Abstract
Aims
To evaluate the histopathological risk factors for lymph node metastasis in cases of pedunculated or semipedunculated submucosal invasive colorectal carcinoma (SICC).
Methods
A total of 48 patients with non‐sessile SICC who underwent systematic lymph node dissection were included. Tumour size, histological grade, angiolymphatic invasion, tumour budding, dedifferentiation, objective submucosal invasion depth from the identified muscularis mucosa, relative invasion depth of the submucosal layer, and depth of stalk invasion were investigated histopathologically.
Results
Lymph node metastasis was observed in seven cases (14.6%). Univariate analysis showed angiolymphatic invasion and tumour budding to be significantly associated with lymph node metastasis. Multivariate analysis showed that tumour budding was the only independent factor associated with lymph node metastasis in cases of non‐sessile SICC.
Conclusions
Results indicate that tumour budding is a useful risk factor for predicting lymph node metastasis in cases of pedunculated or semipedunculated SICC.
Keywords: colorectal carcinoma, pedunculated, lymph node metastasis, risk factors, tumour budding
Recent advances in endoscopic instruments and techniques have led to a marked increase in the number of tumours that are removed endoscopically, and resulted in increased detection of early colorectal carcinoma.1,2,3 Early colorectal carcinoma is defined as carcinoma in which invasion is limited to the mucosa or submucosa, regardless of the presence or absence of lymph node metastases.4 Endoscopic complete resection of intramucosal carcinoma is accepted as a curative therapy, because there is no risk of lymph node metastasis.3,5 However, 6–12% of submucosal invasive colorectal carcinoma (SICC) cases show lymph node metastasis.6,7,8 Thus, cases of SICC that are subject to endoscopic resection need careful pathological examination to determine if there is a clinically significant risk of lymph node metastasis that would warrant additional surgery.7,9
Previously, histopathological factors such as angiolymphatic invasion, poor differentiation, and massive submucosal invasion were reported to be associated with lymph node metastasis in SICC.3,7,10,11,12,13 Measurement of the depth of submucosal invasion is relatively simple in cases of sessile polyps11; however, in pedunculated or semipedunculated polyps it is relatively complicated due to the presence of stalks and the varied length of these stalks. Since Haggitt et al12 proposed a “level of invasion” classification for colorectal carcinoma arising from polyps in 1985, the Haggitt classification of submucosal invasion has been widely used in pathological evaluation. However, this classification has some limitations: it tends to lead to the over‐treatment of cases of SICC without lymph node metastasis13,14; it is not easy to apply to semipedunculated polyps; and it does not consider recently identified risk factors such as tumour budding.9,11,12,13,14 Kudo15 and Kitajima et al11 have proposed classifications according to the relative level of submucosal invasion (namely, sm1, sm2, and sm3) and the objective depth of submucosal invasion, respectively. However, the former method may be difficult to apply in the endoscopically resected specimen, and the latter may lead to over‐treatment in cases with long stalks.
Several studies have shown the prognostic significance of tumour budding or dedifferentiation in colorectal carcinoma. However, the definitions used are somewhat confused, and the prognostic significance in SICC has not yet been sufficiently studied.11,16,17,18,19,20,21 In the present study, we separately defined budding and dedifferentiation according to the number of tumour cells in the invasive nest (<5 for budding, ⩾5 for dedifferentiation), and evaluated their prognostic significance in relation to lymph node metastasis. We also compared the prognostic significance of submucosal invasion depth as determined by Haggitt's classification, Kudo's relative level, and objective depth of submucosal invasion, and of other pathological parameters including tumour size, histological grade, and angiolymphatic invasion in non‐sessile SICC.
Patients and methods
Between May 2000 and June 2006, 113 patients with SICC underwent surgery for systematic lymph node dissection at the National Cancer Center Hospital, Goyang, Korea. Of these, 48 patients with pedunculated (20 cases) or semipedunculated (28 cases) SICC were selected for this study. Exclusion criteria were: (1) macroscopic sessile or flat/depressed type of SICC (36 cases); (2) a broad‐based villous tumour (8 cases); (3) a past history of cancer treatment, including chemoradiation (17 cases); (4) previous or concurrent advanced colorectal carcinoma (1 case); and (5) if precise histological evaluation could not be performed because the polypectomy was performed outside our hospital (3 cases). All pathology slides were examined by two of the authors (HJC and DKS). The clinical data from the colorectal cancer database and clinical charts were also reviewed retrospectively. This study was performed in accordance with the Declaration of Helsinki.
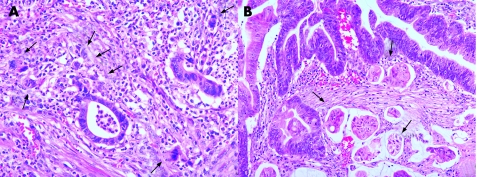
The tumour size, histological type and grade, angiolymphatic invasion, budding, dedifferentiation, and depth of submucosal invasion were investigated. Histological type and grade were classified according to World Health Organization criteria,22 and pathological tumour‐node‐metastasis classification was determined according to the International Union Against Cancer criteria.23 Angiolymphatic invasion was defined as the presence of cancer cells within endothelial‐lined channels. An isolated cell or a small cluster of <5 carcinoma cells in the invasive front was defined as a “budding” focus, and ⩾10 budding foci when viewed at a 200‐fold magnification was considered positive for tumour budding, based on the data from Ueno et al (fig 1).17 Dedifferentiation was originally defined as “microscopic clusters of undifferentiated cells just ahead of the invasive front”19 or “a single carcinoma cell or a solitary trabecular form at the invasive front”.16 These definitions partly overlap with that of “tumour budding”; however, tumour budding alone could not estimate the prognostic significance for solid clusters composed of at least five undifferentiated cells. Thus, we subsequently separately defined dedifferentiation as solid trabecular nests of carcinoma cells (⩾5 cells in each nest) at the invasive front (fig 1).
Figure 1 Histology of budding (A) and dedifferentiation (B). Budding was defined as ⩾10 budding foci, where each focus was composed of an isolated cell or a small cluster of fewer than five cancer cells in the invasive front. Dedifferentiation was defined as solid trabecular nests of cancer cells (five or more cells in each nest) at the invasive front. (A: H&E, ×200; B: H&E, ×100.)
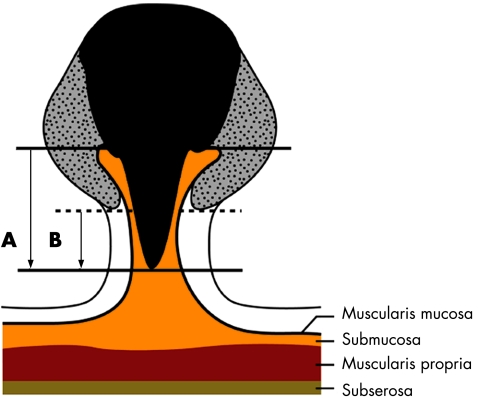
The depth of submucosal invasion was measured in three ways. First, the objective depth of invasion was measured as shown in fig 2. Second, Kudo's classification was evaluated to define the relative invasion depth of the submucosal layer as sm1 (infiltration into the upper third of the submucosal layer), sm2 (middle third), or sm3 (lower third).1 Third, Haggitt's classification was evaluated.12 If the cancer invades the stalk, the depth of stalk invasion was also measured as the length from the neck (Haggitt level 2) to the deepest portion of invasion (fig 2).11
Figure 2 The methods used for measurement of submucosal invasion depth. The objective submucosal invasion depth (A) was measured as the vertical distance from the baseline, defined as the horizontal line between the identified muscularis mucosa, to the deepest portion of invasion. The depth of stalk invasion (B) was measured as the vertical distance from the neck of stalk (the point of level 2 invasion as defined by Haggitt's classification) to the deepest portion of invasion (black solid portion, carcinoma; gray dotted portion, adenoma).
Statistical analyses were performed using the χ2 test, Fisher's exact probability test, or Student's t test to estimate differences in the relationships between histopathological factors and lymph node metastasis. Multivariate logistic regression analyses were then performed to identify factors that were considered to have an effect on lymph node metastasis. Statistical significance was defined as p<0.05.
Results
Patient characteristics
Of the 48 patients, 24 were male and 24 were female; mean age was 54.9 years. Thirty‐seven patients had tumours in the colon and 11 had tumours in the rectum. Lymph node metastasis was observed in seven cases (14.6%). The mean tumour size was 20 (9.0) mm (range 10–48 mm). The histological type was classed as well differentiated adenocarcinoma in 22 cases (45.8%), moderately differentiated adenocarcinoma in 23 cases (47.9%) and poorly differentiated adenocarcinoma in three cases (6.3%). Angiolymphatic invasion was identified in 19 cases (40%). Tumour budding and dedifferentiation were found in 10 (21%) and 12 cases (25%), respectively. The mean objective submucosal invasion depth was 2.8 (1.5) mm (range 0.2–6.5 mm). According to Kudo's classification, the number of cases classed as sm1, sm2, and sm3 tumours was 22 (46%), 13 (27%), and 13 (27%), respectively. According to Haggitt's classification, submucosal invasion was classed as level 1 in 12 cases (25%), level 2 in 11 cases (23%), level 3 in 20 cases (42%) and level 4 in 5 cases (10%). In the cases classed as levels 3 or 4, the mean stalk invasion depth was 1.5 (0.8) mm.
Correlation between histopathological parameters and lymph node metastasis
Table 1 summarises correlations between each parameter and lymph node metastasis. Of the parameters analysed, angiolymphatic invasion (32% vs 3% in lymph node metastasis positive and negative groups, respectively; p = 0.011) and tumour budding (60% vs 3%, respectively; p<0.0001) were significantly correlated with lymph node metastasis. Poorly differentiated histological type and dedifferentiation showed some correlation to lymph node metastasis but with marginal statistical significance (p = 0.052 and p = 0.055, respectively). Multivariate logistic regression analyses showed that tumour budding was a significant risk factor for lymph node metastasis in cases of non‐sessile SICC. Table 2 shows the odds ratio and 95% confidence interval. However, regardless of the classification method used, the depth of submucosal invasion and tumour size did not correlate to lymph node metastasis (table 1). Table 3 summarises the clinicopathological details of the cases with lymph node metastasis.
Table 1 The relationship between histopathological factors and lymph node metastasis in non‐sessile submucosal invasive colorectal cancer.
| Histopathological factors | Total (n = 48) | Lymph node metastasis | ||
|---|---|---|---|---|
| Negative (n = 41) | Positive (n = 7) | p Value | ||
| Tumour size (mean (SD), mm) | 20 (0.9) | 21 (1.0) | 17 (0.4) | NS |
| Histologic type | 0.052 | |||
| Well or moderately differentiated | 45 | 40 | 5 | |
| Poorly differentiated | 3 | 1 | 2 | |
| Angiolymphatic invasion | 0.011 | |||
| Positive | 19 | 13 | 6 | |
| Negative | 29 | 28 | 1 | |
| Tumour budding | <0.001 | |||
| Positive | 10 | 4 | 6 | |
| Negative | 38 | 37 | 1 | |
| Dedifferentiation | 0.055 | |||
| Positive | 12 | 8 | 4 | |
| Negative | 36 | 33 | 3 | |
| Objective submucosal invasion depth (mean (SD), mm) | 2.8 (1.5) | 2.8 (1.6) | 2.9 (1.0) | NS |
| Relative submucosal invasion depth | NS | |||
| sm1 | 22 | 18 | 4 | |
| sm2, sm3 | 26 | 23 | 3 | |
| Haggitt's classification | NS | |||
| Level 1–3 | 43 | 37 | 6 | |
| Level 4 | 5 | 4 | 1 | |
| Depth of stalk invasion (mean (SD), mm) | 1.5 (0.8) | 1.6 (0.9) | 1.5 (0.8) | NS |
Table 2 Multivariate analysis of histopathological risk factors for lymph node metastasis in non‐sessile submucosal invasive colorectal cancer.
| Histopathological factors | p Value | Odds ratio (95% CI) |
|---|---|---|
| Histological type | NS | |
| Angiolymphatic invasion | NS | |
| Tumour budding | 0.010 | 69.516 (2.789 to 1732.795) |
| Dedifferentiation | NS |
Table 3 Detailed data for all cases with non‐sessile SICC with lymph node metastasis.
| Case no | Age (y) | Sex | Location | Size (mm) | HG | AI | TB | De | OSID (mm) | RSID (mm) | HL | DSI (mm) | No of LN (+) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 41 | M | Sigmoid | 12 | Moderate | + | + | – | 3.0 | sm1 | 3 | 1.0 | 1 |
| 2 | 72 | F | Ascending | 25 | Poor | + | + | + | 2.5 | sm1 | 1 | 0 | 1 |
| 3 | 68 | F | Rectum | 15 | Moderate | + | + | – | 2.5 | sm1 | 3 | 1.0 | 1 |
| 4 | 54 | M | Sigmoid | 19 | Well | + | + | + | 2.5 | sm3 | 3 | 1.0 | 1 |
| 5 | 52 | M | Sigmoid | 15 | Well | + | – | – | 3.0 | sm3 | 4 | 2.0 | 3 |
| 6 | 46 | F | Sigmoid | 20 | Poor | + | + | + | 5.0 | sm2 | 3 | 3.0 | 2 |
| 7 | 62 | F | Sigmoid | 15 | Moderate | – | + | + | 2.0 | sm1 | 2 | 0 | 3 |
HG, histological type; AI, angiolymphatic invasion; TB, tumour budding; De, dedifferentiation; OSID, objective submucosal invasion depth; RSID, relative submucosal invasion depth; HL, Haggitt's level; DSI, depth of stalk invasion; LN(+), lymph nodes with metastasis.
Discussion
Several systems have been proposed to determine the depth of submucosal invasion in pedunculated SICC, but there is controversy over which is the best for predicting lymph node metastasis. Level 4 (underlying wall) invasion according to Haggitt's classification has been reported to be an important prognostic factor,12 as well as a risk factor associated with lymph node metastasis in cases of SICC.13 However, previous studies have shown that only 10% of cases classed as level 4 SICC have metastasis to the lymph nodes; hence recommendations to resect the colon may constitute over‐treatment in 90% of patients who would undergo additional surgery.13 In 1993, Kudo15 suggested a new three‐grade classification that divided the thickness of the submucosa into three layer (sm1, sm2, or sm3), and he reported that this classification was useful for predicting lymph node metastasis. Kudo's classification has been used extensively in Japan and Korea due to its simplicity, but it is not useful for analysis of specimens resected by endoscopy because the muscularis propria is not included. Recently, Kitajima et al11 reported that, if there was no lymphatic invasion, cases of pedunculated SICC with submucosal invasion of <3000 μm did not have lymph node metastasis. However, the authors did not critically consider that there could be marked differences in the depth of submucosal invasion depending on the tumour size, stalk length, and the state of the muscularis mucosa in cases of pedunculated polyps. In the present study, we compared the prognostic values of these three classifications for predicting lymph node metastasis, but no significant relationship was found between submucosal depth, as measured by any of these classification, and lymph node metastasis. The total number of cases analysed in the present study was not large, so further results may be needed to confirm the non‐significant relationship between the depth of submucosal invasion and lymph node metastasis in cases of pedunculated SICC. However, we showed that tumour budding might be an important histopathological risk factor for lymph node metastasis in cases of pedunculated or semipedunculated SICC.
Take‐home messages
This study indicates that tumour budding is a useful histopathological factor for predicting lymph node metastasis in cases of non‐sessile SICC.
Further studies are needed to clarify the predictive value of the submucosal invasion depth for lymph node metastasis in cases of pedunculated SICC.
Tumour budding is considered to be a pathological characteristic corresponding to the initial phase of tumour invasion and has been reported to be associated with metastatic activity and prognostic outcome.16,17,18,19,20,21,24,25,26 However, different researchers have proposed several diverse definitions. In the present study, we applied Ueno's grading system,17 in which tumour budding is divided into low and high grades according to the number of budding foci (defined as a cluster of fewer than five cancer cells) in a field of ×250 magnification. Ueno et al17 have previously reported that high‐grade budding (10 or more budding foci in a field of ×250) was an independent prognostic factor associated with a worse survival rate compared with low‐grade budding (five‐year survival rate 41% vs 84%). Our present results confirmed that high‐grade budding in SICC is a prognostic factor for lymph node metastasis. In addition, tumour budding seems to be a better prognostic factor than angiolymphatic invasion for predicting lymph node metastasis (specificity 90% vs 68%; positive predictive value 60% vs 31%, respectively), although the sensitivities of both parameters were similar (86%). Indeed, angiolymphatic invasion might be underestimated or overestimated, since retraction or cauterisation artefacts could be confused with angiolymphatic invasion.27 Confusingly, tumour budding has also been referred to as “sprouting” or “focal dedifferentiation”.16,21 Recently, Tominaga et al16 reported that lymphatic invasion and high‐grade focal dedifferentiation at the submucosal invasive front are important predictors of lymph node metastasis in patients with non‐pedunculated SICC. However, their definition of focal dedifferentiation includes the budding foci. Thus, we separated dedifferentiation from tumour budding and also evaluated the association between dedifferentiation and lymph node metastasis; however, unlike tumour budding, the association between dedifferentiation and lymph node metastasis was only marginally statistically significant (p = 0.055).
In conclusion, tumour budding may be an independent risk factor for lymph node metastasis in cases of pedunculated or semipedunculated SICC. Additional surgical treatment should be considered in patients with non‐sessile SICC showing tumour budding even if pathological analysis is negative for other prognostic factors. Further studies are needed to verify the prognostic significance of the depth of submucosal invasion in cases of pedunculated or semipedunculated SICC.
Abbreviations
SICC - submucosal invasive colorectal carcinoma
Footnotes
Funding: This work was supported by a research grant from the National Cancer Center, Korea.
Competing interests: None declared.
References
- 1.Kudo S, Kashida H, Nakajima T.et al Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg 199721694–701. [DOI] [PubMed] [Google Scholar]
- 2.Iishi H, Tatsuta M, Narahara H.et al Endoscopic resection of large pedunculated colorectal polyps using a detachable snare. Gastrointest Endosc 199644594–597. [DOI] [PubMed] [Google Scholar]
- 3.Morson B C, Whiteway J E, Jones E A.et al Histopathology and prognosis of malignant colorectal polyps treated by endoscopic polypectomy. Gut 198425437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashida H, Kudo S E. Early colorectal cancer: concept, diagnosis, and management. Int J Clin Oncol 2006111–8. [DOI] [PubMed] [Google Scholar]
- 5.Nivatvongs S. Surgical management of early colorectal cancer. World J Surg 2000241052–1055. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi R, Takano M, Takagi K.et al Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 1995381286–1295. [DOI] [PubMed] [Google Scholar]
- 7.Cooper H S. Surgical pathology of endoscopically removed malignant polyps of the colon and rectum. Am J Surg Pathol 19837613–623. [DOI] [PubMed] [Google Scholar]
- 8.Cranley J P, Petras R E, Carey W D.et al When is endoscopic polypectomy adequate therapy for colonic polyps containing invasive carcinoma? Gastroenterology 198691419–427. [DOI] [PubMed] [Google Scholar]
- 9.Egashira Y, Yoshida T, Hirata I.et al Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol 200417503–511. [DOI] [PubMed] [Google Scholar]
- 10.Okabe S, Shia J, Nash G.et al Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg 200481032–1040. [DOI] [PubMed] [Google Scholar]
- 11.Kitajima K, Fujimori T, Fujii S.et al Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 200439534–543. [DOI] [PubMed] [Google Scholar]
- 12.Haggitt R C, Glotzbach R E, Soffer E E.et al Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 198589328–336. [DOI] [PubMed] [Google Scholar]
- 13.Nivatvongs S, Rojanasakul A, Reiman H M.et al The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum 199134323–328. [DOI] [PubMed] [Google Scholar]
- 14.Haggitt R C. Management of the patient with carcinoma in an adenoma. Prog Clin Biol Res 198827989–99. [PubMed] [Google Scholar]
- 15.Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 199325455–461. [DOI] [PubMed] [Google Scholar]
- 16.Tominaga K, Nakanishi Y, Nimura S.et al Predictive histopathologic factors for lymph node metastasis in patients with nonpedunculated submucosal invasive colorectal carcinoma. Dis Colon Rectum 20054892–100. [DOI] [PubMed] [Google Scholar]
- 17.Ueno H, Price A B, Wilkinson K H.et al A new prognostic staging system for rectal cancer. Ann Surg 2004240832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hase K, Shatney C, Johnson D.et al Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 199336627–635. [DOI] [PubMed] [Google Scholar]
- 19.Ono M, Sakamoto M, Ino Y.et al Cancer cell morphology at the invasive front and expression of cell adhesion‐related carbohydrate in the primary lesion of patients with colorectal carcinoma with liver metastasis. Cancer 1996781179–1186. [DOI] [PubMed] [Google Scholar]
- 20.Masaki T, Matsuoka H, Sugiyama M.et al Budding as a useful determinant of the optimal treatment for T1 rectal carcinomas. Hepatogastroenterology 200350388–391. [PubMed] [Google Scholar]
- 21.Okuyama T, Oya M, Yamaguchi M. Budding (sprouting) as a useful prognostic marker in colorectal mucinous carcinoma. Jpn J Clin Oncol 200232412–416. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton S R, Aaltonen L A. eds. WHO classification of tumors; pathology and genetics of tumors of the digestive system. Lyon: IARC Press, 2000
- 23.Greene F L, Page D L, Fleming I D.et al, eds. AJCC cancer staging manual, 6th edn. New York: Springer‐Verlag, 2002
- 24.Ueno H, Mochizuki H, Hashiguchi Y.et al Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004127385–394. [DOI] [PubMed] [Google Scholar]
- 25.Ueno H, Mochizuki H, Shinto E.et al Histologic indices in biopsy specimens for estimating the probability of extended local spread in patients with rectal carcinoma. Cancer 2002942882–2891. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka M, Hashiguchi Y, Ueno H.et al Tumor budding at the invasive margin can predict patients at high risk of recurrence after curative surgery for stage II, T3 colon cancer. Dis Colon Rectum 2003461054–1059. [DOI] [PubMed] [Google Scholar]
- 27.Compton C C, Fielding L P, Burgart L J.et al Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000124979–994. [DOI] [PubMed] [Google Scholar]