Abstract
Objective
To assess whether chronic treatment with carvedilol can increase myocardial blood flow (MBF) and MBF reserve in idiopathic dilated cardiomyopathy (IDC).
Study design
In a double‐blind, placebo‐controlled trial, 16 consecutive patients with IDC were randomised to treatment with either carvedilol up to 25 mg twice a day (n = 8, 7 men, mean (SD) age 60 (9) years, mean (SD) left ventricular ejection fraction (LVEF) 30% (5%)), or placebo (n = 8 , 6 men, mean (SD) age 62 (9) years, mean (SD) LVEF 28% (6%), NS vs carvedilol group). Before and 6 months after treatment, regional MBF was measured at rest and after intravenous injection of dipyridamole (Dip; 0.56 mg/kg in 4 min) by positron emission tomography and using 13N‐ammonia as a flow tracer. Exercise capacity was assessed as the time duration in a maximal bicycle exercise stress test.
Results
Carvedilol induced a significant decrease in heart rate at rest and during maximal exercise, and an increase in exercise capacity. Absolute MBF values did not significantly change after carvedilol or placebo treatment, either at rest or during Dip injection, although Dip‐MBF tended to improve after treatment. Coronary flow reserve significantly increased following carvedilol treatment (from 1.67 (0.63) to 2.58 (1.04), p<0.001), whereas it remained unchanged following the placebo treatment (from 1.80 (0.84) to 1.77 (0.60), NS). Stress‐induced regional perfusion defects decreased after carvedilol treatment (from 38% to 15%).
Conclusions
Long‐term treatment with carvedilol can significantly increase coronary flow reserve and reduce the occurrence of stress‐induced perfusion defects, suggesting a favourable effect of the drug on coronary microvascular function in patients with IDC.
Chronic treatment with carvedilol improves prognosis in patients with heart failure.1,2 It has been shown to be superior to other β‐blockers in reducing mortality3 and improving left ventricular (LV) function.4 The mechanisms of the favourable effects of carvedilol are still debated; they include the known effects of β‐blocking agents in heart failure, such as reversing LV remodelling, increasing myocardial oxygen to supply ratio,5,6 as well as additional mechanisms associated with vasodilatory7 and antioxidant8 properties. In particular, the ancillary effects of carvedilol could directly improve myocardial perfusion by enhancement of endothelium‐dependent and independent coronary vascular function, possibly causing recovery of chronic ischaemic LV dysfunction. This hypothesis has been partially confirmed in the Carvedilol Hibernation Reversible Ischaemia Trial, in which improvement of left ventricular ejection fraction (LVEF) after carvedilol treatment in patients with ischaemic LV dysfunction was found to be proportional to the volume of ischaemic/viable myocardium.9
In patients with idiopathic dilated cardiomyopathy (IDC), the increase in LVEF induced by carvedilol seems to be similar or even more consistent than in patients with heart failure due to ischaemic heart disease,3,10,11,12 and independent of the level of cardiac sympathetic nervous system impairment.13 One possible explanation for these findings in IDC is that the diffuse coronary microvascular dysfunction, consistently demonstrated in these patients14,15 and putatively able to cause myocardial ischaemia,16,17 could provide a homogeneous substrate for the vascular actions of carvedilol, which adds to the haemodynamic and myocardial effects. These combined mechanisms could improve perfusion and function in the homogeneously viable myocardium of patients with IDC.
To test this hypothesis, we assessed the effects of chronic treatment with carvedilol, as compared with placebo, on myocardial perfusion in a randomised trial performed in patients with IDC. We used positron emission tomography (PET) and 13N‐ammonia as the reference technique to quantitatively measure absolute regional myocardial blood flow (MBF) at rest and during pharmacological vasodilation, before and after 6 months of treatment. Changes in MBF were compared with those observed in clinical, functional and haemodynamic variables.
Methods
Population and trial design
This is a substudy of a multicentre, double‐blind, parallel group, placebo‐controlled trial (CAR‐01) carried out to assess the effect of carvedilol on submaximal exercise tolerance after 6 months of maintenance treatment in patients with stable, mild to moderate congestive heart failure (New York Heart Association class II–III) due to IDC. The diagnosis of IDC was based on the presence of reduced LV systolic function (LVEF ⩽35%) and angiographically normal coronary arteries. The study enrolled a total of 84 patients with IDC randomised to carvedilol or placebo in addition to optimal treatment for heart failure (HF) including angiotensin‐converting enzyme‐inhibitors and diuretics.18 Sixteen patients (13 men and 3 women, mean (SD) age 69 (9) years) were enrolled in the PET substudy. The study conformed to good clinical practice guidelines and followed the recommendations of the Declaration of Helsinki. The protocol was approved by the relevant and local ethics review boards. Written informed consent was obtained from all patients before enrolment. Roche (Basel, Switzerland) sponsored the main study, while there was no specific sponsorship for the PET substudy.
Following screening and stabilisation on standard treatment (week‐4 to day 0), baseline evaluations were performed. Admitted patients received an open‐label carvedilol challenge with a dose of 3.125 mg twice daily for 14 days, and patients intolerant to the drug were excluded from the study. Patients who tolerated the open‐label challenge were randomised to receive placebo (eight patients, group 1) or carvedilol (eight patients, group 2) in an up‐titration phase starting from 6.25 mg twice daily, up to 25 mg twice daily, doubling the dose at 2‐week intervals, followed by a 6‐month maintenance period at the highest tolerated dose. Five patients in the carvedilol group reached the maximum dosage of 25 mg twice daily without changes in the 6‐month period. Three patients did not tolerate higher doses, and were treated with 12.5 mg twice daily (n = 2) or 6.25 mg twice daily (n = 1). After 6 months, exercise tolerance and MBF were reassessed with the same protocols used at baseline.
Procedures
Submaximal exercise tolerance was assessed by exercise duration expressed in seconds at a constant workload corresponding to 80% of the peak workload attained during the maximal baseline symptom‐limited bicycle exercise stress test (Bruce protocol).
A PET study was performed to measure specific MBF (ml/min/g) at rest and during pharmacological vasodilation by intravenous dipyridamole (Dip; 0.56 mg/kg in 4 min) using 13N‐ammonia as a flow tracer. Caffeine, theophylline and theophylline derivatives were withdrawn 24 h before imaging. After an overnight fasting period, patients were positioned on the bed of a three‐ring positron tomograph (ECAT 931, CTI, Knoxville, Tennessee, USA), which provided seven simultaneous cross‐sectional planes. Transmission images were acquired up to 60 million counts with a 68Ge source and subsequently used to generate attenuation correction factors. Correct positioning was maintained throughout the study with the use of the light beam and marks on the subject's torso. Thereafter, 7.4 MBq/kg body weight (0.2 mCi/kg) of 13N‐ammonia (physical half‐life, 9.9 min) was infused over a 10–20 s period into the left antecubital vein. Dynamic acquisition was started simultaneously with tracer injection; 28 frames were acquired over 8 min (16 frames of 3 s, 11 frames of 12 s and 1 frame of 300 s). At 50 min after the baseline study, Dip (0.56 mg/kg body weight) was infused intravenously over 4 min; at 3 min after the end of the infusion, 13N‐ammonia was injected. Aminophylline (120–240 mg) was always injected intravenously ⩾3 min after 13N‐ammonia injection to antagonise the effects of Dip. Absolute MBF (ml/min/g) was computed in the best cross‐sectional plane, in six LV regions of interest (two in the septal, two in the anterior and two in the posterolateral wall) by one experienced cardiologist unaware of the clinical findings according to a method already applied in patients with IDC.14,15 Regional coronary flow reserve (CFR) was defined as the ratio between hyperaemic MBF and baseline MBF in each LV region. Normalised resting MBF (nMBF) was obtained in each LV region by correction for rate pressure product (RPP = systolic blood pressure (mm Hg)×heart rate (beats/min)) as nMBF = (MBF/RPP) ×10 000. To assess whether Dip could induce maldistribution of regional myocardial perfusion as compared with the perfusion pattern at rest, MBF values in each LV region and in each condition (resting, Dip) were expressed as percentage of the MBF value measured in that condition in the reference region (corresponding to the best perfused region at rest). Dip‐induced regional perfusion defect (Dip+) was arbitrarily defined if ((%MBF rest)−(%MBF Dip)) >5%. A similar approach is used in the semiquantitative evaluation of rest–stress PET perfusion studies.19
Statistical analysis
All data are presented as mean (SD), unless otherwise indicated. Student's t test for paired samples was used to compare variables in the same patients before and after treatment. MBF, nMBF and CFR values obtained in each region (six values for each patient) were entered into the analysis. Two tailed analysis of variance, followed by Scheffé's F test, was used for comparisons of percentage changes in regional MBF variables before and after treatment between groups. χ2 test was used to compare the incidence of Dip+ before and after treatment between groups. Values of p<0.05 were considered significant.
Results
At enrolment, there was no significant difference between patients randomised to placebo (group 1) and those randomised to carvedilol (group 2) in main clinical and LV functional variables (table 1). After 6 months, 6/8 patients in group 1 and 4/8 in group 2 had showed an improvement in New York Heart Association functional class, whereas 2/8 patients in the placebo group and none in the carvedilol group showed a deterioration. LVEF tended to increase, whereas diastolic and systolic LV dimensions tended to decrease in the carvedilol group even though these changes were not statistically significant.
Table 1 Clinical and echocardiographic left ventricular functional data at enrolment.
| Group 1 (n = 8) | Group 2 (n = 8) | p Value | |
|---|---|---|---|
| Clinical data | |||
| Men (n) | 6 | 7 | NS |
| Mean (SD) age (years) | 62 (9) | 60 (9) | NS |
| NYHA II/III (n/n) | 4/4 | 6/2 | NS |
| ACE inhibitors/sartanics (n) | 8 | 6 | NS |
| Digoxin (n) | 5 | 8 | NS |
| Diuretics (n) | 7 | 7 | NS |
| Amiodarone (n) | 3 | 0 | NS |
| LV functional data | |||
| Mean (SD) LVEDD (mm) | 65 (9) | 65 (8) | NS |
| Mean (SD) LVESD (mm) | 53 (9) | 53 (8) | NS |
| Mean (SD) LVEF (%) | 28 (6) | 30 (5) | NS |
LV, left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVESD, left ventricular end‐systolic diameter; LVEF, left ventricular ejection fraction; NS, not significant.
The two groups of patients did not differ in haemodynamic and exercise variables at enrolment. After 6‐months treatment, carvedilol induced a significant decrease in heart rate and RPP, both at rest and during exercise. These haemodynamic changes were associated with a significant increase in submaximal exercise capacity. Conversely, in the placebo group, haemodynamic variables and exercise capacity remained unchanged after 6 months (table 2).
Table 2 Haemodynamic and exercise data at enrolment and after 6 months of treatment.
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Enrolment | 6 months (placebo) | p Value | Enrolment | 6 months (carvedilol) | p Value | |
| Rest HR (bpm) | 77 (15) | 83 (14) | NS | 74 (14) | 61 (10) | 0.009 |
| Rest SBP (mm Hg) | 131 (21) | 129 (15) | NS | 135 (13) | 130 (9) | NS |
| Rest RPP (mm Hgxbpm) | 10210 (3264) | 10756 (2688) | NS | 10014 (2376) | 7821 (1284) | 0.013 |
| Ex HR (bpm) | 129 (23) | 128 (23) | NS | 127 (29) | 99 (20) | 0.007 |
| Ex SBP (mm Hg) | 139 (19) | 144 (17) | NS | 140 (23) | 139 (23) | NS |
| Ex RPP (mm Hgxbpm) | 18024 (4606) | 18574 (4845) | NS | 17416 (3436) | 13973 (4176) | 0.009 |
| Ex duration (s) | 360 (250) | 428 (213) | NS | 315 (110) | 509 (251) | 0.030 |
Ex, exercise; HR, heart rate; SBP, systolic blood pressure; RPP, rate pressure product.
All values are represented as mean (SD).
The two groups of patients did not differ in any of the PET regional flow variables at enrolment. In carvedilol‐treated patients, at 6 months, Dip‐MBF tended to increase and CFR increased significantly, whereas patients in the placebo group did not show significant changes in any regional MBF variable. After correction for RPP, the carvedilol group also showed a significant increase in normalised MBF values at rest (table 3).
Table 3 Positron emission tomogaphy data at enrolment and after 6 months of treatment.
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Enrolment | 6 months (placebo) | p Value | Enrolment | 6 months (carvedilol) | p Value | |
| Rest MBF | 0.65 (0.28) | 0.63 (0.26) | NS | 0.56 (0.20) | 0.53 (0.21) | NS |
| Dip‐MBF | 1.08 (0.49) | 1.02 (0.34) | NS | 1.16 (0.7) | 1.37 (0.64) | NS |
| CFR | 1.80 (0.84) | 1.77 (0.6) | NS | 2.05 (0.74) | 2.71 (1.37) | 0.003 |
| Rest nMBF | 0.67 (0.20) | 0.68 (0.21) | NS | 0.59 (0.21) | 0.67 (0.30) | 0.041 |
CFR, coronary flow reserve; Dip, dipyridamole; MBF, myocardial blood flow; nMBF, regional MBF at rest normalised to rate pressure product.
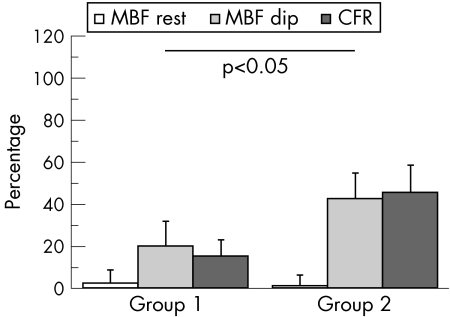
Overall, the two groups significantly differed in percentage changes in MBF variables from enrolment to 6 months of treatment (fig 1).
Figure 1 Percentage changes (mean (SE)) in regional myocardial blood flow (MBF) at rest and during dipyridamole (Dip) infusion as well as in regional coronary flow reserve (CFR) from enrolment to 6 months treatment are presented for patients treated with placebo (group 1) or with carvedilol (group 2). The two groups significantly differed in analysis of variance.
Before treatment, 11 of 48 regions in the placebo group (in 5/8 patients) and 18 of 48 regions in the carvedilol group (in 6/8 patients) showed Dip+ (p = NS, between groups). Dip+ areas in the whole population included 25% of the septal, 31% of the anterior and 34% of the lateral regions, with no between‐group difference in distribution.
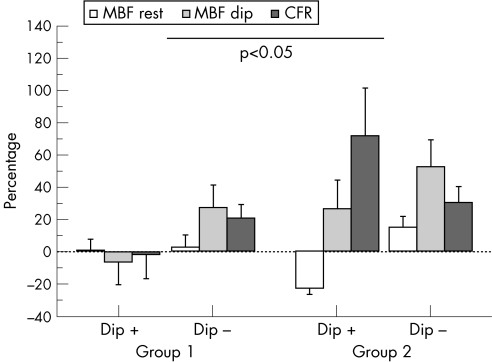
After treatment, the number of Dip+ regions increased in the placebo group (from 23% to 33%) but decreased in the carvedilol group (from 38% to 15%; p<0.05 between groups; fig 2). In the placebo group, regional MBF values did not significantly change at 6 months both in Dip+ and in Dip– myocardial regions. In the carvedilol group, treatment induced a significant decrease in resting MBF in Dip+ myocardial regions. Dip MBF and CFR increased in both Dip+ and Dip− regions, reaching statistical significance for CFR in Dip+ regions (table 4). Overall the two groups significantly differed for percentage MBF changes in Dip+ or Dip– regions from enrolment to 6 months treatment as shown in fig 3.
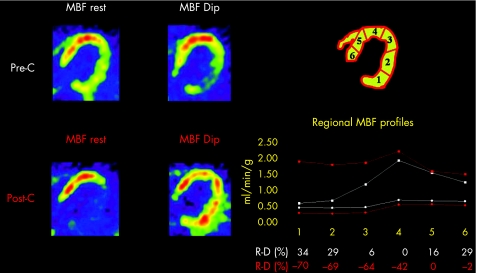
Figure 2 Transaxial positron emission tomography flow images obtained at rest and during dipyridamole (Dip) infusion in a representative patient before (pre‐C) and after treatment (post‐C) with carvedilol are shown (left panel). Absolute myocardial blood flow (MBF) value profiles, together with ((%MBF rest)−(%MBF Dip)) values (R‐D%), in the six left ventricular myocardial regions are also reported before (white colour) and after treatment (red colour; right panel). Before treatment, Dip increased the maldistribution of regional MBF already present at rest, causing stress‐induced flow defects more evident in the posterolateral wall (regions 1–2). After treatment, resting MBF was actually reduced in particular in the posterolateral wall, whereas Dip MBF was increased, in particular in the posterolateral wall, and stress‐inducible regional flow defects were no longer present.
Table 4 Myocardial blood flow changes in regions with or without dipyridamole‐induced perfusion defects.
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Enrolment | 6 months (placebo) | p Value | Enrolment | 6 months (carvedilol) | p Value | |
| Dip+ regions | ||||||
| Rest MBF | 0.55 (0.15) | 0.53 (0.14) | NS | 0.56 (0.18) | 0.44 (0.19) | <0.001 |
| Dip‐MBF | 1.04 (0.37) | 0.87 (0.35) | NS | 1.07 (0.62) | 1.18 (0.62) | NS |
| CFR | 1.98 (0.74) | 1.65 (0.49) | NS | 1.82 (0.58) | 2.95 (1.91) | 0.02 |
| Dip− regions | ||||||
| Rest MBF | 0.68 (0.30) | 0.66 (0.29) | NS | 0.56 (0.22) | 0.59 (0.21) | NS |
| Dip‐MBF | 1.10 (0.52) | 1.07 (0.32) | NS | 1.22 (0.75) | 1.49 (0.63) | NS |
| CFR | 1.75 (0.88) | 1.81 (0.64) | NS | 2.19 (0.80) | 2.57 (0.92) | NS |
CFR, coronary flow reserve; Dip, dipyridamole; Dip+ region, showing dipyridamole‐induced perfusion defects at enrolment; Dip−, regions not showing Dip‐induced perfusion defects at enrolment; MBF, myocardial blood flow.
Figure 3 Percentage changes (average (SE)) in regional myocardial blood flow (MBF) at rest and during dipyridamole (Dip) infusion, as well as in regional coronary flow reserve (CFR), from enrolment to 6 months of treatment, are represented for patients treated with placebo (group 1) or with carvedilol (group 2) in regions with (Dip+) or without (Dip−) defects at enrolment. The two groups significantly differed in analysis of variance. Directional response of maximal MBF and CFR to carvedilol was similar in Dip+ or Dip– regions. By contrast, resting MBF actually decreased after carvedilol in Dip+ regions, whereas it increased in Dip– regions. Accordingly, CFR‐positive changes induced by carvedilol were more evident in Dip+ regions (See section Discussion).
Discussion
This is the first study documenting that, besides the known effects of the drug on LV function and exercise tolerance, chronic treatment with carvedilol improves depressed coronary vasodilatory reserve in patients with IDC. Treatment with carvedilol was also associated with lower occurrence of Dip+, thus supporting an anti‐ischaemic effect of the drug in patients with IDC. Since in such patients MBF impairment due to coronary microvascular dysfunction correlates with severe long‐term prognosis,15 the present results suggest that the effects of carvedilol in improving long‐term outcome in IDC and more generally in heart failure1,2,3 might be, at least in part, dependent on its favourable effects on coronary microcirculation and thus on myocardial perfusion.
Effects of carvedilol on regional MBF in patients with LV dysfunction
Besides its benefits on symptoms and survival,1,2,3 long‐term treatment with carvedilol has favourable effects on LV structure and function in patients with heart failure4 that could be explained, at least in part, by anti‐ischaemic mechanisms. The reduction in heart rate with a prolonged duration of diastolic coronary blood flow and the decrease in myocardial energy demand, as for other β‐blockers, might improve myocardial oxygen balance, in particular during stress, and reduce the occurrence of ischaemia.5,6 The relevance of these potentially anti‐ischaemic effects in patients with post‐ischaemic cardiomyopathy has been supported by the observation that improvement in LVEF after carvedilol treatment was conditioned by the proportion of pre‐existing ischaemic/viable myocardium.9 However, when resting MBF and maximal MBF after Dip were measured by PET in coronary patients with LV dysfunction, treatment with carvedilol actually caused a decrease in MBF values proportional to the decrease in RPP, without any improvement in CFR.20
We hypothesised that the effects of carvedilol on myocardial perfusion could be more evident in a different clinical model of LV dysfunction, that is, IDC, where coronary microvascular dysfunction might contribute to LV impairment.14,15,16 As a matter of fact, the improvement in LV function in response to carvedilol is even more consistent in patients with IDC than in those with post‐ischaemic heart failure.3,10,11,12 The diffuse coronary microvascular dysfunction, consistently demonstrated in IDC, might provide a more homogeneous and specific substrate for the vascular actions of carvedilol in addition to its haemodynamic and myocardial effects.
To test this hypothesis, we measured the effects of carvedilol on PET regional MBF and CFR, as well as on exercise tolerance and haemodynamic variables, in patients with IDC in a double‐blind, placebo‐controlled trial. Carvedilol induced, as expected, a reduction of haemodynamic determinants of LV oxygen demand at rest and during exercise, and improved exercise tolerance. Despite reduced energy requirements, as expressed by RPP values, patients treated with carvedilol did not show a parallel reduction in resting MBF as expected on the basis of MBF‐metabolic demand matching and as documented in patients with heart failure due to coronary artery disease.20 This observation could be due to opposite effects of the drug in IDC, reducing cardiac work and oxygen consumption, on the one hand, thus tending to decrease resting flow, but improving endothelial function and abnormal coronary vascular tone on the other hand, thus tending to increase flow. As a matter of fact, resting MBF corrected for RPP, an estimate of cardiac work, actually increased after carvedilol treatment, thus proving that the drug is operating also by mechanisms other than pure changes in myocardial work and metabolic requirements. Moreover, carvedilol induced an increase in Dip‐MBF, resulting in a significant improvement of MBF reserve. Increased MBF reserve may well be one of the mechanisms of the observed increase in exercise tolerance in patients treated with carvedilol.
The favourable effects of carvedilol on MBF in patients with IDC might be due to different combined mechanisms. The reduction in heart rate, observed after treatment both at rest and during Dip could have prolonged the diastolic phase of the cardiac cycle and increased diastolic perfusion. This single mechanism, however, seems unable to explain the different results obtained in this study as compared with the study of Bøttcher et al20 in post‐ischaemic LV dysfunction, as in both series a similar decrease in heart rate was observed after carvedilol treatment. It is possible that the combined effects of carvedilol in reducing α‐adrenergic vascular tone7 and increasing coronary endothelial function by its antioxidant properties8 are more effective in IDC than in post‐ischaemic patients with post‐ischaemic cardiomyopathic problems. A severe dysfunction of the coronary endothelium, such as that previously observed in IDC,21 may directly limit regional MBF at rest and during adenosine‐induced vasodilation.22,23 Accordingly, carvedilol, by improving endothelial function, might have increased a severely depressed MBF, and α‐adrenergic inhibition may have added its effects in restoring adenosine‐induced myocardial hyperaemia.24 By contrast, in patients with coronary artery disease, the presence of coronary stenosis and the heterogeneity of microvascular impairment may have blunted the potential effect of carvedilol on MBF.9,20
In this study, carvedilol improved mean MBF and also reduced the occurrence of regional imbalance in myocardial perfusion during Dip infusion. Before treatment, 38% of LV regions showed Dip+, as previously described in patients with IDC,14 and interpreted as potential trigger for regional myocardial ischaemia.17 After treatment with carvedilol, only 15% of LV regions showed Dip+, thus suggesting an anti‐ischaemic effect of the drug.
We also analysed the effects of carvedilol in Dip+ and Dip− regions at enrolment. Resting MBF, not corrected for RPP, tended to increase in Dip− regions, but significantly decreased in Dip+ regions, as described in patients with post‐ischaemic cardiomyopathy.20 Dip‐MBF increased in both regions, so that MBF reserve was more evidently improved in previously ischaemic areas (fig 2). To explain these results, it can be hypothesised that microvascular dysfunction was more severe in Dip+ regions, causing repetitive episodes of myocardial ischaemia.17 In these areas, the combined antioxidant and α‐adrenergic blocking properties of carvedilol might be relevant to Dip‐induced hyperaemia.24 In the same regions, resting tone could be increased by additional mechanisms secondary to chronic repetitive ischaemia and/or hibernation being less responsive to α‐blockade. Moreover, the reduction in energy requirements, already induced by chronic repetitive ischaemia, might be further reduced by carvedilol. The combination of these mechanisms in Dip+ areas could explain the evident decrease in resting MBF after treatment which, together with improvement in flow reserve, might represent a protective effect of the drug on myocardium at ischaemic risk.
Whether the pronounced effects of carvedilol on regional MBF demonstrated in this study depend on the ability of the drug to reverse coronary microvascular impairment cannot be definitely stated on the basis of the present results. However, if this is the mechanism in IDC, it could also be relevant in post‐ischaemic cardiomyopathy, where it cannot clearly emerge owing to the higher heterogeneity of the myocardial/vascular status and the presence of stenosis in patients with ischaemia.
Limitations
A major limitation of this study is the relatively small population enrolled from the larger trial to the PET substudy. These small numbers probably did not permit detection, in patients treated with carvedilol, of significant changes in LV functional parameters which were clearly observed in the whole population of the CAR‐01 trial.18
Another limitation of the study is the use of only one transaxial slice of the PET examination to define the six regions of interest and compute regional MBF values. Only a small, even if representative, portion of the LV was in fact evaluated, and this approach may have limited the evaluation of the extent of Dip+, as well as of the active drug effect or placebo effect on these defects.
Evaluation of LV stress and LV work, as estimates of myocardial oxygen consumption, was not a specific aim of this study, since relevant parameters for these measurements were not included in the database. Resting regional MBF values were corrected for RPP, which is a clinical approximation of cardiac work and oxygen consumption but does not take into account the possible effect of treatment in reducing oxygen consumption by decreasing myocardial contractility or wall stress. PET may directly estimate myocardial oxygen consumption from the rate of oxidative metabolism of 11C‐acetate. Using this approach, β‐blocker treatment was demonstrated to improve myocardial metabolic efficiency in patients with LV dysfunction, that is to reduce myocardial oxygen consumption beyond the reduction in measured cardiac work.25 Possibly, carvedilol had the same energy‐sparing effect in our patients with IDC, and actual reduction in myocardial oxygen consumption could have exceeded the measured reduction in RPP. Accordingly, the correction of resting MBF for a better estimate of oxygen consumption would have further increased normalised MBF following carvedilol.
Conclusions
Carvedilol was able to improve CFR in patients with LV dysfunction due to IDC, independently of its haemodynamic effects. Even if the present results do not directly clarify the mechanism, this is the first study that strongly suggests a favourable effect of carvedilol on coronary microvascular function and myocardial–microvascular interplay. Since the detection of coronary microvascular dysfunction is relevant in predicting long‐term prognosis in IDC,15 it could also serve to select those patients who could most benefit from carvedilol treatment.
Acknowledgements
We thank Dr Susanna Carabba for her invaluable help in the organisation of all the activities connected to the study.
Abbreviations
CFR - coronary flow reserve
Dip - dipyridamole
Dip+ - region with Dip‐induced regional perfusion defect
Dip− - region without Dip‐induced regional perfusion defect
IDC - idiopathic dilated cardiomyopathy
LV - left ventricular
LVEF - left ventricular ejection fraction
MBF - myocardial blood flow
nMBF - normalised resting myocardial blood flow
PET - positron emission tomography
RPP - rate pressure product
Footnotes
This is a substudy of a multicentre trial (CAR‐01) sponsored by Roche. Roche did not specifically fund the substudy, and had no role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Competing interests: None declared.
References
- 1.The CAPRICORN investigators Effect of carvedilol on outcome after myocardial infarction in patients with left‐ventricular dysfunction: the CAPRICORN randomised trial. Lancet 20013571385–1390. [DOI] [PubMed] [Google Scholar]
- 2.Packer M, Coats A J S, Fowler M B, for the Carvedilol Prospective Randomized Cumulative Survival Study Group et al Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 20013441651–1658. [DOI] [PubMed] [Google Scholar]
- 3.Poole‐Wilson P A, Swedberg K, Cleland J G F, for the COMET investigators et al Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 20033627–13. [DOI] [PubMed] [Google Scholar]
- 4.Packer M, Antonopoulos G V, Berlin J A.et al Comparative effects of carvedilol and metoprolol on left ventricular ejection fraction in heart failure: results of a meta‐analysis. Am Heart J 2001141899–907. [DOI] [PubMed] [Google Scholar]
- 5.Bristow M R. Why does the myocardium fail? Insights from basic science. Lancet 1998352(Suppl I)8–14. [DOI] [PubMed] [Google Scholar]
- 6.Cleland J G F, Bristow M, Erdman E.et al Beta‐blocking agents in heart failure: should they be used and how? Eur Heart J 1996171629–1639. [DOI] [PubMed] [Google Scholar]
- 7.Sponer G, Strein K, Bartsch W.et al Vasodilatory action of carvedilol. J Cardiovasc Pharmacol 199219(Suppl 1)S5–11. [DOI] [PubMed] [Google Scholar]
- 8.Yue T L, Ruffolo R R, Jr, Feuerstein G. Antioxidant action of carvedilol: a potential role in treatment of heart failure. Heart Fail Rev 1999439–51. [Google Scholar]
- 9.Cleland J G, Pennell D J, Ray S G.et al Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet 200336214–21. [DOI] [PubMed] [Google Scholar]
- 10.Green P, Anshelevich M, Talreja A.et al Long‐term effects of carvedilol or metoprolol on left ventricular function in ischemic and nonischemic cardiomyopathy. Am J Cardiol 2005951114–1116. [DOI] [PubMed] [Google Scholar]
- 11.Metra M, Nardi M, Giubbini R.et al Effects of short‐ and long‐term carvedilol administration on rest and exercise hemodynamic variables, exercise capacity and clinical conditions in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1994241678–1687. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe J H, Jr, Magalski A, Stevens T L.et al Predictors of improvement in left ventricular ejection fraction with carvedilol for congestive heart failure. J Nucl Cardiol 200073–7. [DOI] [PubMed] [Google Scholar]
- 13.Gerson M C, Craft L L, McGuire N.et al Carvedilol improves left ventricular function in heart failure patients with idiopathic dilated cardiomyopathy and a wide range of sympathetic nervous system function as measured by iodine 123 metaiodobenzylguanidine. J Nucl Cardiol 20029608–615. [DOI] [PubMed] [Google Scholar]
- 14.Neglia D, Parodi O, Gallopin M.et al Myocardial blood flow response to pacing tachycardia and to dipyridamole infusion in patients with dilated cardiomyopathy without overt heart failure. A quantitative assessment by positron emission tomography. Circulation 199592796–804. [DOI] [PubMed] [Google Scholar]
- 15.Neglia D, Michelassi C, Trivieri M G.et al Prognostic role of myocardial blood flow impairment in idiopathic left ventricular dysfunction. Circulation 2002105186–193. [DOI] [PubMed] [Google Scholar]
- 16.Unverferth D V, Magorien R D, Lewis R P.et al The role of subendocardial ischemia in perpetuating myocardial failure in patients with nonischemic congestive cardiomyopathy. Am Heart J 1983105176–179. [DOI] [PubMed] [Google Scholar]
- 17.van den Heuvel A F, van Veldhuisen D J, van der Wall E E.et al Regional myocardial blood flow reserve impairment and metabolic changes suggesting myocardial ischemia in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 20003519–28. [DOI] [PubMed] [Google Scholar]
- 18.De Maria R, Gavazzi A, Sinagra G.et al Long‐term carvedilol treatment in idiopathic dilated cardiomyopathy: biological effects beyond pharmacological activity. J Am Coll Cardiol 200239(Suppl B)409B [Google Scholar]
- 19.Gould K L. Identifying and measuring severity of coronary artery stenosis: quantitative coronary angiography and positron emission tomography. Circulation 198878237–245. [DOI] [PubMed] [Google Scholar]
- 20.Bøttcher M, Refsgaard J, Gøtzsche O.et al Effect of carvedilol on microcirculatory and glucose metabolic regulation in patients with congestive heart failure secondary to ischemic cardiomyopathy. Am J Cardiol 2002891388–1393. [DOI] [PubMed] [Google Scholar]
- 21.Treasure C B, Vita J A, Cox D A.et al Endothelium‐dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation 199081772–779. [DOI] [PubMed] [Google Scholar]
- 22.Duffy S J, Castle S F, Harper R W.et al Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow‐mediated dilation in human coronary circulation. Circulation 19991001951–1957. [DOI] [PubMed] [Google Scholar]
- 23.Quyyumi A A, Dakak N, Andrews N P.et al Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation 199592320–326. [DOI] [PubMed] [Google Scholar]
- 24.Buus N H, Bøttcher M, Hermansen F.et al Influence of nitric oxide synthase and adrenergic inhibition on adenosine‐induced myocardial hyperemia. Circulation 20011042305–2310. [DOI] [PubMed] [Google Scholar]
- 25.Beanlands R S, Nahmias C, Gordon E.et al The effects of beta(1)‐blockade on oxidative metabolism and the metabolic cost of ventricular work in patients with left ventricular dysfunction: a double‐blind, placebo‐controlled, positron‐emission tomography study. Circulation 20001022070–2075. [DOI] [PubMed] [Google Scholar]