectively). Haematological and biochemical values, age, body mass index, left ventricular diastolic function variables, plasma NT‐proBNP, NYHA functional class and treadmill results did not show any statistical change.
Table 1 Clinical characteristics of the 74 patients with heart failure over the entire study (basal, stage I = at 12 months, stage II = at 24 months).
| Variable | Basal | Stage I | Stage II |
|---|---|---|---|
| Age (years) | 65 (11) | 66 (11) | 67 (11) |
| SBP (mm Hg) | 128 (20) | 130 (22) | 125 (19)* |
| Heart rate (bpm) | 76 (14) | 73 (11) | 75 (12) |
| Total cholesterol (mg/l) | 1940 (4460) | 1860 (310) | 1830 (370) |
| Sodium (mEq/l) | 139 (3) | 140 (3) | 140 (3) |
| Potassium (mEq/l) | 4.6 (0.5) | 4.7 (0.4) | 4.5 (0.5) |
| GFR (ml/min/1.73 m2) | 71 (22) | 74 (21) | 71 (21) |
| Packed cell volume (%) | 42 (5) | 42 (5) | 42 (5) |
| BMI (kg/m2) | 28 (5) | 28 (6) | 28 (4) |
| Hypertension (%) | 50 | 54 | 57 |
| Diabetes mellitus (%) | 41 | 46 | 49 |
| Smoking (%) | 13 | 7 | 8 |
| NYHA class | 2 (0.5) | 2.1 (0.5) | 2.1 (0.6) |
| Treatment (%): | |||
| ACE inhibitors | 79 | 75 | 74 |
| Diuretics | 76 | 79 | 77 |
| β Blockers | 60 | 65 | 70 |
| Aldosterone antagonists | 46 | 49 | 43 |
| Digoxin | 25 | 20 | 26 |
| Calcium channel blockers | 14 | 13 | 16 |
| ARA II | 10 | 11 | 15 |
| Modified Naughton protocol (s) | 632 (36) | 611 (34) | 585 (31) |
| EF (%) | 38 (10) | 36 (11)* | 36 (9)* |
| E/A | 1.0 (0.6) | 1.0 (0.6) | 1.2 (0.8) |
| DT (ms) | 214 (71) | 199 (54) | 201 (69) |
| Plasma NT‐proBNP (pg/ml) | 1237 (1737) | 1241 (1748) | 1043 (1153) |
| Urine NT‐proBNP (pg/ml) | 83 (23) | 92 (28)† | 89 (17)† |
| Plasma/urine NT‐proBNP ratio | 13 (15) | 11 (11) | 10 (9)* |
Results are shown as mean (SD) unless otherwise indicated.
ACE, angiotensin‐converting enzyme; ARA II, angiotensin receptor antagonist; BMI, body mass index; DT, deceleration time; E/A, flow velocity in early diastole and during atrial contraction ratio; EF, ejection fraction; GFR, glomerular filtration rate; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide levels; NYHA, New York Heart Association; SBP, systolic blood pressure.
Significant difference versus basal levels: *p<0.05; †p<0.01.
When we analysed the scatter of NT‐proBNP levels at each stage—basal, stage I and stage II—we noted that few patients had NT‐proBNP levels >±1 SD in basal (plasma 27%, urine 20%), stage I (plasma 27%, urine 7%) and stage II (plasma 28%, urine 20%). Urinary levels of NT‐pro‐BNP showed less variability between our patients with HF than between plasma levels at all stages (basal: 1.90 (0.1) vs 2.85 (0.43); stage I: 1.95 (0.09) vs 2.84 (0.46); stage II: 1.94 (0.07) vs 2.83 (0.41), respectively).
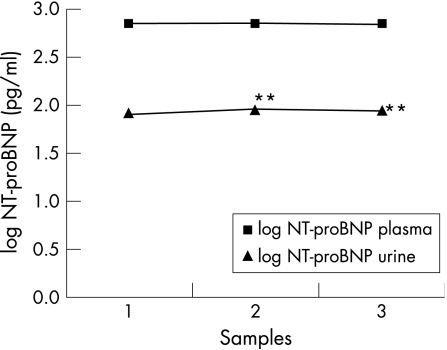
Figure 1 shows the mean levels of urine and plasma NT‐proBNP over the entire study. Urinary levels were lower than NT‐proBNP plasma levels (p<0.001). We found significant changes in urinary levels (p<0.01), but no differences were found in NT‐proBNP plasma levels.
Figure 1 Urinary and plasma logarithm of NT‐proBNP levels during a 24‐month follow‐up. Measurements represent the mean at basal (1), stage I (2) and stage II (3). NT‐proBNP, N‐terminal pro‐brain natriuretic peptide levels; stage I, 12‐month follow‐up; stage II, 24‐month follow‐up. **p<0.01 with respect to basal levels.
We also calculated the mean (SD) plasma/urine NT‐proBNP ratio over the entire study (basal: 13 (15); stage I: 11 (11), stage II: 10 (9)) and found significant differences between stage II and basal levels (p<0.05).
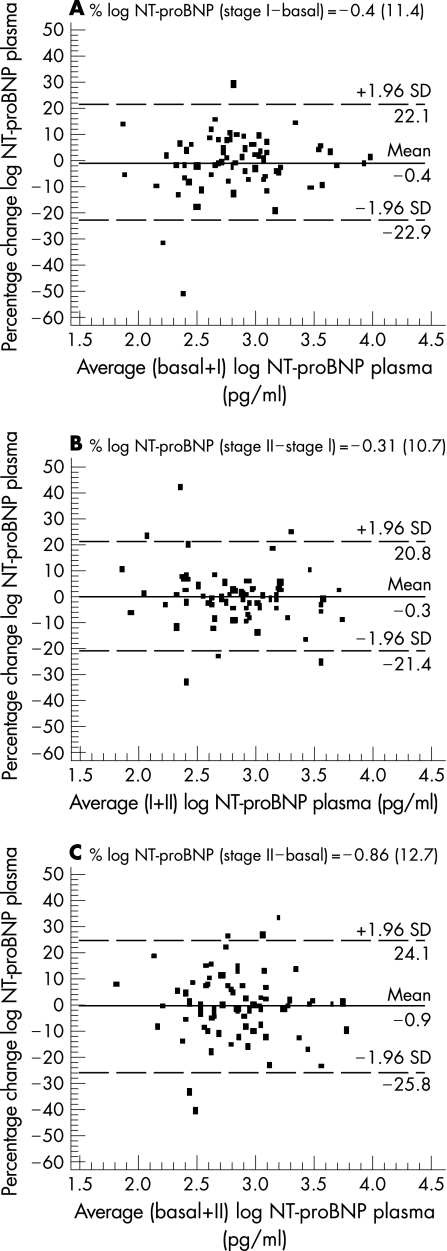
Figure 2 shows the Bland–Altman plots for changes in NT‐proBNP plasma levels over each of the intervals studied (A: stage I – basal; B: stage II – stage I; C: stage II – basal). In the A interval, 70 patients (94.6%) fell within 1.96 SDs of the mean. The mean (SD) percentage change of peptide levels agreement was –0.4 (11.4), with a CR of 22.4%. In the B interval, 69 patients (93.2%) fell within range of 1.96 SD with a mean percentage of change of –0.31 (10.7) and CR of 21%. Finally, in the C interval, 68 patients (91.9%) fell within 1.96 standard deviations of the mean, with a mean change of –0.86 (12.7) and CR of 24.9%.
Figure 2 Bland–Altman plot showing agreement between the logarithm of NT‐proBNP plasma levels percentage change against the average of the logarithm of NT‐proBNP plasma levels in basal + stage I (A), stage I + stage II (B) and basal + stage II (C). The solid line represents the mean of the percentage change. The dashed lines define the limits of agreement (standard deviation of percentage of change ×1.96 SD). NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SD, standard deviation; stage I, 12‐month follow‐up; stage II, 24‐month follow‐up.
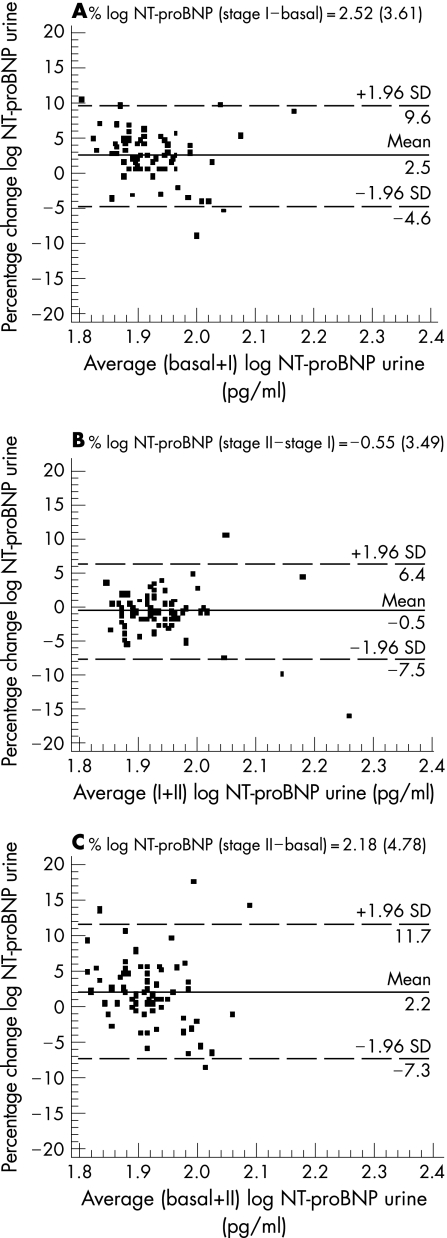
Figure 3 shows the changes in NT‐proBNP urinary levels in the A, B and C intervals. The percentage of patients within 1.96 SDs of the mean was slightly higher than plasma levels (94.6%, 96% and 94.6%, respectively). Furthermore, the values of the mean (SD) percentage change and CR were 2.52 (3.61) with a CR of 7.1%; −0.55 (3.49) with a CR 6.8% and 2.18 (4.78) with a CR 9.4%, respectively. For both plasma and urine samples agreement, the Bland–Altman plots did not show a skewed distribution on NT‐proBNP. Thus, high reproducibility within different measurements, without analytical bias, was established in our group of patients with clinically and functionally stable HF. This reproducibility was better in urine samples than in plasma.
Figure 3 Bland–Altman plot showing agreement between the logarithm of the NT‐proBNP urine levels percentage change against the average of the logarithm of NT‐proBNP urine levels in basal + stage I (A), stage I + stage II (B) and basal + stage II (C). The solid line represents the mean of the percentage change. The dashed lines define the limits of agreement (mean of percentage of change × 1.96 SD). NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SD, standard deviation; stage I, 12‐month follow‐up; stage 2, 24‐month follow‐up.
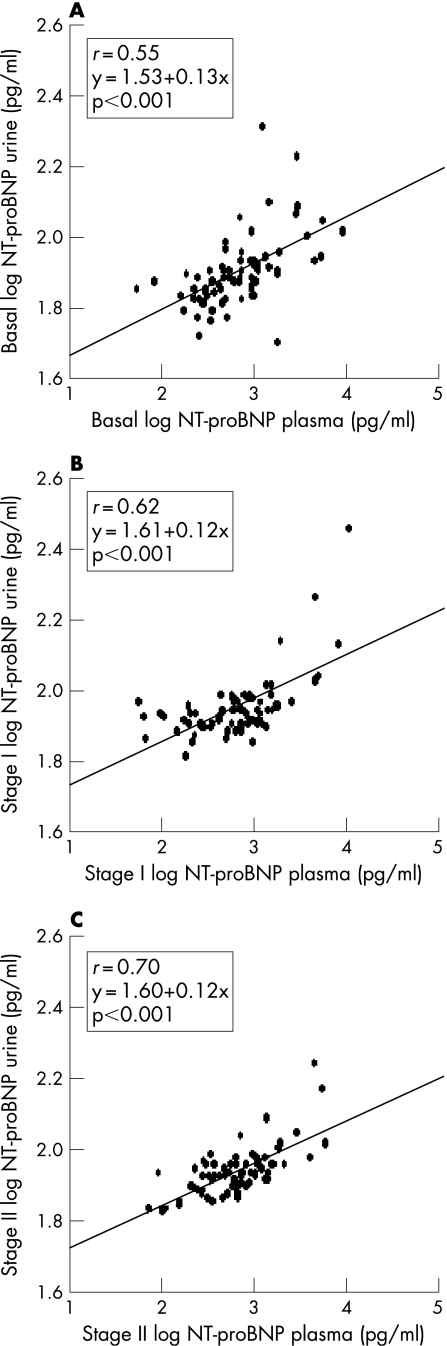
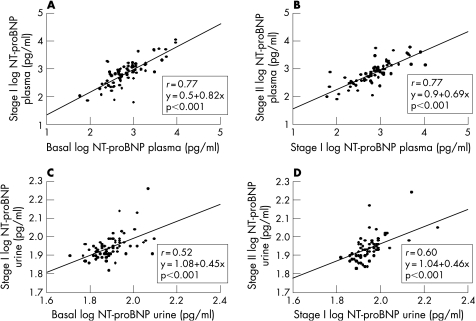
We calculated (fig 4), the correlation between plasma and urinary peptide levels at the three stages (p<0.001). Furthermore, when we analysed the correlation between the different NT‐proBNP measurements, we also found good correlation between plasma (p<0.001) and urine levels (p<0.001) (fig 5). Nevertheless, a slightly higher coefficient of correlation was obtained in plasma than in urine NT‐proBNP: basal versus stage I (r = 0.77 and r = 0.52) and stage I versus stage II (r = 0.77 and r = 0.60) (fig 5).
Figure 4 Scatter plots of the association between log‐transformed values of plasma and urine NT‐proBNP levels in basal (A), stage I (B) and stage II (C). NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; stage I, 12‐month follow‐up; stage II, 24‐month follow‐up.
Figure 5 Correlation between basal and stage I (A); and stage I and stage II (B) log‐transformed values of NT‐proBNP plasma levels and for NT‐proBNP urine (C) and (D), respectively. NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; stage I, 12‐month follow‐up; stage II, 24‐month follow‐up.
Discussion
NT‐proBNP is a useful marker for diagnosis of left ventricular dysfunction, offers diagnostic and prognostic value and may be useful for guiding treatment in patients with HF either in plasma7,9,23 or urine samples,15,24 on the basis of a high negative predictive value. Previous studies have evaluated the biological variation of BNP and NT‐proBNP plasma levels in both healthy people and patients with chronic HF over a short interval of time (within a day and week to week).10,11 Recently, Bruins et al have found higher variability in BNP than in NT‐proBNP plasma levels,11 because of its pulsatile pattern of release and its rapid turnover,13 in patients with chronic HF.
In this work, we investigated simultaneously the biological variation of plasma and urine NT‐proBNP levels in a 24‐month follow‐up of patients with stable HF who were given standard medical treatments. Any influences deriving from fluid intake, fluid infusion, exercise and circadian rhythm25,26,27,28 were minimised by using the first urine of the day and by taking the blood samples at the same time interval (08:00–10:00). Furthermore, we believe that the data obtained from our treated patients are more clinically useful for judging plasma and urine NT‐proBNP variation than the data reported from untreated or asymptomatic patients.
In our 74 patients with clinically and functionally stable HF there were neither clinical events nor significant differences in the functional class or ventricular function. But a 2% change in EF in stages I and II with respect to basal values was seen and could potentially be attributed to the methodology used.29 For the natriuretic peptide levels, our data indicate that both plasma and urine NT‐proBNP remain quite stable over time. Nevertheless, we have found significant differences for urine determinations in stages I and II compared with basal levels, whereas plasma NT‐proBNP remains unchanged. As a consequence, the plasma/urine NT‐proBNP ratio also showed significant changes in stage II with respect to basal levels (10 (9) versus 13 (15)).
Another consideration of natriuretic peptide stability is that NT‐proBNP plasma and urine levels are strongly correlated in the three different stages. As we have shown in previous work,16 urinary levels reflect plasma levels,15 and this finding was confirmed in the three stages. Furthermore, we obtained a good correlation over the entire study between plasma levels and urine levels at the three stages, the correlation coefficients being slightly higher for the plasma levels. These findings show the high stability seen in both NT‐proBNP urine and plasma samples.
On the other hand, previous studies have established that the estimated biological variation (within a day or week to week) for natriuretic peptide should be ∼30%.10,12 However, the percentage change in our patients with HF over the entire study (24‐month follow‐up) did not reach an SD value >13% for plasma levels and >5% for urine levels over any of the three intervals. Only a small group of patients showed a percentage change surpassing these percentages at the different intervals of time, in blood and urine samples. This difference between results may occur because NT‐proBNP levels were closely regulated by specific pathophysiological mechanisms and these variations reflect changes in the activation of the neuroendocrine system, and there were no changes in our left ventricular haemodynamic and functional parameters. Furthermore, the NT‐proBNP immunoassay methods were not precise14,30 and this influence on total variability has to be taken into account in analysing our results.
One important practical consequence of this study is the establishment of an NT‐proBNP percentage change, from which we can monitor the progress of these patients. Thus, we suggest that all NT‐proBNP measured variations, with a coefficient of reproducibility (>1.96 SD percentage change) above 22% in NT‐proBNP plasma concentration and 7% for urine, may be considered of potential clinical value in a 12‐month follow‐up study. Both plasma and urine NT‐proBNP showed an expected increase in percentages of 3% and 2%, respectively, for a longer follow‐up of 24 months. Increases greater than this must be evaluated by the clinicians because the natriuretic peptide system has a close relationship with neuroendocrine network activity and this variability may indicate the presence of a clinical change.
Study limitations
We have to admit that a larger group of patients would have provided additional information. However, the strict inclusion criteria to be followed to form a group of 74 stable patients during a 2‐year follow‐up study give our results even greater value.
Another important consideration is that we selected patients with stable HF without clinical or functional changes, but we cannot rule out the possibility of subtle changes in neurohormonal and immunology systems that might potentially influence the variability of the natriuretic peptide levels. However, we think that because of this, our data are more useful for judging the clinical variations in plasma and urine NT‐proBNP levels, and they have evident practical application.
It is known that the drugs given can influence peptide levels. Some studies show a decrease in plasma BNP levels in patients who receive treatment with spironolactone31 or with furosemide.32 Furthermore, one study concludes that renal excretion of NT‐proBNP is modified by enalapril,33 but it remains to be determined if this is a direct effect of ACE‐I. Thus, in order to extrapolate our results, sample collection during a follow‐up study should be carried out in a stable therapeutic setting similar to that presented in our work. Another aspect to consider is the influence that age may have on our results. The age of our patients is representative of a usual cohort of subjects with this diagnosis. However, the results possibly would not be the same in a follow‐up of patients of different ages.34
It has been reported that the measurement of ventricular function is more precise when magnetic resonance is used.35 Nevertheless, the fact that examinations were all performed in a centralised manner by a cardiologist helps us have confidence in the reliability of our results.
Conclusions
This study shows that there is good stability in NT‐proBNP plasma and urine levels in a 24‐month follow‐up study of patients with clinically and functionally stable HF. As a consequence, assessment of urinary and plasma NT‐proBNP concentrations may be a useful tool for monitoring the follow‐up of patients with HF. Measured variations in peptide concentration, exceeding 22% in plasma and 7% in urine in a 12‐month follow‐up and 25% and 9% in a 24‐month follow‐up, may indicate pathophysiological changes.
Acknowledgements
This research was supported by the National Institute of Health Fondo de Investigaciones Sanitarias del Instituto de Salud Carlos III, FIS 01/0943 Project, Spain.
Abbreviations
CR - coefficient of reproducibility
E/A - flow velocity in early diastole and during atrial contraction ratio
EF - ejection fraction
HF - heart failure
NT‐proBNP - N‐terminal pro‐brain natriuretic peptide
Footnotes
Conflict of interest: None declared
References
- 1.de Bold A J, Bruneau B G, Kuroski de Bold M L. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc Res 1996317–18. [PubMed] [Google Scholar]
- 2.de Lemos J A, McGuire D K, Drazner M H. B‐type natriuretic peptide in cardiovascular disease. Lancet 2003362316–322. [DOI] [PubMed] [Google Scholar]
- 3.Levin E R, Gardner D G, Samson W K. Natriuretic peptides. N Engl J Med 1998339321–328. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura M, Endo H, Nasu M.et al Value of plasma B type natriuretic peptide measurement for heart disease screening in a Japanese population. Heart 200287131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koglin J, Pehlivanli S, Schwaiblmair M.et al Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol 2001381934–1941. [DOI] [PubMed] [Google Scholar]
- 6.Lubien E, DeMaria A, Krishnaswamy P.et al Utility of B‐natriuretic peptide in detecting diastolic dysfunction comparison with Doppler velocity recordings. Circulation 2002105595–601. [DOI] [PubMed] [Google Scholar]
- 7.Talwar S, Squire I B, Davies J E.et al Plasma N‐terminal pro‐brain natriuretic peptide levels and the ECG in the assessment of left‐ventricular systolic dysfunction in a high risk population. Eur Heart J 1999201736–1744. [DOI] [PubMed] [Google Scholar]
- 8.Morrison L K, Harrison A, Krishnaswamy P.et al Utility of a rapid B‐natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol 200239202–209. [DOI] [PubMed] [Google Scholar]
- 9.Swedberg K, Cleland J, Dargie H.et al Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005261115–1140. [DOI] [PubMed] [Google Scholar]
- 10.Melzi d'Eril G, Tagnochetti T, Nauti A.et al Biological variation of N‐terminal pro‐brain natriuretic peptide in healthy individuals. Clin Chem 2003491554–1555. [DOI] [PubMed] [Google Scholar]
- 11.Bruins S, Fokkema M R, Romer J W.et al High intraindividual variation of B‐type natriuretic peptide (BNP) and amino‐terminal proBNP in patients with stable chronic heart failure. Clin Chem 2004502052–2058. [DOI] [PubMed] [Google Scholar]
- 12.Wu A H B, Smith A, Wieczoreck S.et al Biological variation for N‐terminal pro‐and B‐type natriuretic peptide and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 200392628–631. [DOI] [PubMed] [Google Scholar]
- 13.Hunt P J, Richards A M, Nicholls M G.et al Immunoreactive amino‐terminal pro‐brain natriuretic peptide (NT‐PROBNP): a new marker of cardiac impairment. Clin Endocrinol (Oxf) 199747287–296. [DOI] [PubMed] [Google Scholar]
- 14.Clerico A, Emdin M. Diagnostic accuracy and prognostic relevance of the measurement of cardiac natriuretic peptides: a review. Clin Chem 20045033–50. [DOI] [PubMed] [Google Scholar]
- 15.Ng L L, Geeranavar S, Jennings S C.et al Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci 2004106129–133. [DOI] [PubMed] [Google Scholar]
- 16.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005461–82. [DOI] [PubMed] [Google Scholar]
- 17.Levey A S, Bosch J P, Lewis J B.et al A more accurate method to estimate glomerular rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999130461–470. [DOI] [PubMed] [Google Scholar]
- 18.Sirera R, Salvador A, Roldán I.et al Quantification of proinflammatory cytokines in the urine of congestive heart failure patients. Its relationship with plasma levels. Eur J Heart Fail 2003527–31. [DOI] [PubMed] [Google Scholar]
- 19.Collinson P O, Bames S C, Gaze D C.et al Analytical performance of the N terminal pro B type natriuretic peptide (NT‐proBNP) assay on the Elecsys 1010 and 2010 analyzers. Eur J Heart Fail 20046365–368. [DOI] [PubMed] [Google Scholar]
- 20.Heringlake M, Heide C, Bahlmann L.et al Effects of tilting and volume loading on plasma levels and urinary excretion of relaxin, NT‐pro‐ANP, and NT‐pro‐BNP in male volunteers. J Appl Physiol 200497173–179. [DOI] [PubMed] [Google Scholar]
- 21.Bland J M, Altman D G. Statistical method for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 22.Bland J M, Altman D G. Measuring agreement in method comparison studies. Statistical Methods in Medical Research 19998135–160. [DOI] [PubMed] [Google Scholar]
- 23.Steele I C, McDowell G, Moore A.et al Responses of atrial natriuretic peptide and brain natriuretic peptide to exercise in patients with chronic heart failure and normal control subjects. Eur J Clin Invest 199727270–276. [DOI] [PubMed] [Google Scholar]
- 24.Mudambo K S, Coutie W, Rennie M J. Plasma arginine vasopressin, atrial natriuretic peptide and brain natriuretic peptide responses to long‐term field training in the heat: effects of fluid ingestion and acclimatization. Eur J Appl Physiol Occup Physiol 199775219–225. [DOI] [PubMed] [Google Scholar]
- 25.Heringlake M, Heide C, Bahlmann L.et al Effects of tilting and volume loading on plasma levels and urinary excretion of relaxin, NT‐proBNP, and NT‐proBNP in male volunteers. J Appl Physiol 200497173–179. [DOI] [PubMed] [Google Scholar]
- 26.Richards M, Troughton R W. NT‐proBNP in heart failure: therapy decisions and monitoring. Eur Heart J 20046351–354. [DOI] [PubMed] [Google Scholar]
- 27.Ng L L, Loke I W, Davies J E.et al Community screening for left ventricular dysfunction using plasma and urinary natriuretic peptides. J Am Coll Cardiol 2005451043–1050. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y F, Stein P K. Circadian rhythm in the cardiovascular system: chronocardiology. Am Heart J 2003145779–786. [DOI] [PubMed] [Google Scholar]
- 29.Sievers B, Kirchberg S, Franken U.et al Visual estimation versus quantitative assessment of left ventricular ejection fraction: a comparison by cardiovascular magnetic resonance imaging. Am Heart J 2005150737–742. [DOI] [PubMed] [Google Scholar]
- 30.Clerico A, Prontera C, Emdin M.et al Analytical performance and diagnostic accuracy of immunometric assays for the measurement of plasma B‐type natriuretic peptide (BNP) and N‐terminal proBNP. Clin Chem 200551445–447. [DOI] [PubMed] [Google Scholar]
- 31.Tsutamoto T, Wada A, Maeda K.et al Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol 2001382132–2133. [DOI] [PubMed] [Google Scholar]
- 32.Paterna S, Di Pasquale P, Parrinello G.et al Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high‐dose furosemide and hypertonic saline solution versus high‐dose furosemide alone in refractory congestive heart failure: a double‐blind study. J Am Coll Cardiol 2005452004–2007. [DOI] [PubMed] [Google Scholar]
- 33.Heringlake M, Will B, Klaus S.et al The effects of angiotensin‐converting enzyme inhibition on the urinary excretion of NTproBNP in male volunteers. Kidney Blood Press Res 200629294–305. [DOI] [PubMed] [Google Scholar]
- 34.Redfield M M, Rodeheffer R J, Jacobsen S J.et al Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 200240976–982. [DOI] [PubMed] [Google Scholar]
- 35.Eicken A, Frazt S, Gutfried C.et al Hearts late after fontan operation have normal mass, normal volume, and reduced systolic function: a magnetic resonance imaging study. J Am Coll Cardiol 2003421061–1065. [DOI] [PubMed] [Google Scholar]