Abstract
Aim
To establish the regional delay of contrast arrival in magnetic resonance perfusion imaging (MRPI) for the detection of collateral‐dependent myocardium in patients with coronary artery disease.
Design and setting
Observational study, case series; single centre, university hospital.
Patients
30 patients with coronary artery disease and collateral‐dependent myocardium and 17 healthy volunteers.
Methods
Resting and hyperaemic (adenosine) MRPI was used to determine the delay time (Δtd) of contrast arrival between the left ventricle and collateral‐dependent or antegradely perfused myocardium, and myocardial perfusion (MP, ml/min/g).
Results
In healthy volunteers, mean (SD) Δtd at rest and during hyperaemia were 0.8 (0.4) and 0.3 (0.3) s, and MP was 1.14 (0.21) and 4.23 (1.12) ml/min/g. In patients Δtd in antegradely perfused vs collateral‐dependent myocardium was 0.9 (0.7) vs 1.7 (1.0) s at rest (p<0.001), and 0.4 (0.3) vs 1.1 (0.6) s (p<0.001) during hyperaemia. MP was 1.12 (0.11) and 0.98 (0.28) ml/min/g (p = NS) at rest and 2.46 (0.85) vs 1.86 (0.91) ml/min/g (p<0.01) during hyperaemia. Receiver operating characteristics analysis showed the best sensitivity and specificity of 90% and 83% for hyperaemic Δtd of >0.6 s (area under the curve (AUC) = 0.89) to detect collateral‐dependent myocardium, while resting Δtd (AUC = 0.77) and perfusion (AUC = 0.69 at rest or 0.70 during hyperaemia) were less accurate.
Conclusions
MRPI‐derived hyperaemic delay of contrast arrival detects collateral‐dependent myocardium with high sensitivity and specificity. Perfusion was less sensitive, emphasising the clinical role of Δtd in non‐invasive detection of collateral‐dependent myocardium.
The impact of coronary collaterals on prognosis and their protective role in patients with coronary artery disease has been described.1,2 Detection and assessment of coronary collaterals is the domain of invasive coronary angiography,3 as clinical and electrocardiographic responses during exercise testing failed to show a correlation with the presence of collateral circulation.4 Prospective, non‐invasive detection of collateral perfusion is cumbersome, since perfusion might be normal if collateralisation is sufficient.
Quantitative magnetic resonance first‐pass perfusion imaging (MRPI) has been used to assess collateral flow in animals.5 Pearlman et al6 used MRI and determined a delay time measured from the appearance of the contrast in the left ventricle (LV) to the time when the signal reaches a tissue region. Recent animal data suggest that MRPI might be able to detect collateral‐dependent myocardium by an increased delay time of contrast arrival.7
The purpose of this study was to assess the delay time of contrast arrival with MRPI as a novel parameter to detect collateral‐dependent myocardium in patients. In a population of patients with one chronically occluded coronary artery, we assessed regional delay of contrast arrival to distinguish antegradely perfused and collateral‐dependent myocardium. We aimed to define a threshold value for the delay parameter to distinguish collateral‐dependent from antegradely perfused myocardium. We hypothesised that, despite a similar myocardial perfusion (MP), the arrival of contrast is delayed in collateral‐dependent myocardium and therefore better distinguishes it from antegradely perfused myocardium.
Methods
In total 30 patients (mean (SD) age 63 (11) years, 20 men) with collateral‐dependent myocardium due to a chronic total coronary occlusion of one main coronary artery (left anterior descending (LAD) n = 8, left circumflex coronary (LCx) n = 12, right coronary occlusion (RCA) n = 10) were enrolled. The remaining coronary arteries, including the feeding vessel of the collateral‐dependent myocardium, had <50% diameter stenosis. Clinical history, 12‐lead electrocardiogram and serum troponin I level were used to exclude acute or recent (<3 month) myocardial infarction. Patients underwent MRPI within 48 h of their coronary angiogram. Antianginal medication was discontinued at least 24 h before testing. MRPI was also performed in 17 healthy volunteers (mean (SD) age 34 (9) years, 11 men). At the time of the study, all patients were in stable clinical condition. Subjects with contraindications to MRPI imaging, such as metal implants, and patients with prior coronary bypass surgery were not enrolled. All subjects gave written informed consent in accordance with the requirements of the local institutional ethics committee.
Coronary angiography
Coronary angiography was performed using standard Judkins's technique. Coronary collaterals were graded according to Rentrop's classification8 (0 = no filling of the distal vessel, 1 = small side branches filled, 2 = major side branches filled and 3 = main epicardial vessel filled). Quantitative coronary angiography was performed to exclude >50% diameter stenosis of the collateral feeding vessels using the ACOM software package (Siemens Medical Solutions, Erlangen, Germany).
Magnetic resonance image acquisition
A 1.5 T MR scanner (Siemens Medical Solutions) with eight receiver channels and a 12‐element phased‐array body coil was used. Myocardial function was assessed in the short‐axis plane covering the entire LV from base to apex using a segmented single‐slice cine sequence (repetition time (TR)/echo time (TE) 3.0/1.5 ms, flip angle 50°, slice thickness 8 mm, field of view 280×380 mm2, matrix size 190×256). Perfusion imaging was performed with a single‐shot gradient‐echo technique with saturation‐recovery preparation for T1 weighting (TR/TE/flip angle of 2.4 ms/1.2 ms/18°, acquisition of 1 image/slice/heart beat, spatial resolution 2–3 mm, slice thickness 10 mm). Sixty images per slice were acquired. Patients were asked to hold their breath as long as they could comfortably do so. This reduces breathing motions, which hamper postprocessing of the images. Perfusion was determined in three LV short‐axis slices: one slice located close to the base of the heart just below the aortic outflow tract, a second in the middle of the LV and a third close to the apex just below the base of the papillary muscles. Perfusion imaging was performed first during adenosine‐induced hyperaemia and then at rest, with an interstudy delay of a minimum of 10 min. An intravenous bolus of 0.05 mmol/kg body weight (BW) gadolinium‐diethylene triamine pentaacetic acid (Gd‐DTPA) (Magnevist, Schering, Germany) injected at a rate of 6 ml/s was applied for perfusion imaging. An additional 20 ml saline flush was given after contrast injection. Adenosine was given for a minimum of 4 min at a dose of 140 μg/kg/min. Heart rate and cuff‐blood pressure (at rest and during adenosine infusion) were recorded.
Although a dose of 0.05 mmol/kg BW Gd‐DTPA can result in some signal saturation in the LV, we found it best for visual assessment of the images, while maintaining the possibility for quantitative analysis with the imaging protocol outlined above. To ascertain whether there was signal saturation in the blood pool, we compared the peak amplitudes and the width at half height of the first‐pass peak. In all cases, the peak signal intensity (SI) was higher in the right and lower in the LV, proportionate to the dispersion of the bolus between right ventricle and LV. We concluded that signal saturation effects were, if at all present, relatively minor in these studies, although we cannot completely exclude the possibility of overestimating blood flow. For the delay parameter, any saturation of the signal in the LV will not have any material effect on the delay estimation, as the delay pertains to the lag between contrast enhancement in the LV and the myocardium, but not the degree of contrast enhancement itself.
Following perfusion imaging, a third contrast injection with 0.1 mmol/kg BW Gd‐DTPA was given. After 10 min, delayed contrast‐enhanced images were acquired in the short‐axis view according to the orientation of the perfusion images, using an inversion‐recovery TurboFLASH sequence (TR/TE 11/4.4 ms, inversion temperature set to 260–290 ms (adjusted to null the myocardial signal), slice thickness 10 mm, field of view 280×340 mm2, matrix 166×256).
Image analysis
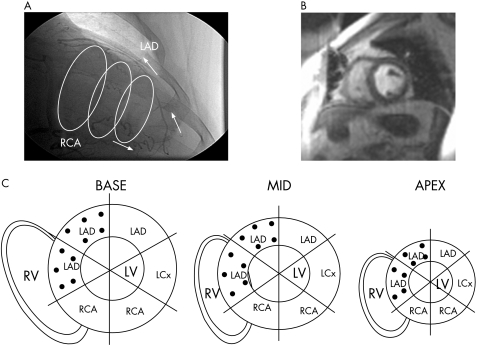
Magnetic resonance image analysis was performed by definition of collateral‐dependent myocardium based on the assessment of the coronary angiogram, the individual anatomy, size, dominance and location of the occluded vessel (fig 1A, C). Regions that appeared only partially collateral‐supplied were assigned to antegradely perfused myocardium.
Figure 1 (A) The angiogram shows an occluded left anterior descending (LAD) occlusion (after the first diagonal branch), which is filled by Rentrop grade III collaterals from the right coronary occlusion (RCA); the three rings indicate basal, midventricular and apical short‐axis slices of the left ventricle (LV) as assessed by magnetic resonance perfusion imaging (MRPI). (B) A representative MRPI in a midventricular slice position showing the peak enhancement in the LV. For generation of the signal intensity curves, endocardial and epicardial borders are manually drawn in the image with the brightest contrast enhancement in the LV cavity. An automated edge detection algorithm is then applied for segmentation to the remainder of the image frames, with subsequent manual adjustment of the contours to avoid signal spill‐over from the LV. The programme then calculates the signal/time intensity values in the six sectors. (C) The schematic plot shows the three slices (basal, midventricular and apical short‐axis position) of the LV divided into six regions; the black dots indicate collateral‐dependent regions according to the angiogram. LCx, left circumflex coronary; RV, right ventricle.
Image analysis was performed blinded to the clinical status and the coronary angiogram. Cine and perfusion studies were analysed using a dedicated software (ARGUS, Siemens Medical Solutions). LV ejection fraction (%) was calculated from the end systolic and end diastolic volumes derived from the short‐axis cine study. For perfusion imaging, the short‐axis images were automatically divided into six sectors of equal size, with the reference sector defined at the anterior junction of the left and right ventricles (fig 1C). Spatially averaged, baseline‐corrected SI values from each region were used to plot SI versus time curves from the perfusion images (fig 2 and Muehling et al9). The software interpolated the curves automatically to 100 data points, resulting in an interpolated resolution equal to 60% of the relative risk interval. An additional region of interest in the centre of the LV cavity was used to obtain the arterial input.
Figure 2 The signal intensity curve for a left ventricular region of interest, used here as arterial input, is shown with tissue curves for collateral‐dependent and antegradely perfused myocardial regions, respectively. td is the time between the appearance of the contrast in the left ventricle and antegradely perfused myocardium; tdcollat. is the time between the appearance of the contrast in the left ventricle and collateral‐dependent myocardium.
An impulse response was calculated by deconvolution of each measured tissue curve with the arterial input10 using custom‐written software (Matlab V.6.5.1, Mathworks, Natick, MA, USA). A tissue response was calculated from this impulse response. The software allows the calculation of delay time and perfusion in a one‐step approach. The delay parameter was defined as the delay time (td, in seconds) between contrast arrival in a myocardial region of interest and the LV input (fig 2). The software adjusted the delay for best agreement with the measured SI curve—that is, the intersection of a regression line of increasing flow versus time shift over the range in which flow remains constant.7
According to our experience in an animal model,7 the td had to be corrected for the dependence of the delay time on the distance of the short‐axis slices from the base of the heart (corrected delay time (Δtd) = delay time of the individual sector (td)−minimum delay in that slice). MP (ml/min/g) was determined from the maximum amplitude of the impulse response curve.10 MP was normalised to the rate pressure product (mm Hg/min×10 000) as an index of cardiac work.11
Infarct was identified by a signal intensity >2 SDs above the signal intensity in antegradely perfused myocardium 10 min after contrast injection12 in the delayed contrast‐enhanced images. Where applicable, the transmural extent of infarction (TEI, %) was determined by planimetry using the scanner software. TEI was calculated as the contrast‐enhanced area in collateral‐dependent myocardium (mm2)×100/total area of collateral‐dependent myocardium (mm2) per region.
Statistics
Data were analysed using SPSS V.13. Student's t test for paired samples was used to test levels of significance between antegradely perfused and collateral‐dependent regions in one patient. Two‐way analysis of variance was used to test levels of significance between regions in patients and volunteers. If significance was indicated by analysis of variance, a Bonferroni correction was used as a post‐hoc test for multiple pairwise comparisons. Threshold values were determined by receiver operating characteristics (ROC) and the area under the curve. Associations between Rentrop grade and Δtd or perfusion were evaluated by Spearman's rank correlation coefficient. Continuous data were expressed as mean (SD). A p value of <0.05 was considered significant.
Results
Demographic characteristics and haemodynamic data of patients and volunteers are shown in table 1.
Table 1 Demographic characterstics and haemodynamic data of patients and volunteers.
| Patients n = 30 | Volunteers n = 17 | p Value | |
|---|---|---|---|
| Age (years) | 61 (11) | 34 (9) | <0.001 |
| Male | 67% | 65% | NS |
| Ejection fraction (%) | 52 (14) | 65 (3) | <0.05 |
| Heart rate (min−1) | |||
| At rest | 65 (12) | 64 (9) | NS |
| During hyperaemia | 76 (13)* | 87 (14)* | 0.05 |
| Pressure rate product (mm Hg/min) | |||
| At rest | 6373 ((1445) | 6137 (967) | NS |
| During hyperaemia | 7531 (1774)* | 7919 (1862)* | NS |
| Occluded vessel | |||
| LAD | 27% | – | |
| LCx | 40% | – | |
| RCA | 33% | – | |
| Risk factors | |||
| Smoking | 62% | No | |
| Hyperlipidaemia | 80% | No | |
| Diabetes | 7% | No | |
| Hypertension | 80% | No |
LAD, left anterior descending; LCx, left circumflex coronary; NS, non‐significant; RCA, right coronary occlusion.
*p<0.01 vs resting measurement.
In the group of volunteers, a total of 306 regions (6 regions ×3 slices ×17 volunteers) were analysed. In patients, 135 regions were assigned to collateral‐dependent and 405 regions to antegradely perfused myocardium. Uncorrected resting delay time (td) was 1.6 (1.0) and 1.7 (0.7) s in antegradely perfused myocardium and in the myocardium of healthy volunteers. Table 2 shows the mean resting and hyperaemic data of the slice‐corrected delay time (Δtd) and perfusion in collateral‐dependent and antegradely perfused myocardium and the myocardium of healthy volunteers.
Table 2 Mean resting and hyperaemic data of slice‐corrected delay time and perfusion.
| Regions (n) | Patients | Volunteers | p Value | |
|---|---|---|---|---|
| Antegradely perfused n = 405 | Collateral‐dependent n = 135 | Normal myocardium n = 306 | ||
| Δtd | ||||
| Rest | 0.9 (0.7)* | 1.7 (1.0) | 0.8 (0.4)# | *p<0.01 and #p<0.001 vs collateral‐dependent |
| Hyperaemia | 0.4 (0.3)# | 1.1 (0.6) | 0.3 (0.3)# | |
| Perfusion | ||||
| Rest | 1.12 (0.11) | 0.98 (0.28) | 1.14 (0.21) | *p<0.001 vs patients #p<0.01 vs antegradely perfused |
| Hyperaemia | 2.46 (0.85) | 1.86 (0.91)# | 4.23 (1.12)* | |
p<0.001 rest vs hyperaemia in patients.
p<0.001 rest vs hyperaemia in all groups.
p<0.001 rest vs hyperaemia in volunteers.
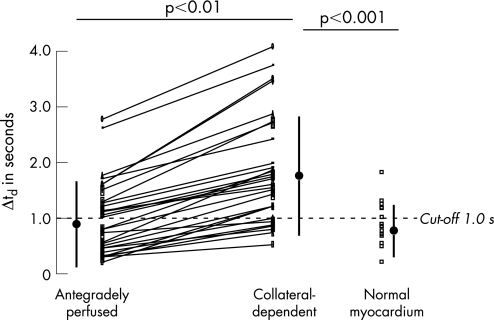
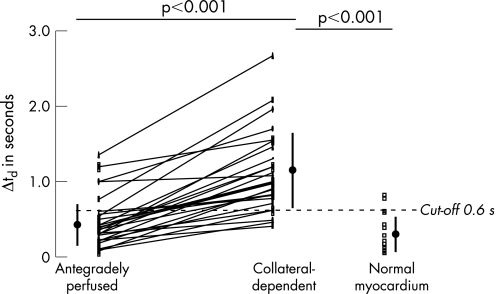
Data of the slice‐corrected delay time in individual patients and volunteers in collateral‐dependent, antegradely perfused and normal myocardium are shown in figs 3 (at rest) and 4 (during hyperaemia).
Figure 3 Analysis of slice‐corrected delay time at rest in antegradely perfused and collateral‐dependent myocardium per patient and in myocardium of healthy volunteers. The cut‐off value for resting Δtd of >1.0 s was derived from receiver operating characteristics analysis, and indicates the best sensitivity and specificity to detect collateral‐dependent myocardium (see text and table 3 for details).
The results of a prospective analysis of a regional‐based ROC analysis detecting collateral‐dependent myocardium by the delay of contrast arrival or myocardial perfusion are shown in table 3.
Table 3 The cut‐off value for hyperaemia Δtd of >0.6 s derived from receiver operating characteristics.
| Parameter | Cut‐off value | Sensitivity | Specificity | AUC | 95% CI |
|---|---|---|---|---|---|
| Δtd rest | >1.0 s | 77 | 67 | 0.77 | 0.66 to 0.88 |
| Δtd hyperaemia | >0.6 s | 90 | 83 | 0.89 | 0.78 to 0.96 |
| MPrest | <1.0 ml/min/g | 65 | 85 | 0.69 | 0.54 to 0.84 |
| MPhyperaemia | <1.6 ml/min/g | 55 | 93 | 0.70 | 0.52 to 0.84 |
AUC, area under the curve; MP, myocardial perfusion; Δtd, slice‐corrected delay time.
Relationship between extent of infarction, angiographic collateral vessel filling, MP and slice‐corrected delay time
Δtd was not significantly different in 43 collateral‐dependent regions with ⩾50% TEI, compared with 92 regions with <50% TEI at rest (1.8 (0.8) vs 1.6 (0.8) s, p = NS) or during hyperaemia (1.2 (1.0) vs 1.0 (0.4) s, p = NS). In contrast, perfusion in collateral‐dependent myocardium with ⩾50% TEI was significantly lower compared with regions with <50% TEI at rest (0.39 (0.12) and 1.12 (0.28) ml/g/min, p<0.001) and during adenosine‐induced hyperaemia (0.93 (0.44) and 2.21 (0.73) ml/min/g, p<0.001).
Δtd in collateral‐dependent myocardium was longer in 12 patients with Rentrop grades 0–1 (52 regions) compared with 18 patients with Rentrop grades 2–3 (83 regions) at rest (2.1 (0.9) vs 1.4 (0.8) s, p<0.04) and during hyperaemia (1.4 (0.7) vs 0.8 (0.5) s, p<0.03). At rest, no significant difference in perfusion of collateral‐dependent myocardium was found in patients with Rentrop grades 2–3 compared with patients with Rentrop grades 0–1 (1.00 (0.25) vs 0.96 (0.29) ml/min/g, p = NS), and a similar pattern during hyperaemia (1.88 (0.86) for Rentrop grades 2–3, vs 1.82 (0.81) ml/min/g for Rentrop grades 0–1, p = NS). Rentrop grades were not significantly different in patients with ⩾50% TEI, compared with those with <50% TEI (1.7 (0.8) vs 1.9 (1.1), p = NS).
There was a significant inverse correlation between Rentrop grade and Δtd in collateral‐dependent myocardium at rest (r = –0.49, p<0.04) and during hyperaemia (r = −0.56, p<0.03), but not between Rentrop grade and perfusion (at rest: r = 0.02, p = 0.94, hyperaemia: r = 0.09, p = 0.72).
Discussion
This is the first study to show that collateral‐dependent myocardium in patients with a chronic occluded coronary artery can be non‐invasively detected using the delay of contrast‐arrival MRPI. The slice‐corrected delay time is a stronger predictor of collateral‐dependent myocardium than MP. The uncorrected delay of contrast arrival in antegradely perfused myocardium is 1.6–1.7 s at rest in our study. Based on the Stewart–Hamilton principle and a flow of 1 ml/min/g, a theoretical estimate of the delay time between the LV and the myocardium is about 1.5 s7 at rest, which compares well with our measured delay for antegradely perfused myocardium. The higher sensitivity and specificity of the hyperaemic myocardium over the resting delay for the detection of collateral‐dependent myocardium demonstrated by the ROC analysis can be best explained by an increasing heterogeneity to the delay parameter in relation to the resistance of the microcirculation. The influence of the microcirculation on the delay parameter can be decreased by adenosine, and results in a reduced data overlap between collateral‐dependent and antegradely perfused myocardium. This can be best perceived considering figs 3 and 4. In addition, there is a slightly smaller variability of the slice‐corrected delay time during adenosine‐induced hyperaemia compared with the data at rest. Our findings are supported by an increasing variability of baseline, but not hyperaemic, coronary Doppler‐flow velocity in response to adenosine with increasing atherosclerosis in an earlier study.13
Figure 4 Analysis of slice‐corrected delay time during hyperaemia in antegradely perfused and collateral‐dependent myocardium per patient and in the myocardium of healthy volunteers. The cut‐off value for hyperaemic Δtd of >0.6 s for hyperaemia was derived from receiver operating characteristics (ROC) analysis, and indicates the best sensitivity and specificity to detect collateral‐dependent myocardium (see text and table 3 for details).
Advantage of the delay time over perfusion
Our data demonstrate that perfusion at rest may not be reduced in collateral‐dependent myocardium, if significant stenosis of the coronary feeding vessel and extensive (⩾50%) transmural infarction are excluded. Hyperaemic perfusion may be significantly lower in collateral‐dependent compared with antegradely perfused myocardium; however, this can be related to other pathologies, such as infarction, as demonstrated in our study, or coronary stenosis, as demonstrated elsewhere.14 Furthermore, hyperaemic perfusion was similar in collateral‐dependent and antegradely perfused myocardium if the transmural extent of infarction was limited (<50%). This explains to some extent the higher sensitivity of the delay time compared with MP for the detection of collateral‐dependent myocardium. Thus, perfusion, either at rest or under hyperaemic conditions, is not an adequate parameter to distinguish collateral‐dependent from antegradely perfused myocardium. Accordingly, we, as well as others,15 believe that even quantification of myocardial flow or flow reserve may be inadequate for the detection of collateral‐dependent myocardium.
Correlation between perfusion and angiographic collateral vessel filling
A weak correlation between perfusion and angiographic collateral vessel filling has been demonstrated earlier with echocardiography.16 It was explained by the limited spatial resolution of a coronary angiogram, which visualises only vessels of >100 μm diameter.17 Thus, many collateral vessels are smaller and not visualised by this technique. The delay of contrast arrival in collateral‐dependent myocardium had an inverse relationship to the angiographic collateral vessel filling (Rentrop grade). An increase in Rentrop grade translates into a decrease in delay time, whereas MP did not allow a differentiation between regions with good and poor angiographic collateral vessel filling. In addition, and despite the significant difference in antegrade hyperaemic perfusion, the delay Δtd was not significantly different between patients and volunteers. For non‐invasive assessment of myocardial blood flow, it therefore becomes important to consider both flow and delays in contrast arrival together, rather than assuming a constant delay.
Limitations
Our patient population included only two patient with diabetes. It is unknown how significant microcirculatory disease—for example, in patients with diabetes—will influence the results. In this context, Seiler3 recently reported identical collateral flow indices in patients with and in those without diabetes. Furthermore, we can only speculate how a flow‐limiting stenosis of the collateral‐feed vessel will affect the difference in delay time between antegradely perfused and collateral‐dependent myocardium. A previous magnetic resonance study demonstrated that a haemodynamically significant epicardial stenosis does reduce perfusion reserve, but will not result in a noticeable delay of contrast arrival.18 Patients with bypass vessels were not included in our study. Although this is speculative, with the relationship of the delay time to the length of the vessel, we believe that a long bypass vessel will most probably increase the delay time of contrast arrival.
Conclusion
Using the delay of contrast arrival during adenosine‐induced hyperaemia, MRPI offers a novel non‐invasive parameter to detect collateral‐dependent myocardium with high sensitivity and specificity in patients without a flow‐limiting stenosis in the collateral feeding vessel, and independent of the TEI. The delay of contrast arrival is related to angiographic collateral vessel filling, but seems to be mostly independent of MP. Resting and hyperaemic MP in collateral‐dependent myocardium can be maintained if significant stenosis of the coronary feeding vessel and extensive transmural infarction are excluded. In this context, the clinical impact of the delay time becomes evident as it allows distinction between antegradely perfused and collateral‐dependent myocardium, and therefore might be a useful parameter for further clinical decision making—for example, interventional/surgical versus conservative approach.
Acknowledgements
We thank Ruth Robertson for editing the manuscript. MJH gratefully acknowledges support for his work by NIH RO1 HL65394‐01.
Abbreviations
BW - body weight
DTPA - diethylene triamine pentaacetic acid
LV - left ventricle
MP - myocardial perfusion
MR - magnetic resonance
MRPI - magnetic resonance perfusion imaging
ROC - receiver operating characteristics
SI - signal intensity
TEI - transmural extent of infarction
TR/TE - repetition time/echo time
Footnotes
Competing interests: None.
References
- 1.Habib G B, Heibig J, Forman S A.et al Influence of coronary collateral vessels on myocardial infarct size in humans. Results of phase I thrombolysis in myocardial infarction (TIMI) trial. The TIMI Investigators. Circulation 199183739–746. [DOI] [PubMed] [Google Scholar]
- 2.Hansen J F. Coronary collateral circulation: clinical significance and influence on survival in patients with coronary artery occlusion. Am Heart J 1989117290–295. [DOI] [PubMed] [Google Scholar]
- 3.Seiler C. The human coronary collateral circulation. Heart 2003891352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman S B, Dunn R F, Bernstein L.et al Influence of coronary collateral blood flow on the development of exertional ischemia and Q wave infarction in patients with severe single‐vessel disease. Circulation 198571681–686. [DOI] [PubMed] [Google Scholar]
- 5.Jerosch‐Herold M, Wilke N. MR first pass imaging: quantitative assessment of transmural perfusion and collateral flow. Int J Cardiac Imaging 199713205–218. [DOI] [PubMed] [Google Scholar]
- 6.Pearlman J D, Hibberd M G, Chuang M L. Magnetic resonance mapping demonstrates benefits of VEGF‐induced myocardial angiogenesis. Nat Med 199511085–1089. [DOI] [PubMed] [Google Scholar]
- 7.Jerosch‐Herold M, Hu X, Murthy N S.et al Time delay for arrival of MR contrast agent in collateral‐dependent myocardium. IEEE Trans Med Imaging 200423881–890. [DOI] [PubMed] [Google Scholar]
- 8.Rentrop K P, Cohen M, Blanke H.et al Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol 19855587–592. [DOI] [PubMed] [Google Scholar]
- 9.Muehling O, Dickson M, Zenovich A.et al Quantitative magnetic resonance first‐pass perfusion analysis: inter‐ and intraobserver agreement. J Cardiovasc Magn Reson 20013247–256. [DOI] [PubMed] [Google Scholar]
- 10.Jerosch‐Herold M, Wilke N, Stillman A E. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys 19982573–84. [DOI] [PubMed] [Google Scholar]
- 11.Czernin J, Muller P, Chan S.et al Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 19938862–69. [DOI] [PubMed] [Google Scholar]
- 12.Kim R J, Wu E, Rafael A.et al The use of contrast‐enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 20003431445–1453. [DOI] [PubMed] [Google Scholar]
- 13.Wieneke H, Schmermund A, Ge J.et al Increased heterogeneity of coronary perfusion in patients with early coronary atherosclerosis. Am Heart J 2001142691–697. [DOI] [PubMed] [Google Scholar]
- 14.Gould K L, Lipscomb K, Hamilton G W. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol 19743387–94. [DOI] [PubMed] [Google Scholar]
- 15.Sambuceti G, Parodi O, Giorgetti A.et al Microvascular dysfunction in collateral‐dependent myocardium. J Am Coll Cardiol 199526615–623. [DOI] [PubMed] [Google Scholar]
- 16.Vernon S M, Camarano G, Kaul S.et al Myocardial contrast echocardiography demonstrates that collateral flow can preserve myocardial function beyond a chronically occluded coronary artery. Am J Cardiol 199678958–960. [DOI] [PubMed] [Google Scholar]
- 17.Gensini G G, Bruto da Costa B C. The coronary collateral circulation in living man. Am J Cardiol 196924393–400. [DOI] [PubMed] [Google Scholar]
- 18.Kroll K, Wilke N, Jerosch‐Herold M.et al Modeling regional myocardial flows from residue functions of an intravascular indicator. Am J Physiol 1996271H1643–H1655. [DOI] [PMC free article] [PubMed] [Google Scholar]