Abstract
Background
Leptin is an adipocyte‐derived neurohormone, high levels of which are found in obese individuals. Leptin controls energy expenditure, acting in the brain, and regulates different processes in peripheral organs. Recent studies have suggested that leptin may be involved in cancer development and progression.
Aims
To analyse leptin expression in human colorectal cancer as well as in colorectal mucosa and colorectal adenomas.
Methods
Leptin expression was assessed by immunohistochemistry in 166 colorectal cancers, 101 samples of colorectal mucosa and 41 adenomas. Leptin concentration in colorectal cancer was correlated with selected clinicopathological features.
Results
Immunoreactivity for leptin was observed in 51.2% (85/166) of primary colorectal cancers. In adenomas leptin expression was observed in 14.6% (6/41) of studied cases. In normal mucosa, leptin was present at low levels, except in tumour bordering areas where its concentration appeared to reflect levels in the adjacent cancer tissue. Leptin expression in colorectal cancer significantly correlated with tumour G2 grade (p = 0.002) as well as with histological type (adenocarcinoma) of tumours (p = 0.044).
Conclusions
Results indicate that leptin is overexpressed in human colorectal cancer, which suggests that the hormone might contribute to colorectal cancer development and progression.
Keywords: leptin, normal colorectal mucosa, adenoma, colorectal cancer
Leptin, the product of the ob (obese) gene, is a 16‐kDa peptide cytokine; it plays a central role in mammalian feeding behaviour and energy expenditure.1 In addition to its neurohormonal action in the brain, the cytokine is involved in numerous processes in peripheral organs. Although leptin is produced mainly by the adipose tissue, its expression has also been detected in other tissues, including the digestive tract.2,3,4,5,6,7 In humans, leptin levels are proportional to body mass index and are raised in obese individuals.8 Since obesity is known to increase the risk of certain cancers, much effort has been directed at elucidating the possible role of leptin in cancer development.9,10 Several in vitro studies showed that leptin can act as a mitogenic, antiapoptotic and tumourigenic factor for different cancer cell lines.11,12,13,14 Data obtained with clinical samples suggested that leptin and its receptor (ObR) are expressed in cancer tissues; thus the leptin system might promote cancer progression in an autocrine and paracrine manner.15,16,17,18,19 Our latest results with breast cancer samples showed that leptin and ObR are overexpressed in tumour tissue with respect to non‐tumour tissue. Furthermore, we have shown that in breast cancer cells, leptin and ObR expression can be induced by obesity‐related stimuli: oestrogens, insulin‐like growth factors and hypoxia.18
Previous studies have suggested that paracrine and autocrine leptin might regulate diverse gastric epithelial cell functions.7 For instance, leptin appears to control local inflammatory responses and might prevent gastric ulcer formation by increasing the activities of the cyclo‐oxygenase and nitric oxide pathways and by enhancing mucus secretion.20,21,22 In human colonic goblet‐like HT29‐MTX cells, leptin increased mucin secretion by the activation of protein kinase C (PKC) and phosphatidylinositol 3‐kinase (PI‐3K)‐dependent pathways.23
Studies in cellular models suggested involvement of leptin in colorectal carcinogenesis. For example, leptin stimulated the proliferation and migration of normal intestinal epithelial cells and colorectal cancer cells, and exerted antiapoptotic properties in HT‐29 cells.11,12,14 In different colorectal cancer cell lines (HT‐29, LoVo, Caco2, SW 480), leptin treatment activated the mitogen‐activated protein kinase (MAPK) and PI‐3K pathways, and induced DNA synthesis.11,12,13 Hong et al found leptin expression in gastric adenocarcinomas and suggested a pathophysiological role in carcinogenesis.24 Additionally, leptin mRNA expression was detected in normal mucosa, polyps and colorectal adenocarcinomas.11
The involvement of endocrine leptin in gastric cancer development is still controversial. For instance, Liu et al postulated that a high fat diet might promote carcinogenesis by increasing circulating leptin levels.25 In that study on rats, high concentrations of serum leptin corresponded to increased colonic cell proliferation, c‐fos protein expression and aberrant crypt foci.25 Similarly, Stattin et al observed that high serum leptin levels in patients correlated with increased risk of colon, but not rectal, cancer.26 However, in another study, there was no evidence of increased serum leptin levels in patients with colorectal cancer.27 Moreover, Bolukbas et al observed a significantly lower serum leptin concentration in the gastrointestinal cancer group compared with controls.28 Similarly, general hyperleptinaemia did not enhance the development of intestinal adenomas and did not promote the growth of human cancer xenografts in nude mice.13 In another study, leptin reduced the development of preneoplastic aberrant crypt foci, which were induced by colon carcinogens in the rat colonic epithelium.29
These data suggest that further studies are required to explain the potential role of leptin in the pathogenesis of colorectal cancer. Because the concentration of local leptin in colorectal cancer has never been investigated, we investigated whether leptin is expressed in colorectal tumours, and whether its levels correlate with tumour progression and other clinicopathological parameters.
Materials and methods
Tissue samples
The expression of leptin was assessed in colorectal mucosa, colorectal adenomas and colorectal cancer. Tissue samples were obtained from 166 patients (88 men and 78 women) who underwent surgical resection because of colon (87 cases) and rectal (79 cases) carcinomas. Immediately after excision, tissue samples were fixed in 10% buffered formaldehyde solution, embedded in paraffin blocks at 56°C and stained with H&E. The protocol of the present study was reviewed and approved by the local ethical committee.
Colorectal mucosa
A total of 101 samples of colorectal mucosa were analysed: 28 were from the surgical resection margin and 73 directly bordered (within 1.0 cm) on colorectal tumours.
Adenomas
A total of 41 samples of adenomas were studied, including samples from low to high grade of dysplasia.
Colorectal cancer samples
A total of 166 colorectal cancer samples were analysed: 141 adenocarcinomas and 25 mucinous adenocarcinomas. Within all cancer samples, 118 were G2 grade and 48 cases were G3 grade; 3 tumours were in pT1 stage, 14 in pT2 stage, 128 in pT3 stage and 21 in pT4 stage. For statistical analyses, because of both clinical staging and the relatively small number of pT1, pT2 and pT4 cases, the samples were divided into two groups, pT1 + pT2 and pT3 + pT4. Involved lymph nodes at the time of diagnosis were present in 84 of 166 (50.6%) patients. The age of patients ranged from 35 to 92 years (mean 65.6 years).
Immunohistochemistry
Leptin expression was analysed by immunohistochemistry in 5 μm tissue sections obtained from tissue samples using previously described methodology.18 After antigen unmasking and endogenous peroxidase removal, non‐specific binding was blocked by incubating the slides for 1 h with 1.5% normal serum in phosphate‐buffered saline. The sections were then incubated with an anti‐leptin rabbit polyclonal antibody A‐20 (Santa Cruz Biotechnology, Santa Cruz, California, USA) at dilution 1:100. Antibody–antigen reaction was revealed with avidin–biotin–peroxidase complex (ABC Staining System, Santa Cruz, California, USA); the slides were then counterstained with haematoxylin. Specimens previously classified by us as positive for the expression of leptin were used as a positive control.18 In negative controls, the leptin antibody was omitted. The expression of leptin was analysed by light microscopy in 10 different section fields and the mean percentage of tumour cells displaying positive staining was scored. The expression of leptin in cancer samples was classified using a 3‐point scale: 0, <10% cells with positive staining; 1+, 10–50% cells with positive staining; 2+, >50% cells with positive staining. The expression of leptin in adenomas and colorectal mucosa was classified as negative (<5% of positive cells) or positive (>5% positive cells).
Statistical analysis
The associations of leptin with clinicopathological features were evaluated using the χ2 test. Values of p<0.05 were taken as statistically significant.
Results
Leptin expression in colorectal mucosa
Leptin was absent from all 28 studied sections of normal colorectal mucosa from the surgical resection margin (at a distance at least 10 cm from tumour). However, in several cases, colorectal mucosa directly bordering on colorectal tumours (within 1 cm) showed leptin immunoreactivity. The concentration of leptin in the bordering mucosa appeared to reflect the level of leptin expression in the adjacent cancer tissue (fig 1). In mucosa bordering on leptin‐negative colorectal tumours, focal expression of leptin was found in only 5 out of 40 cases (12.5%) (mainly at the luminal surface) (table 1). In cases of cancers expressing leptin at 1+ or 2+ levels, leptin was more often expressed in the bordering colorectal mucosa (in 20% and 23.1%, respectively), in all parts (superficial and basal) of the colorectal crypts (table 1).
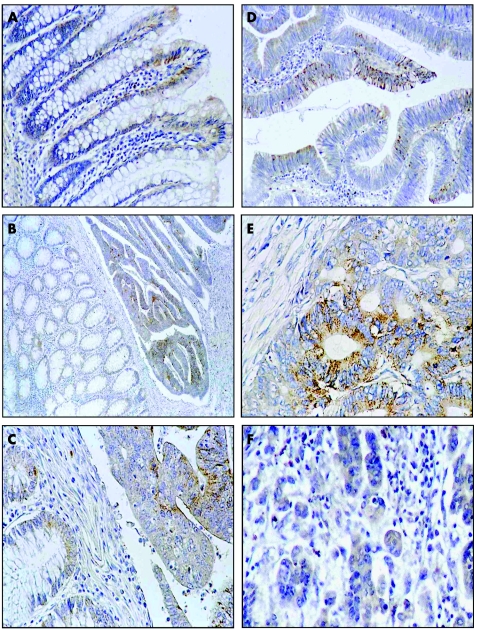
Figure 1 Immunohistochemical detection of leptin expression in colorectal mucosa, adenoma and colorectal cancer. Cytoplasmic leptin expression, mainly at the luminal surface, in colorectal mucosa (A). Weak, focal leptin expression in all parts of colorectal crypts in colorectal mucosa adjacent to cancer tissue expressing leptin at 1+ level (B and C). Focal leptin expression in a few epithelial cells of colorectal adenoma (D). Strong leptin immunoreactivity is present in colorectal cancer in grade G2 of histological differentiation (E), while significantly lower immunostaining can be seen in G3 tumours (F). Original magnification: 200× (A, C, D, E. F); 100× (B).
Table 1 Leptin expression in normal colorectal mucosa directly bordering on colorectal tumours.
| Colorectal mucosa | Adjacent cancer | |
|---|---|---|
| Negative (n) | Positive (n) | |
| 35 | 5* | 0 |
| 16 | 4 | 1+ |
| 3 | 10 | 2+ |
n, number of cases in each staining category.
*Focal expression only; bordering normal tissue was within 1.0 cm of cancer tissue.
Leptin expression in adenomas
The frequency and intensity of leptin immunostaining in tubular, villous and tubulovillous adenomas of different degrees of dysplasia were similar, therefore all cases were treated as one group of adenomas. Of the 41 adenomas, 20 (48.8%) were negative for leptin expression, while leptin expression was detected in 21 (51.2%) (fig 1). In 15 of these latter sections, leptin was expressed in <50% of colorectal epithelial cells; however in 6 cases, it was found in >50% of cells.
Leptin expression in colorectal cancers
Positive leptin expression was detected in 51.2% (85/166) of primary colorectal cancers. In 33.7% (56/166) of studied cases, leptin immunostaining was classified as 1+, while high (2+) leptin expression was found in 17.5% (29/166) of colorectal cancer cases (fig 1). The expression of leptin was undetectable in the control samples when immunostaining was performed with the omission of the primary antibody.
Associations of leptin with clinicopathological features
Analysis of associations revealed a statistically significant relationship between leptin expression in colorectal cancer and tumour differentiation (table 2). Leptin was expressed significantly less in tumours in grade G3 of histological differentiation than in grade G2 (p = 0.002; table 2). A statistically significant weak association was observed between leptin expression and histological type of tumours (p = 0.044; table 2). The expression of leptin did not correlate significantly with age, sex of patients, tumour localisation or lymph node involvement (table 2).
Table 2 Leptin expression in colorectal cancer and clinicopathological characteristics of patients.
| Clinicopathological features | Leptin expression | p‐Value | ||
|---|---|---|---|---|
| 0 | 1+ | 2+ | ||
| n = 81 | n = 56 | n = 29 | ||
| Age (years) | ||||
| ⩽60 (n* = 52) | 25 (48.1%) | 16 (30.8%) | 11 (21.1%) | 0.672 |
| >60 (n* = 114) | 56 (49.1%) | 40 (35.1%) | 18 (15.8%) | |
| Gender | ||||
| Male (n* = 88) | 41 (46.6%) | 33 (37.5%) | 14 (15.9%) | 0.539 |
| Female (n* = 78) | 40 (51.3%) | 23 (29.5%) | 15 (19.2%) | |
| Tumour localisation | ||||
| Rectum (n* = 79) | 38 (48.2%) | 26 (32.9%) | 15 (18.9%) | 0.885 |
| Colon (n = 87; 100%) | 43 (49.4%) | 30 (34.5%) | 14 (16.1%) | |
| Histological type | ||||
| Adc (n* = 141) | 66 (46.8%) | 46 (32.6%) | 29 (20.6%) | 0.044 |
| Adc muc (n* = 25) | 15 (60.0%) | 10 (40.0%) | 0 (0%) | |
| Histological differentiation | ||||
| G2 (n* = 118) | 51 (43.2%) | 39 (33.1%) | 28 (23.7%) | 0.002 |
| G3 (n* = 48) | 30 (62.5%) | 17 (35.4%) | 1 (2.1%) | |
| Tumour size | ||||
| pT1+pT2 (n* = 17) | 7 (41.2%) | 7 (41.2%) | 3 (17.6%) | 0.764 |
| pT3+pT4 (n* = 149) | 74 (49.7%) | 49 (32.9%) | 26 (17.4%) | |
| Lymph node involvement | ||||
| Negative (n* = 82) | 40 (48.8%) | 26 (31.7%) | 16 (19.5%) | 0.746 |
| Positive (n* = 84) | 41 (48.8%) | 30 (35.7%) | 13 (15.5%) | |
n*, number of cases taken as 100%.
Adc, adenocarcinoma; Adc muc, adenocarcinoma mucinosum.
Discussion
Epidemiological data have suggested that obesity is a risk factor for colon cancer development25,26; however, possible molecular mechanisms underlying this phenomenon remain unclear. In this context, several recent studies examined the link between the obesity hormone leptin and colorectal cancer. The in vitro work clearly suggested that colorectal cancer cells can express leptin receptors and respond to leptin with the activation of downstream signalling pathways and subsequent induction of DNA synthesis, cell proliferation, migration and invasion.11,12,13,14 However, studies using animal models as well as epidemiological data yielded controversial results, which noted either a positive or a negative correlation between serum leptin levels and colorectal cancer (or colorectal cancer risk). Additionally, some reports indicated that circulating leptin has no association with colorectal cancer.26,27,28,30
Previous work of our and other laboratories suggested that local, rather then endocrine, leptin might play a significant role in breast tumourigenesis. Specifically, both leptin and ObR (mRNA and protein) were found in breast tumours, indicating that leptin could influence cancer cells through autocrine or paracrine mechanisms.15,17,18 Importantly, the levels of leptin and ObR were significantly raised in breast cancer cells relative to normal mammary epithelial cells, and this overexpression appeared to be induced by obesity‐related stimuli.18
Until now, the expression of leptin protein in colorectal cancer has not been investigated. In the current immunohistochemistry study we showed that, similarly to breast tumours, leptin is overexpressed in human colorectal cancers relative to colorectal mucosa. Our results suggested a progressive increase in leptin expression during colorectal carcinogenesis. Weak or undetectable leptin immunostaining was observed in colorectal mucosa, while higher leptin levels were found in tissues adjacent to colorectal cancer. We also noted decreased leptin expression in poorly differentiated (G3) tumours relative to better differentiated (G2) tumours. Interestingly, downregulation of leptin expression in less differentiated tumours was also observed by other authors in gastric adenocarcinomas, which could indicate a trend for silencing leptin expression during dedifferentiation of gastric cancers.24
Take‐home messages
Obesity is a risk factor for cancer development. In vitro investigation showed that leptin may be involved in colorectal carcinogenesis. However, studies using animal models as well as epidemiological data have yielded controversial results, noting any correlation between serum leptin levels and colorectal cancer risk.
In the current study, leptin expression was evaluated by immunohistochemistry during colorectal carcinogenesis. Results suggest that in normal colorectal epithelium and colorectal adenomas, leptin expression is relatively weak, but the hormone is overproduced in colorectal cancer.
Leptin may correlate with tumour G2 grade as well as with histological type of tumours. It is postulated that leptin might contribute to colorectal cancer growth and progression.
The molecular mechanism responsible for leptin overexpression in colorectal cancer is presently unknown; however, we speculate that it could be related to local hypoxia in cancer tissue. Indeed, in several cellular systems, including breast cancer cells, leptin mRNA expression is induced by hypoxia, and the leptin gene promoter is regulated by hypoxia‐inducible factor‐1α (HIF‐1α).18,31,32 Our preliminary analysis of colorectal cancer tissues suggested that, similar to leptin, HIF‐1α was significantly associated with the G2 grade tumours (data not shown).
In summary, our study is the first to evaluate leptin protein expression in a relatively high number of samples of normal mucosa and colorectal cancer. Other investigators described leptin mRNA in normal mucosa, polyps and colorectal adenocarcinomas, but they did not quantify leptin protein expression and its correlation with cancer progression.11 Our results suggest that in normal epithelium and colorectal adenomas leptin expression is relatively weak, but the hormone is overproduced in colorectal cancer. We postulate that this overabundance of local leptin might contribute to colorectal cancer growth and progression.
Footnotes
Funding: This work was supported in part by the Foundation for Polish Science (MK) and the WW Smith Charitable Trust (ES).
Competing interests: None declared.
References
- 1.Attele A S, Shi Z Q, Yuan C S. Leptin, gut, and food intake. Biochem Pharmacol 2002631579–1583. [DOI] [PubMed] [Google Scholar]
- 2.Smith‐Kirwin S M, O'Connor D M, De Johnston J.et al Leptin expression in human mammary epithelial cells and breast milk. J Clin Endocrinol Metab 1998831810–1813. [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Liu R, Hawkins M.et al A nutrient‐sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998393684–688. [DOI] [PubMed] [Google Scholar]
- 4.Senaris R, Garcia‐Caballero T, Casabiell X.et al Synthesis of leptin in human placenta. Endocrinology 19971384501–4504. [DOI] [PubMed] [Google Scholar]
- 5.Aparicio T, Kermorgant S, Darmoul D.et al Leptin and Ob‐Rb receptor isoform in the human digestive tract during fetal development. J Clin Endocrinol Metab 2005906177–6184. [DOI] [PubMed] [Google Scholar]
- 6.Bohlender J, Rauh M, Zenk J.et al Differential distribution and expression of leptin and the functional leptin receptor in major salivary glands of humans. J Endocrinol 2003178217–223. [DOI] [PubMed] [Google Scholar]
- 7.Mix H, Widjaja A, Jandl O.et al Expression of leptin and leptin receptor isoforms in the human stomach. Gut 200047481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauter E R, Garofalo C, Hewett J.et al Leptin expression in breast nipple aspirate fluid (NAF) and serum is influenced by body mass index (BMI) but not by the presence of breast cancer. Horm Metab Res 200436336–340. [DOI] [PubMed] [Google Scholar]
- 9.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol 200620712–22. [DOI] [PubMed] [Google Scholar]
- 10.Sulkowska M, Golaszewska J, Wincewicz A.et al Leptin—from regulation of fat metabolism to stimulation of breast cancer growth. Pathol Oncol Res 20061269–72. [DOI] [PubMed] [Google Scholar]
- 11.Attoub S, Noe V, Pirola L.et al Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3‐kinase‐, rho‐, and rac‐dependent signaling pathways. FASEB J 2000142329–2338. [DOI] [PubMed] [Google Scholar]
- 12.Hardwick J C, Van Den Brink G R, Offerhaus G J.et al Leptin is a growth factor for colonic epithelial cells. Gastroenterology 200112179–90. [DOI] [PubMed] [Google Scholar]
- 13.Aparicio T, Kotelevets L, Tsocas A.et al Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in Apc(Min/+) mice. Gut 2005541136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouet‐Benzineb P, Aparicio T, Guilmeau S.et al Leptin counteracts sodium butyrate‐induced apoptosis in human colon cancer HT‐29 cells via NF‐kappaB signaling. J Biol Chem 200427916495–16502. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB‐R) in human breast cancer. Clin Cancer Res 2004104325–4331. [DOI] [PubMed] [Google Scholar]
- 16.Bogusiewicz M, Semczuk A, Gogacz M.et al Lack of correlation between leptin receptor expression and PI3‐K/Akt signaling pathway proteins immunostaining in endometrioid‐type endometrial carcinomas. Cancer Lett 200623861–68. [DOI] [PubMed] [Google Scholar]
- 17.Frankenberry K A, Skinner H, Somasundar P.et al Leptin receptor expression and cell signaling in breast cancer. Int J Oncol 200628985–993. [PubMed] [Google Scholar]
- 18.Garofalo C, Koda M, Cascio S.et al Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity‐related stimuli. Clin Cancer Res 2006121447–1453. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Shen Z X, Luo H S.et al Possible involvement of leptin and leptin receptor in developing gastric adenocarcinoma. World J Gastroenterol 2005117666–7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bado A, Levasseur S, Attoub S.et al The stomach is a source of leptin. Nature 1998394790–793. [DOI] [PubMed] [Google Scholar]
- 21.Barrachina M D, Martinez V, Wang L.et al Synergistic interaction between leptin and cholecystokinin to reduce short‐term food intake in lean mice. Proc Natl Acad Sci USA 19979410455–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adeyemi E O, Bastaki S A, Chandranath I S.et al Mechanisms of action of leptin in preventing gastric ulcer. World J Gastroenterol 2005114154–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plaisancie P, Ducroc R, Homsi M E.et al Luminal leptin activates mucin‐secreting goblet cells in the large bowel. Am J Physiol Gastrointest Liver Physiol 2006290805–812. [DOI] [PubMed] [Google Scholar]
- 24.Hong S J, Kwon K W, Kim S G.et al Variation in expression of gastric leptin according to differentiation and growth pattern in gastric adenocarcinoma. Cytokine 20063366–71. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Uesaka T, Watanabe H.et al High fat diet enhances colonic cell proliferation and carcinogenesis in rats by elevating serum leptin. Int J Oncol 2001191009–1014. [DOI] [PubMed] [Google Scholar]
- 26.Stattin P, Lukanova A, Biessy C.et al Obesity and colon cancer: does leptin provide a link? Int J Cancer 2004109149–152. [DOI] [PubMed] [Google Scholar]
- 27.Tessitore L, Vizio B, Jenkins O.et al Leptin expression in colorectal and breast cancer patients. Int J Mol Med 20005421–426. [DOI] [PubMed] [Google Scholar]
- 28.Bolukbas F F, Kilic H, Bolukbas C.et al Serum leptin concentration and advanced gastrointestinal cancers: a case controlled study. BMC Cancer 2004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aparicio T, Guilmeau S, Goiot H.et al Leptin reduces the development of the initial precancerous lesions induced by azoxymethane in the rat colonic mucosa. Gastroenterology 2004126499–510. [DOI] [PubMed] [Google Scholar]
- 30.Stattin P, Soderberg S, Biessy C.et al Plasma leptin and breast cancer risk: a prospective study in northern Sweden. Breast Cancer Res Treat 200486191–196. [DOI] [PubMed] [Google Scholar]
- 31.Grosfeld A, Andre J, Hauguel‐De Mouzon S.et al Hypoxia‐inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem 200227742953–42957. [DOI] [PubMed] [Google Scholar]
- 32.Meissner U, Ostreicher I, Allabauer I.et al Synergistic effects of hypoxia and insulin are regulated by different transcriptional elements of the human leptin promoter. Biochem Biophys Res Commun 2003303707–712. [DOI] [PubMed] [Google Scholar]