Abstract
Background
Transforming growth factor‐β1 (TGF‐β1) has the potential to induce acute inflammation and apoptosis in lung epithelial cells and plays a central role in subsequent fibrosis.
Aims
To examine a new anti‐TGF‐β1 therapy against lung injury and fibrosis, which comprises the transfection of soluble TGF type II receptor (sTGFRII) gene into skeletal muscles by in vivo electroporation.
Methods
Soluble TGFRII was detectable between 1 and 14 days in the serum and significantly increased between 3 and 10 days after gene transfer into muscles. Based on these findings, the sTGFRII gene was injected at 3 days before or 4 days after the bleomycin instillation in order to examine the significance of TGF‐β1 on the early inflammatory phase (day 0 to day 7) or the fibrotic phase (day 7 to day 14) in this model.
Results
Transfection of sTGFRII gene at 3 days before or 4 days after bleomycin instillation significantly attenuated apoptosis, injury, and fibrosis at 7 or 14 days, respectively. This method does not require the use of viral vector or neutralising antibody, and it is therefore possible to avoid problems regarding the pathogenicity of the viral vector or immunocomplex.
Conclusions
This novel anti‐TGF‐β1 strategy may have clinical application in the treatment of lung injury and fibrosis.
Keywords: in vivo electroporation, pulmonary fibrosis, transforming growth factor‐β1, apoptosis, gene therapy
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic fibrosing interstitial pneumonia associated with the histopathological appearance of usual interstitial pneumonia on surgical lung biopsy. The median survival of patients with IPF is reported to be 3–4 years from the onset of respiratory symptoms.1 In spite of such poor prognosis, the aetiology of IPF is as yet unknown and no effective therapeutic strategy has been established. The effects of current immunosuppressive therapy with corticosteroids and cytotoxic agents are limited and the adverse effects cannot be ignored. Thus, establishment of an alternative therapeutic strategy is urgently needed.
Transforming growth factor‐β1 (TGF‐β1) has multiple effects that may exacerbate fibrosis. There is a consistent increase in TGF‐β1 production in epithelial cells and macrophages in lung tissue from patients with IPF.2 Transient overexpression of active TGF‐β1 through the transfection of porcine TGF‐β1 cDNA to the rat lung, results in prolonged and severe interstitial and pleural fibrosis.3 In the bleomycin‐induced pulmonary fibrosis model, TGF‐β1 is expressed in alveolar macrophages at the acute phase of inflammatory cell infiltration, and in epithelial cells at the later phase of pulmonary fibrosis.4 TGF‐β1 is also reported to be a critical mediator of pulmonary oedema in acute lung injury.5 We previously shown that TGF‐β1 could induce apoptosis of small airway epithelial cells.6 TGF‐β1 seems to be a primary factor which induces lung injury, which subsequently leads to pulmonary fibrosis.
We previously shown that mutant MCP‐1 gene transfection into muscle cells by in vivo electroporation prevents the development of bleomycin‐induced pulmonary fibrosis in mice.7 Skeletal muscle cells infected with an expression plasmid can produce a secreted protein into the circulating blood.8 Soluble TGFRII has been shown to inhibit bleomycin‐induced pulmonary fibrosis in the hamster.9 To investigate the new anti‐TGF‐β1 therapy in this model, we developed a transfection strategy using in vivo electroporation that comprises transfection of the sTGFRII gene into skeletal muscles as a biofactory for anti‐TGF‐β1 therapy in the lungs. We hypothesised that muscle cells infected with the sTGFRII gene would secrete sTGFRII protein into the circulating blood, and that this protein would then capture TGF‐β1 in the lung tissue, thereby blocking its signalling. This novel strategy to inhibit TGF‐β1 signalling should be considered in the treatment of lung injury and fibrosis.
Methods
Soluble TGFRII gene transfection into muscle cells by in vivo electroporation
The entire extracellular domain of TGFRII fused to the FC portion of human IgG1 was cloned into the Xho1 and Xba1 sites of the eukaryotic expression vector pCDM. Mice were anaesthetised by an intraperitoneal injection of pentobarbital sodium (Schering‐Plough, San Diego, California, USA) and in vivo electroporation was performed as previously described.7 Briefly, the sTGFRII expression plasmid vector (50 μg/50 μl of saline) or empty vector pCDM was injected into the femoral muscle with a 27‐gauge needle. Immediately after the plasmid injection, a pair of electrode needles (Tokiwa Science, Fukuoka, Japan) spaced 5 mm apart were inserted into the femoral muscle, one on each side of the injected site. Six 100‐V square wave pulses (spaced 1 s apart) were applied with an electric pulse generator CUY201 (BTX Corp., San Diego, California, USA), and the wound was closed.
Analysis of sTGFRII expression by measuring human IgG in serum
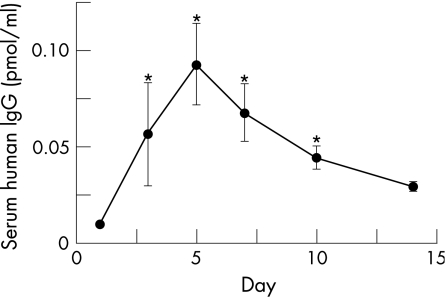
At 1, 3, 5, 7, 10 and 14 days after gene transfection, three mice were killed at each time point and serum was obtained. Soluble TGFRII concentrations in serum were assayed with ELISA for human IgG1. Soluble TGFRII was detectable between 1 and 14 days in the serum; it significantly increased between 3 and 10 days after gene transfer (fig 1). Based on these findings, we injected the sTGFRII gene at 3 days before or 4 days after the bleomycin instillation in order to examine the significance of TGF‐β1 signalling on the early inflammatory phase (day 0 to day 7) or the fibrotic phase (day 7 to day 14) in this model, respectively.
Figure 1 Time course of human IgG concentration in the serum after intramuscular gene transfection. Data are shown as mean (SEM) from five mice. Significance was compared with mice of day 0 (*p<0.05).
Model of bleomycin‐induced pneumopathy
The present experiments were approved by the Committee on Ethics regarding Animal Experiments of Kyushu University Faculty of Medicine, and were performed according to the guidelines of the American Physiological Society. Eight‐week‐old C57BL/6 male mice were purchased from KBT Oriental (Tosu, Japan) and used in all the experiments. The body weight of mice was almost 20–25 mg. After measurement of their weight, mice were anaesthetised with an intraperitoneal injection of pentobarbital sodium (Schering‐Plough). The anesthetised mice received 50 μl of bleomycin hydrochloride (Nippon Kayaku, Tokyo, Japan) solution containing 1.5 U bleomycin/kg body weight in sterile saline intratracheally. We injected the sTGFRII expression plasmid vector to 13 mice at 3 days before or to 9 mice at 4 days after the bleomycin instillation. As controls, we injected the empty vector pCDM to 14 mice at 3 days before or to 11 mice at 4 days after the bleomycin instillation.
Histopathological examination
After thoracotomy at 7 or 14 days after bleomycin instillation, the pulmonary circulation was flushed with saline and the lungs were explored. The lung samples were fixed with 10% formalin overnight and embedded in paraffin. A 3 μm paraffin section was adhered to slides and stained with H&E. The pathological grade of inflammation and fibrosis in the whole area of the mid‐sagittal section was evaluated under ×40 magnification, and determined according to the following criteria: 0, no lung abnormality; 1, presence of inflammation and fibrosis involving <25% of the lung parenchyma; 2, lesions involving 25–50% of the lung; and 3, lesions involving >50% of the lung as previously described.7 As control mice with normal histology, we instilled saline to six mice and obtained lung tissues from three mice at 7 and 14 days, respectively. The slides were stained with Sirius red to assess the collagen deposition.
DNA damage and apoptosis in lung tissues
The number of apoptotic cells detected by the TUNEL method has been considered to reflect the degree of lung injury.10,11 The TUNEL method was performed using the DeadEnd Colorimetric Apoptosis Detection system (Promega, Michigan, USA) as previously described.12 The number of positive cells for terminal transferase‐mediated dUTP nick‐end‐labelling (TUNEL) was counted in the whole field of each section under a microscope with ×200 magnification.
Statistics
Serum human IgG levels, histopathological grade, and the number of TUNEL positive cells were analysed by the Kruskal–Wallis test followed by the Mann–Whitney U test. Results were analysed using Prism (Graphpad Software Inc., San Diego, California, USA); p<0.05 was considered statistically significant.
Results
Effect of sTGFRII gene transfer on histological findings
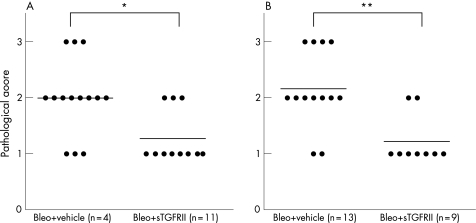
The alveolar wall had begun to thicken with infiltration of neutrophils and lymphocytes at 7 days after bleomycin instillation (fig 2A), compared with the wall of saline‐instilled mice. After 14 days, a large number of lymphocytes infiltrated into the lung interstitium, and thickening of the alveolar septa, collapse of the alveolar spaces, and proliferation of fibroblasts were all observed (fig 2B). Soluble TGFRII gene transfection at 3 days before the bleomycin instillation significantly attenuated histological findings at 7 days after the bleomycin instillation (fig 2D). Soluble TGFRII gene transfection at 4 days after the instillation significantly attenuated histological findings at 14 days (fig 2E). Empty vector pCDM treatment at 7 days after the administration did not affect the histological findings at 14 days. Sirius red staining shows collagen deposition in fibrotic lesions, which was attenuated by sTGFRII gene transfer (fig 2C and 2F, respectively). Figure 3 shows that the pathological grade at 7 and 14 days (fig 3A and fig 3B, respectively) was significantly decreased by sTGFRII gene transfer at 3 days before or 4 days after the instillation compared with the grade in mice treated with empty vector pDCM.
Figure 2 Effect of soluble transforming growth factor type II receptor (sTGFRII) gene transfer on microscopic findings. Representative results of histological findings at day 7 (A) and day 14 (B) after bleomycin instillation. (C) Collagen deposition at 14 days after bleomycin instillation stained with Sirius red. (D) Effect of sTGFRII gene transfer at 3 days before bleomycin instillation on histological findings at 7 days. (E) Effect of sTGFRII gene transfer at 4 days after bleomycin instillation on histological findings at 14 days. (F) Effect of sTGFRII gene transfer on collagen deposition stained by Sirius red. (Original magnification ×100.)
Figure 3 Effect of soluble transforming growth factor type II receptor (sTGFRII) gene transfer on pathological grade. (A) Effect of sTGFRII gene transfer at 3 days before bleomycin instillation on pathological grade at 7 days. Each circle corresponds to the data of one mouse (*p<0.05). (B) Effect of sTGFRII gene transfer at 4 days after bleomycin instillation on pathological grade at 14 days. Each circle corresponds to the data of one mouse (**p<0.01).
Effect of sTGFRII gene transfer on the number of TUNEL positive cells
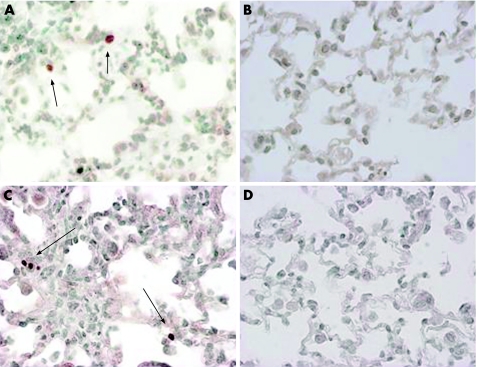
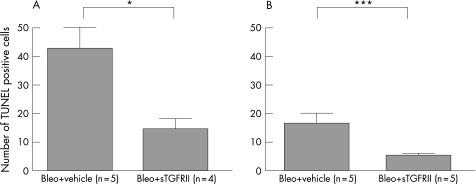
Although the type of cells was not clearly identified, some of the bronchiolar and alveolar epithelial cells or inflammatory cells in the inflammatory lesions showed evidence of DNA damage and apoptosis at 7 and 14 days after the bleomycin instillation (fig 4A and 4C, respectively). TUNEL positive cells were found in only a few bronchiolar epithelial cells in whole lung sections of untreated mice. Soluble TGFRII gene transfer at 3 days before or 4 days after the bleomycin instillation significantly decreased the number of TUNEL positive cells at 7 or 14 days, respectively (fig 4B and 4D, respectively), whereas empty vector pCDM transfer did not. Figure 5 shows that the number of TUNEL positive cells at 7 and 14 days (fig 5A and fig 5B, respectively) was significantly decreased by sTGFRII gene transfer at 3 days before or 4 days after the instillation compared with that in mice treated with empty vector pCDM treatment.
Figure 4 Effect of soluble transforming growth factor type II receptor (sTGFRII) gene transfer on TUNEL staining in lung tissues after bleomycin instillation. TUNEL‐positive cells in lung tissues at 7 days (arrows in A) and at 14 days (arrows in C). These positive signals for TUNEL at 7 or 14 days were abrogated by sTGFRII gene transfer at 3 days before or 4 days after bleomycin instillation (B and D, respectively) (original magnification ×200).
Figure 5 Quantification of the number of TUNEL positive cells. (A) Effect of sTGFRII gene transfer at 3 days before bleomycin instillation on the number of TUNEL positive cells at 7 days (*p<0.05). (B) Effect of sTGFRII gene transfer at 4 days after bleomycin instillation on the number of TUNEL positive cells at 14 days (***p<0.001). Bleo, bleomycin; sTGFRII, soluble transforming growth factor type II receptor.
Discussion
We showed that sTGFRII gene transfer by in vivo electroporation attenuated the development of bleomycin‐induced pneumopathy in mice. We chose this method because it does not require either repeated administrations of sTGFRII or viral vector transfection. Acute alveolitis develops at 2–3 days followed by interstitial inflammation at 4–12 days after intratracheal instillation of bleomycin in rodents,12,13 and fibroblast proliferation and collagen synthesis is initiated at 4–14 days after bleomycin instillation.12 We showed that muscle cells infected with the sTGFRII gene secreted its protein into the circulating blood by demonstrating that sTGFRII protein was detectable in serum at 1–14 days, and significantly increased at 3–10 days after transfection. Based on these findings, we injected sTGFRII gene at 3 days before or 4 days after bleomycin instillation in order to examine the significance of TGF‐β1 on the early inflammatory phase (day 0 to day 7) or the fibrotic phase (day 7 to 14) in this model, respectively. We showed that sTGFRII gene transfer at 3 days before or 4 days after the bleomycin instillation attenuated lung injury, fibrosis, and apoptosis. Accordingly, TGF‐β1 may play an important role in both the initiation of inflammation and the development of fibrosis.
There are three TGF‐β receptors, type I (TGFRI), type II (TGFRII) and type III (TGFRIII). All three receptors bind to all three TGF‐βs with a high affinity. TGFRII is a constitutive active kinase that is recognised by TGFRI only after TGF‐β has bound directly to TGFRII. TGFRI is recruited into the complex and phosphorylated by TGFRII.14 Smad proteins regulate intracellular signals from the membrane to the nucleus of TGF‐β.15 TGF‐β1 is not only a key cytokine in fibrogenesis, but also activates monocytes and macrophages to release a number of cytokines such as platelet‐derived growth factor, interleukin‐1β (IL‐1β), basic fibroblast growth factor, tumour necrosis factor‐α, and TGF‐β1 itself. TGF‐β1 is reported to be a critical mediator of pulmonary oedema in acute lung injury.5 In contrast, TGF‐β1 also has an anti‐inflammatory effect, since TGF‐β1 knockout mice show severe inflammatory reactions in the lung.16 We showed here that inhibition of TGF‐β1 signalling prevented acute lung injury in this model. Recently, it is proposed that inflammation is required to trigger fibrosis but is not essential in the development of fibrosis.17 In fact, activation of the TGF‐β‐Smad3 signalling pathway is indispensable to promote late progressive fibrotic response following transient IL‐1β overexpression.18 TGF‐β1 may be a primary factor, which induces lung injury in the course of pulmonary fibrosis. These results suggest that anti‐TGF‐β1 strategy may be an effective treatment against not only acute lung injury but also progressive pulmonary fibrosis, although it seems likely that we have to transfer this gene every 2 weeks in order to prevent the progression of IPF.
We have previously shown that bronchoalveolar lavage fluid (BALF) TGF‐β1 levels and soluble Fas ligand levels were significantly higher in IPF patients than in BALF from normal volunteers.6,19 Anti‐TGF‐β1 or anti‐Fas ligand neutralising antibody inhibited apoptosis of small airway epithelial cells induced by BALF administration in vitro.6 TGF‐β1 enhanced lung epithelial cell apoptosis and inflammation induced by low‐dose anti‐Fas antibody in vivo.6 In addition, TGF‐β1 plays an important role in epithelial repair and regeneration as well as epithelial cell injury. Exogenous TGF‐β1 inhibits the wound closure of primary airway epithelial cells, and blocking of TGF‐β1 by antibodies accelerates the rate of stretch wound closure in primary airway epithelial monolayers.20,21 The increased production of TGF‐β1 could not be suppressed by high‐dose corticosteroid treatment in bleomycin‐induced pulmonary fibrosis.22 These results may explain the ineffectiveness of corticosteroid treatment in patients with IPF, and suggest the requirement of novel therapy based on the inhibition of TGF‐β1 signalling.
Take‐home messages
Transforming growth factor‐β1 (TGF‐β1) has the potential to induce acute inflammation and apoptosis in lung epithelial cells and plays a central role in subsequent fibrosis.
A new anti‐TGF‐β1 therapy against lung injury and fibrosis, which comprises the transfection of soluble TGF type II receptor gene into skeletal muscles by in vivo electroporation, attenuated the development of bleomycin‐induced pulmonary fibrosis in mice.
Since this method does not require the use of viral vector or neutralising antibody, it may have clinical application in the treatment of lung injury and fibrosis.
In conclusion, sTGFRII gene transfer ameliorated both the early inflammatory phase and late fibrotic phase of bleomycin‐induced pneumopathy in mice. The inhibition of TGF‐β1 activity may be an essential strategy for treating acute lung injury and chronic interstitial inflammation and fibrosis. Because this method does not require the use of viral vector or TGF‐β1 neutralising antibody, it is possible to avoid problems regarding the pathogenicity of viral vector and immunocomplex. Although the bleomycin model does not reproduce the histological pattern of IPF, soluble TGFRII gene transfer into muscles may have clinical application in the treatment of lung injury and fibrosis.
Abbreviations
IPF - idiopathic pulmonary fibrosis
TGF - transforming growth factor
TGFR - transforming growth factor receptor
Footnotes
Funding: This work was supported by a Grant‐in‐Aid for Scientific Research (17590793) from the Ministry of Education, Science and Culture of Japan.
Competing interests: None declared.
References
- 1.American Thoracic Society and European Respiratory Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med 2000161646–664. [DOI] [PubMed] [Google Scholar]
- 2.Khalil N, O'Connor R N, Unruh H W.et al Increased production and immunohistochemical localization of transforming growth factor‐beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 19915155–162. [DOI] [PubMed] [Google Scholar]
- 3.Sime P J, Xing Z, Graham F L.et al Adenovector‐mediated gene transfer of active transforming growth factor‐beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997100768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalil N, Bereznay O, Sporn M.et al Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med 1989170727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittet J F, Griffiths M J, Geiser T.et al TGF‐beta is a critical mediator of acute lung injury. J Clin Invest 20011071537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagimoto N, Kuwano K, Inoshima I.et al TGF‐beta 1 as an enhancer of Fas‐mediated apoptosis of lung epithelial cells. J Immunol 20021686470–6478. [DOI] [PubMed] [Google Scholar]
- 7.Inoshima I, Kuwano K, Hamada N.et al Anti‐monocyte chemoattractant protein‐1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2004286L1038–L1044. [DOI] [PubMed] [Google Scholar]
- 8.Lawson B R, Prud'homme G J, Chang Y.et al Treatment of murine lupus with cDNA encoding IFN‐γR/Fc. J Clin Invest 2000106207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Wang Y, Hyde D M.et al Reduction of bleomycin induced lung fibrosis by transforming growth factor beta soluble receptor in hamsters. Thorax 199954805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeyama T, Kuwano K, Kawasaki M.et al Attenuation of bleomycin‐induced pneumopathy in mice by monoclonal antibody to interleukin‐12. Am J Physiol Lung Cell Mol Physiol 2001280L1128–L1137. [DOI] [PubMed] [Google Scholar]
- 11.Tsuburai T, Suzuki M, Nagashima Y.et al Adenovirus‐mediated transfer and overexpression of heme oxygen 1 cDNA in lung prevents bleomycin‐induced pulmonary fibrosis via a Fas‐Fas ligand‐independent pathway. Hum Gene Ther 2002131945–1960. [DOI] [PubMed] [Google Scholar]
- 12.Smith R E, Strieter R M, Zhang K.et al A role for C‐C chemokines in fibrotic lung disease. J Leukoc Biol 199557782–787. [DOI] [PubMed] [Google Scholar]
- 13.Chandler D B, Hyde D M, Giri S N. Morphometric estimates of infiltrative cellular changes during the development of bleomycin‐induced pulmonary fibrosis in hamsters. Am J Pathol 1983112170–177. [PMC free article] [PubMed] [Google Scholar]
- 14.Wrana J L, Attisano L, Wieser R.et al Mechanism of activation of the TGF‐beta receptor. Nature 1994370341–347. [DOI] [PubMed] [Google Scholar]
- 15.Heldin C H, Miyazono K, ten Dijke P. TGF‐beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997390465–471. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni A B, Huh C G, Becker D.et al Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 199390770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 200329S93–S97. [PubMed] [Google Scholar]
- 18.Bonniaud P, Margetts P J, Ask K.et al TGF‐beta and Smad3 signaling link inflammation to chronic fibrogenesis. J Immunol 20051755390–5395. [DOI] [PubMed] [Google Scholar]
- 19.Kuwano K, Kawasaki M, Maeyama T, Soluble form of fas and fas ligand in BAL fluid from patients with pulmonary fibrosis and bronchiolitis obliterans organizing pneumonia et alChest 2000118451–458. [DOI] [PubMed] [Google Scholar]
- 20.Spurzem J R, Sacco O, Rickard K A.et al Transforming growth factor‐beta increases adhesion but not migration of bovine bronchial epithelial cells to matrix proteins. J Lab Clin Med 199312292–102. [PubMed] [Google Scholar]
- 21.Neurohr C, Nishimura S L, Sheppard D. Activation of transforming growth factor‐beta by the integrin alphavbeta8 delays epithelial wound closure. Am J Respir Cell Mol Biol 200635252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil N, Whitman C, Zuo L.et al Regulation of alveolar macrophage transforming growth factor‐beta secretion by corticosteroids in bleomycin‐induced pulmonary inflammation in the rat. J Clin Invest 1993921812–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]